Diagnostic Accuracy of LDBIO-Toxo II IgG and IgM Western Blot in Suspected Seroconversion in Pregnancy: A Multicentre Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert-Gangneux, F.; Darde, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2000, 25, 264–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyron, F.; Wallon, M.; Kieffer, F.; Garweg, J. Toxoplasmosis. In Infectious Diseases of the Fetus and Newborn Infant; Remington, J.S., Klein, J.O., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2016; pp. 949–1042. [Google Scholar]
- Peyron, F.; L’Ollivier, C.; Mandelbrot, L.; Wallon, M.; Piarroux, R.; Kieffer, F.; Hadjadje, E.; Paris, L.; Garcia-Meric, P. Maternal and Congenital Toxoplasmosis: Diagnosis and Treatment Recommendations of a French Multidisciplinary Working Group. Pathogens 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallon, M.; Peyron, F. Effect of Antenatal Treatment on the Severity of Congenital Toxoplasmosis. Clin. Infect. Dis. 2016, 62, 811–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusa, A.R.; Kasper, D.C.; Sawers, L.; Walter, E.; Hayde, M.; Stillwaggon, E. Congenital toxoplasmosis in Austria: Prenatal screening for prevention is cost-saving. PLoS Negl. Trop. Dis. 2017, 10, e0005648. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, L.R.; Meroni, V.; Bonfanti, C.; Bollani, L.; Lanzarini, P.; Frusca, T.; Castelli, F. Multidisciplinary approach to congenital Toxoplasma infection: An Italian nationwide survey. New Microbiol. 2014, 37, 347–354. [Google Scholar] [PubMed]
- Armengol, C.; Cassaing, S.; Roques-Malecaze, C.; Chauvin, P.; Iriart, X.; Berry, A.; Fillaux, J. Time before anti-Toxoplasma IgG seroconversion detection by 7 commercial assays in French pregnant women. Diagn. Microbiol. Infect. Dis. 2017, 87, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Douet, T.; Armengol, C.; Charpentier, E.; Chauvin, P.; Cassaing, S.; Iriart, X.; Berry, A.; Fillaux, J. Performance of seven commercial automated assays for the detection of low levels of anti-Toxoplasma IgG in French immunocompromised patients. Parasite 2019, 26, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murat, J.B.; Dard, C.; Fricker-Hidalgo, H.; Dardé, M.L.; Brenier-Pinchart, M.P.; Pelloux, H. Comparison of the Vidas system and two recent fully automated assays for diagnosis and follow-up of toxoplasmosis in pregnant women and newborns. Clin. Vaccine Immunol. 2013, 20, 1203–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villard, O.; Cimon, B.; L’Ollivier, C.; Fricker-Hidalgo, H.; Godineau, N.; Houze, S.; Paris, L.; Pelloux, H.; Villena, I. Serological diagnosis of Toxoplasma gondii infection: Recommendations from the French National Reference Center for Toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2016, 84, 22–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meroni, V.; Genco, F.; Tinelli, C.; Lanzarini, P.; Bollani, L.; Stronati, M.; Petersen, E. Spiramycin treatment of Toxoplasma gondii infection in pregnant women impairs the production and the avidity maturation of T. gondii-specific immunoglobulin G antibodies. Clin. Vaccin. Immunol. 2009, 16, 1517–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franck, J.; Garin, Y.J.F.; Dumon, H. LDBio-Toxo II immunoglobulin G Western blot confirmatory test for anti-toxoplasma antibody detection. J. Clin. Microbiol. 2008, 46, 2334–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, C.; Touafek, F.; Fekkar, A.; Courtin, R.; Ribeiro, M.; Mazier, D.; Paris, L. Utility of immunoblotting for early diagnosis of toxoplasmosis seroconversion in pregnant women. Clin. Vaccin. Immunol. 2011, 18, 1908–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khammari, I.; Saghrouni, F.; Lakhal, S.; Bouratbine, A.; Ben Said, M.; Jalel, B.A. New IgG Immunoblot Kit for Diagnosis of Toxoplasmosis in Pregnant Women. Korean J. Parasitol. 2014, 52, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Khammari, I.; Saghrouni, F.; Yacoub, A.; Gaied Meksi, S.; Ach, H.; Garma, L.; Fathallah, A.; Ben Said, M. IgG Western Blot for Confirmatory Diagnosis of Equivocal Cases of Toxoplasmosis by EIA-IgG and Fluorescent Antibody Test. Korean J. Parasitol. 2013, 51, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Leslé, F.; Touafek, F.; Fekkar, A.; Mazier, D.; Paris, L. Discrepancies between a new highly sensitive Toxoplasma gondii ELISA assay and other reagents: Interest of Toxo IgG Western blot. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Maudry, A.; Chene, G.; Chatelain, R.; Patural, H.; Bellete, B.; Tisseur, B.; Raberin, H.; Beretta, S.; Sung, R.T.M.; Hafid, J. Expertise du nouveau test Access® TOXO-IgGII et comparaison avec trois autres techniques automatisées et la technique Western Blot LDBIO TOXO II IgG®. Immuno-Anal. Biol. Spécialisée 2009, 24, 42–49. [Google Scholar] [CrossRef]
- Maudry, A.; Chene, G.; Chatelain, R.; Patural, H.; Bellete, B.T.; Hafid, J. Bicentric evaluation of six anti-toxoplasma immunoglobulin G (IgG) automated immunoassays and comparison to the Toxo II IgG Western blot. Clin. Vaccin. Immunol. 2009, 16, 1322–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, L.; Fillaux, J.; Guigon, A.; Lavergne, R.A.; Villard, O.; Villena, I.; Marty, P.; Pomares, C.; Toxoplasma p35 Study Group. Serological diagnosis of Toxoplasma gondii: Analysis of false-positive IgG results and implications. Parasite 2020, 27, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dard, C.; Bailly, S.; Drouet, T.; Fricker-Hidalgo, H.; Brenier-Pinchart, M.P.; Pelloux, H. Long-term sera storage does not significantly modify the interpretation of toxoplasmosis serologies. J. Microbiol. Methods 2017, 134, 38–45. [Google Scholar] [CrossRef] [PubMed]
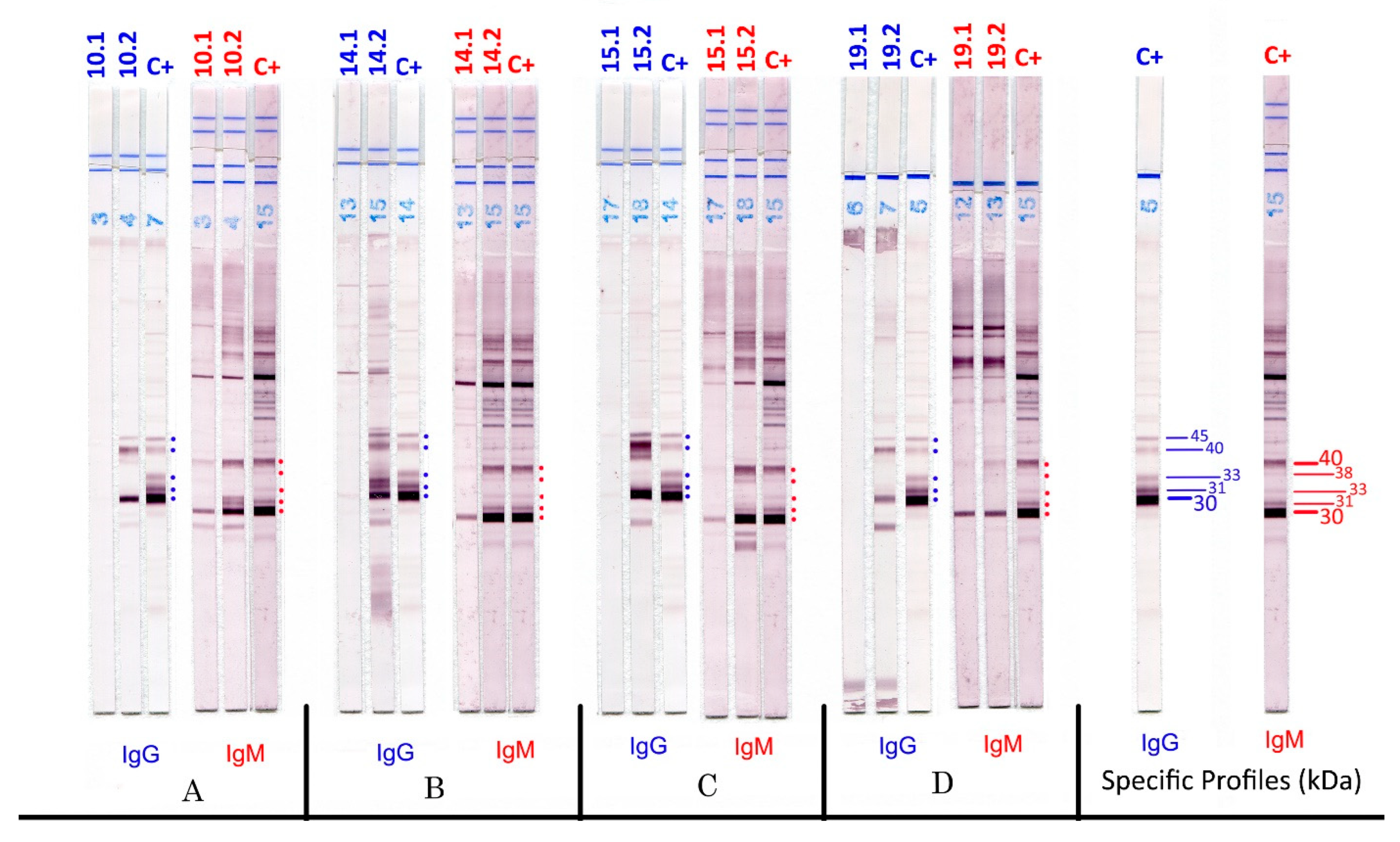
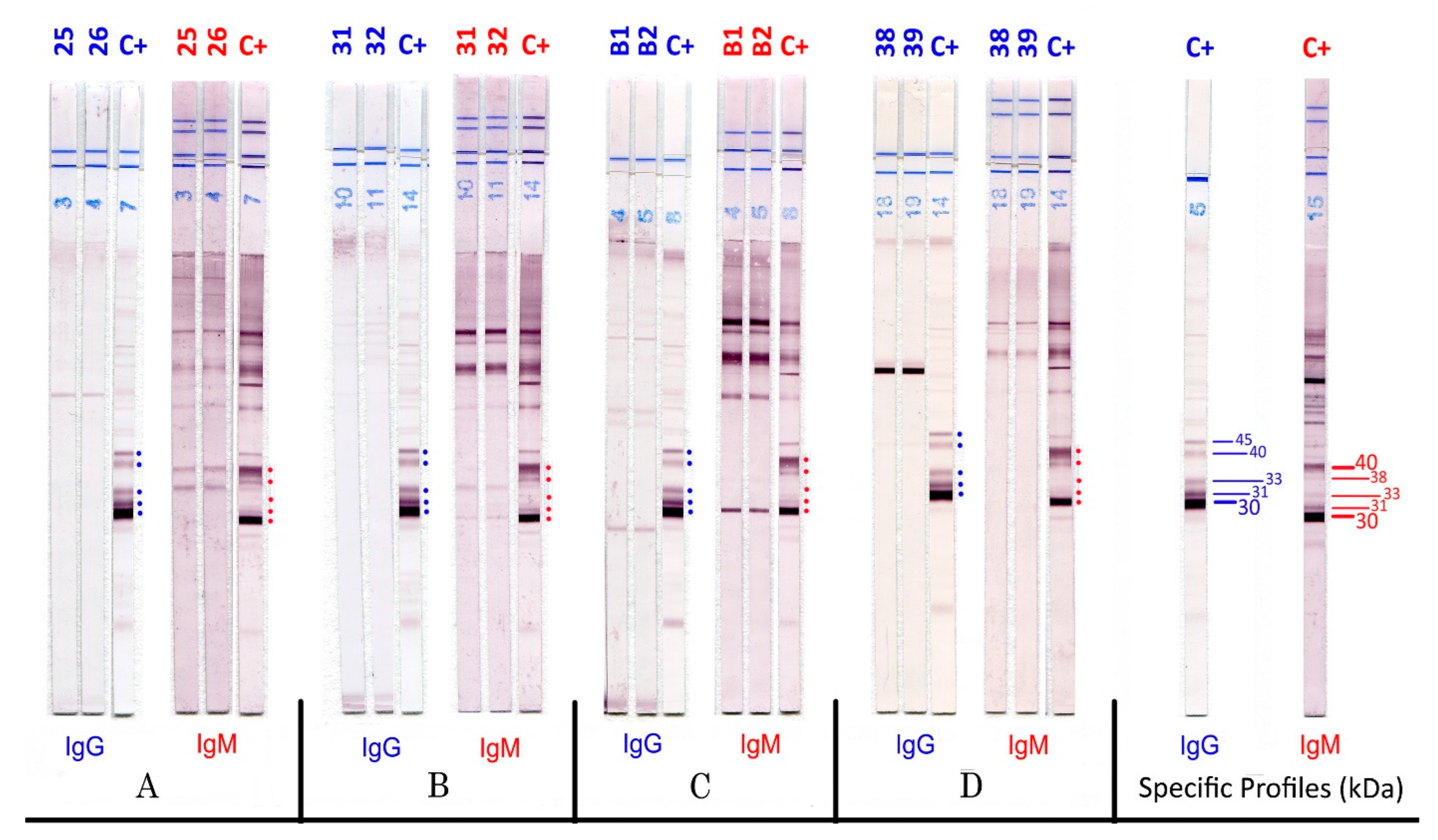
| Centre | Seroconversions | Aspecific IgM | Tests | ||
|---|---|---|---|---|---|
| Samples | Patients | Samples | Patients | ||
| Grenoble | 48 | 20 | 38 | 17 | IgM ISAGA (bioMérieux, France) VIDAS TOXO IgG-IgM (bioMérieux, France), Toxo IgG-IgM Architect (Abbott Diagnostic, Germany) |
| Marseille | 69 | 25 | 4 | 2 | IgM ISAGA (bioMérieux, France) Toxo IgG-IgM Architect (Abbott Diagnostic, Germany) |
| Paris | 45 | 20 | 60 | 21 | IgM ISAGA (bioMérieux, France) LIAISON® Toxo IgG, IgM, (Diasorin, Italy) Toxo IgG-IgM Platelia (Bio Rad, France) |
| Pavia | 67 | 28 | 56 | 28 | IgM ISAGA (bioMérieux, France) VIDAS Toxo IgG, VIDAS Toxo Avidity,) LIAISON® Toxo IgG, IgM, (Diasorin, Italy), Home-made IGRA (Interferon gamma release assay) |
| Total | 229 | 93 | 158 | 68 | |
| Diagnosis | Toxo II IgM WB | Toxo II IgG WB | |||||
|---|---|---|---|---|---|---|---|
| Positive | Equivocal | Negative | Total | Positive | Negative | Total | |
| Seroconversion | 91 | 2 | 0 | 93 | 92 | 1 | 93 |
| False-positive/ nonspecific IgM | 7 | 19 1 | 42 | 68 | 0 | 68 | 68 |
| Total | 98 | 21 | 42 | 161 | 92 | 69 | 161 |
| Sensitivity (equivocal considered negative) | 97.8% (95CI 91.7–99.6%) | 98.9% (95CI 93.3–99.9%) | |||||
| Sensitivity (equivocal considered positive) | 100% (95CI 95.0–100%) | ||||||
| Specificity (equivocal considered negative) | 89.7% (95CI 79.3–95.4%) | 100% (95CI 93.3–100%) | |||||
| Specificity (equivocal considered positive) | 61.8% (95CI 49.1–73.1%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meroni, V.; Genco, F.; Scudeller, L.; Brenier-Pinchart, M.-P.; Fricker-Hidalgo, H.; L’Ollivier, C.; Paris, L.; Pelloux, H. Diagnostic Accuracy of LDBIO-Toxo II IgG and IgM Western Blot in Suspected Seroconversion in Pregnancy: A Multicentre Study. Pathogens 2022, 11, 665. https://doi.org/10.3390/pathogens11060665
Meroni V, Genco F, Scudeller L, Brenier-Pinchart M-P, Fricker-Hidalgo H, L’Ollivier C, Paris L, Pelloux H. Diagnostic Accuracy of LDBIO-Toxo II IgG and IgM Western Blot in Suspected Seroconversion in Pregnancy: A Multicentre Study. Pathogens. 2022; 11(6):665. https://doi.org/10.3390/pathogens11060665
Chicago/Turabian StyleMeroni, Valeria, Francesca Genco, Luigia Scudeller, Marie-Pierre Brenier-Pinchart, Hélène Fricker-Hidalgo, Coralie L’Ollivier, Luc Paris, and Hervé Pelloux. 2022. "Diagnostic Accuracy of LDBIO-Toxo II IgG and IgM Western Blot in Suspected Seroconversion in Pregnancy: A Multicentre Study" Pathogens 11, no. 6: 665. https://doi.org/10.3390/pathogens11060665
APA StyleMeroni, V., Genco, F., Scudeller, L., Brenier-Pinchart, M.-P., Fricker-Hidalgo, H., L’Ollivier, C., Paris, L., & Pelloux, H. (2022). Diagnostic Accuracy of LDBIO-Toxo II IgG and IgM Western Blot in Suspected Seroconversion in Pregnancy: A Multicentre Study. Pathogens, 11(6), 665. https://doi.org/10.3390/pathogens11060665







