Monitoring and Surveillance of Small Ruminant Health in The Netherlands
Abstract
1. Introduction
2. Results
2.1. Overview of Findings
2.2. Elaborated Examples of Three Findings
2.2.1. Detection of the Incursion of a Known Disease: The Case of Bluetongue
2.2.2. Detection of an Unknown Disease: The Case of Schmallenberg Virus
2.2.3. Monitoring Trends and Developments: The Case of Coxiella burnetii Infections
3. Discussion
4. Materials and Methods
4.1. Small Ruminant Population
4.2. Collection of Information
4.2.1. Helpdesk
4.2.2. Diagnostic Services
4.2.3. National and International Networks
Knowledge Network of Veterinary Practitioners
Signalling Forum Zoonoses (SOZ)
European Veterinary Surveillance Network (EVSN)
4.2.4. Data Analysis
- the animal identification and registration (I&R) database operated by The Netherlands Enterprise Agency (Rijksdienst voor Ondernemend Nederland; RVO Assen, The Netherlands),
- the Trade Control and Expert System (TRACES) of NVWA,
- the database of the Dutch rendering plant Rendac (Son, The Netherlands) in which collected fallen stock is recorded,
- the database of GD in which post-mortem submissions to GD are recorded, and
- the customer relationship management system of GD in which herd characteristics like location (2-digit postal code) and production purpose are recorded.
4.3. Aggregation and Assessment
4.4. Communication and Dissemination
4.5. Representativeness and Efficacy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brückner, G.; Knopf, L.; Mac Diarmid, S.C.; Munstermann, A.-S.; Cameron, A.; Mariner, J.C.; Paisley, L.; Parmley, J.; Roger, F.; Scott, A.; et al. Guide to Terrestrial Animal Health Surveillance; Brückner, G., Mac Diarmid, S.C., Munstermann, A.-S., Eds.; OIE: Paris, France, 2014. [Google Scholar]
- Salman, M.D. Animal Disease Surveillance and Survey Systems: Methods and Applications, 1st ed.; Wiley InterScience, M.D., Ed.; Iowa State Press: Ames, IA, USA, 2003. [Google Scholar]
- Vrbova, L.; Stephen, C.; Kasman, N.; Boehnke, R.; Doyle-Waters, M.; Chablitt-Clark, A.; Gibson, B.; Fitzgerald, M.; Patrick, D.M. Systematic Review of Surveillance Systems for Emerging Zoonoses. Transbound. Emerg. Dis. 2010, 57, 154–161. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; Brouwer-Middelesch, H.; van Wuijckhuise, L.; De Bont-Smolenaars, A.J.G.; van Schaik, G. Surveillance of Cattle Health in the Netherlands: Monitoring Trends and Developments Using Routinely Collected Cattle Census Data. Prev. Vet. Med. 2016, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No. 2016/429. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2016.084.01.0001.01.ENG (accessed on 3 March 2022).
- Commission Regulation (EC) No. 2018/1882. Available online: https://eur-lex.europa.eu/eli/reg_impl/2018/1882/oj (accessed on 2 March 2022).
- European Medicine Agency. Scientific Conclusions and Grounds to Vary the Markteting Authorisations Presented by the EMEA. Available online: https://ec.europa.eu/health/documents/community-register/2000/200007103685/anx_3685_en.pdf (accessed on 1 March 2022).
- Annual Report of Monitoring and Surveillance of Small Ruminant Health. Available online: https://www.gddiergezondheid.nl/diergezondheid/monitoring/hoofdpunten-monitoring-kleine-herkauwers?sc_database=web (accessed on 27 February 2022).
- Eysker, M.; Borgsteede, F.H.M.; Ploeger, H.W.; Vellema, P. Drug Resistance Makes New Control Measures of Stomach Parasites in Small Ruminants Necessary. Tijdschr. Diergeneeskd. 2005, 130, 205–209. [Google Scholar] [PubMed]
- Borgsteede, F.H.M.; Moll, L.; Vellema, P.; Gaasenbeek, C.P.H. Lack of Reversion in Triclabendazole-resistant Fasciola Hepatica. Vet. Rec. 2005, 156, 350–351. [Google Scholar] [CrossRef]
- Borgsteede, F.H.M.; Dercksen, D.D.; Huijbers, R. Doramectin and Albendazole Resistance in Sheep in The Netherlands. Vet. Parasitol. 2007, 144, 180–183. [Google Scholar] [CrossRef]
- Van den Brom, R.; Moll, L.; Borgsteede, F.H.M.; van Doorn, D.C.K.; Lievaart-Peterson, K.; Dercksen, D.P.; Vellema, P. Short Communication: Multiple Anthelmintic Resistance of Haemonchus Contortus, Including a Case of Moxidectin Resistance, in a Dutch Sheep Flock. Vet. Rec. 2013, 173, 552. [Google Scholar] [CrossRef] [PubMed]
- Borgsteede, F.; Verkalk, J.; Moll, L.; Dercksen, D.; Vellema, P.; Bavinck, G. How Widespread Is Resistance to Invermectin among Gastrointestinal Nematodes in Sheep in The Netherlands? Tijdschr. Diergeneeskd. 2010, 135, 782–785. [Google Scholar]
- Moll, L.; Gaasenbeek, C.P.H.; Vellema, P.; Borgsteede, F.H.M. Resistance of Fasciola Hepatica against Triclabendazole in Cattle and Sheep in The Netherlands. Vet. Parasitol. 2000, 91, 153–158. [Google Scholar] [CrossRef]
- Meijer, A.; Brandenburg, A.; de Vries, J.; Beentjes, J.; Roholl, P.; Dercksen, D. Chlamydophila Abortus Infection in a Pregnant Woman Associated with Indirect Contact with Infected Goats. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 487–490. [Google Scholar] [CrossRef]
- Van den Brom, R.; Lievaart-Peterson, K.; Luttikholt, S.; Peperkamp, K.; Wouda, W.; Vellema, P. Abortion in Small Ruminants in the Netherlands between 2006 and 2011. Tijdschr. Diergeneeskd. 2012, 137, 450–457. [Google Scholar]
- Van Engelen, E.; Luttikholt, S.; Peperkamp, K.; Vellema, P.; van den Brom, R. Small Ruminant Abortions in the Netherlands during Lambing Season 2012-2013. Vet. Rec. 2014, 174, 506. [Google Scholar] [CrossRef] [PubMed]
- Van den Brom, R.; Moll, L.; Kappert, C.; Vellema, P. Haemonchus Contortus Resistance to Monepantel in Sheep. Vet. Parasitol. 2015, 209, 278–280. [Google Scholar] [CrossRef]
- Wouda, W.; Dercksen, D.P. Abortion and stillbirth among dairy goats as a consequence of Coxiella burnetii. Tijdschr. Diergeneeskd. 2007, 132, 908–911. [Google Scholar] [PubMed]
- Schimmer, B.; Dijkstra, F.; Vellema, P.; Schneeberger, P.M.; Hackert, V.; ter Schegget, R.; Wijkmans, C.; van Duynhoven, Y.; van der Hoek, W. Sustained Intensive Transmission of Q Fever in the South of the Netherlands, 2009. Euro Surveill. 2009, 14, 19210. [Google Scholar] [CrossRef] [PubMed]
- Van den Brom, R.; Vellema, P. Q Fever Outbreaks in Small Ruminants and People in the Netherlands. Small Rumin. Res. 2009, 86, 74–79. [Google Scholar] [CrossRef]
- Schimmer, B.; ter Schegget, R.; Wegdam, M.; Züchner, L.; de Bruin, A.; Schneeberger, P.M.; Veenstra, T.; Vellema, P.; van der Hoek, W. The Use of a Geographic Information System to Identify a Dairy Goat Farm as the Most Likely Source of an Urban Q-Fever Outbreak. BMC Infect. Dis. 2010, 10, 69. [Google Scholar] [CrossRef]
- Van den Wijngaard, C.C.; Dijkstra, F.; van Pelt, W.; van Asten, L.; Kretzschmar, M.; Schimmer, B.; Nagelkerke, N.J.D.; Vellema, P.; Donker, G.A.; Koopmans, M.P.G. In Search of Hidden Q-Fever Outbreaks: Linking Syndromic Hospital Clusters to Infected Goat Farms. Epidemiol. Infect. 2011, 139, 19–26. [Google Scholar] [CrossRef][Green Version]
- Hogerwerf, L.; van den Brom, R.; Roest, H.I.J.; Bouma, A.; Vellema, P.; Pieterse, M.; Dercksen, D.; Nielen, M. Reduction of Coxiella Burnetii Prevalence by Vaccination of Goats and Sheep, the Netherlands. Emerg. Infect. Dis. 2011, 17, 379. [Google Scholar] [CrossRef]
- Van den Brom, R.; van Engelen, E.; Luttikholt, S.; Moll, L.; van Maanen, K.; Vellema, P. Coxiella Burnetii in Bulk Tank Milk Samples from Dairy Goat and Dairy Sheep Farms in The Netherlands in 2008. Vet. Rec. 2012, 170, 310. [Google Scholar] [CrossRef]
- Van den Brom, R.; Moll, L.; van Schaik, G.; Vellema, P. Demography of Q Fever Seroprevalence in Sheep and Goats in The Netherlands in 2008. Prev. Vet. Med. 2013, 109, 76–82. [Google Scholar] [CrossRef]
- Van den Brom, R.; van Engelen, E.; Vos, J.; Luttikholt, S.J.M.; Moll, L.; Roest, H.I.J.; van der Heijden, H.M.J.F.; Vellema, P. Detection of Coxiella Burnetii in the Bulk Tank Milk from a Farm with Vaccinated Goats, by Using a Specific PCR Technique. Small Rumin. Res. 2013, 110, 150–154. [Google Scholar] [CrossRef]
- Vellema, P.; van den Brom, R. The Rise and Control of the 2007–2012 Human Q Fever Outbreaks in the Netherlands. Small Rumin. Res. 2014, 118, 69–78. [Google Scholar] [CrossRef]
- Van den Brom, R.; Santman-Berends, I.; Luttikholt, S.; Moll, L.; van Engelen, E.; Vellema, P. Bulk Tank Milk Surveillance as a Measure to Detect Coxiella Burnetii Shedding Dairy Goat Herds in the Netherlands between 2009 and 2014. J. Dairy Sci. 2015, 98, 3814–3825. [Google Scholar] [CrossRef]
- Van den Brom, R.; Roest, H.J.; De Bruin, A.; Dercksen, D.; Santman-Berends, I.; Van Der Hoek, W.; Dinkla, A.; Vellema, J.; Vellema, P. A Probably Minor Role for Land-Applied Goat Manure in the Transmission of Coxiella Burnetii to Humans in the 2007–2010 Dutch Q Fever Outbreak. PLoS ONE 2015, 10, e0121355. [Google Scholar] [CrossRef]
- Van den Brom, R.; van Engelen, E.; Roest, H.I.J.; van der Hoek, W.; Vellema, P. Coxiella Burnetii Infections in Sheep or Goats: An Opinionated Review. Vet. Microbiol. 2015, 181, 119–129. [Google Scholar] [CrossRef]
- Van Wuijckhuise, L.; Dercksen, D.; Muskens, J.; de Bruijn, J.; Scheepers, M.; Vrouwenraets, R. Bluetongue in The Netherlands; description of the first clinical cases and differential diagnosis. Common symptoms just a little different and in too many herds. Tijdschr. Diergeneeskd. 2006, 131, 649–654. [Google Scholar] [PubMed]
- Dercksen, D.; Groot Nibbelink, N.; Paauwe, R.; Backx, A.; van Rijn, P.; Vellema, P. First Outbreak of Bluetongue in Goats in The Netherlands. Tijdschr. Diergeneeskd. 2007, 132, 786–790. [Google Scholar]
- Vellema, P. Bluetongue in Sheep: Question Marks on Bluetongue Virus Serotype 8 in Europe. Small Rumin. Res. 2008, 76, 141–148. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Popma, J.; Oosterwolde, S.; van Rijn, P.A.; Vellema, P.; Van Rooij, E.M.A. A Cross-Sectional Study to Determine the Seroprevalence of Bluetongue Virus Serotype 8 In Sheep and Goats In 2006 and 2007 In the Netherlands. BMC Vet. Res. 2008, 4, 33. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; van Wuijckhuise, L.; Vellema, P.; van Rijn, P.A. Vertical Transmission of Bluetongue Virus Serotype 8 Virus in Dutch Dairy Herds in 2007. Vet. Microbiol. 2010, 141, 31–35. [Google Scholar] [CrossRef][Green Version]
- Santman-Berends, I.; Luttikholt, S.; Van den Brom, R.; Van Schaik, G.; Gonggrijp, M.; Hage, H.; Vellema, P. Estimation of the Use of Antibiotics in the Small Ruminant Industry in the Netherlands in 2011 and 2012. PLoS ONE 2014, 9, e105052. [Google Scholar] [CrossRef]
- Noronha, L.E.; Wilson, W.C. Comparison of Two Zoonotic Viruses from the Order Bunyavirales. Curr. Opin. Virol. 2017, 27, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Van den Brom, R.; Luttikholt, S.J.; Lievaart-Peterson, K.; Peperkamp, N.H.; Mars, M.H.; van der Poel, W.H.; Vellema, P. Epizootic of Ovine Congenital Malformations Associated with Schmallenberg Virus Infection. Tijdschr. Diergeneeskd. 2012, 137, 106–111. [Google Scholar]
- Lievaart-Peterson, K.; Luttikholt, S.J.M.; van den Brom, R.; Vellema, P. Schmallenberg Virus Infection in Small Ruminants—First Review of the Situation and Prospects in Northern Europe. Small Rumin. Res. 2012, 106, 71–76. [Google Scholar] [CrossRef]
- Van Maanen, C.; Kooi, B.; van der Heijden, H.; Wellenberg, G.J.; Witteveen, G.; Luttikholt, S.; Bouwstra, R.; Kooi, B.; Vellema, P.; Peperkamp, K.; et al. Schmallenberg Virus Antibodies in Bovine and Ovine Fetuses. Vet. Rec. 2012, 171, 299. [Google Scholar] [CrossRef] [PubMed]
- Reusken, C.; van den Wijngaard, C.; van Beek, P.; Beer, M.; Bouwstra, R.; Godeke, G.J.; Isken, L.; van den Kerkhof, H.; van Pelt, W.; van der Poel, W.; et al. Lack of Evidence for Zoonotic Transmission of Schmallenberg Virus. Emerg. Infect. Dis. 2012, 18, 1746. [Google Scholar] [CrossRef]
- Veldhuis, A.M.B.; van Schaik, G.; Vellema, P.; Elbers, A.R.W.; Bouwstra, R.; van der Heijden, H.M.J.F.; Mars, M.H. Schmallenberg Virus Epidemic in the Netherlands: Spatiotemporal Introduction in 2011 and Seroprevalence in Ruminants. Prev. Vet. Med. 2013, 112, 35–47. [Google Scholar] [CrossRef]
- Luttikholt, S.; Veldhuis, A.; van den Brom, R.; Moll, L.; Lievaart-Peterson, K.; Peperkamp, K.; van Schaik, G.; Vellema, P. Risk Factors for Malformations and Impact on Reproductive Performance and Mortality Rates of Schmallenberg Virus in Sheep Flocks in the Netherlands. PLoS ONE 2014, 9, e100135. [Google Scholar] [CrossRef]
- Lievaart-Peterson, K.; Luttikholt, S.; Peperkamp, K.; van den Brom, R.; Vellema, P. Schmallenberg Disease in Sheep or Goats: Past, Present and Future. Vet. Microbiol. 2015, 181, 147–153. [Google Scholar] [CrossRef]
- Letko, A.; Dijkman, R.; Strugnell, B.; Häfliger, I.M.; Paris, J.M.; Henderson, K.; Geraghty, T.; Orr, H.; Scholes, S.; Drögemüller, C. Deleterious AGXT Missense Variant Associated with Type 1 Primary Hyperoxaluria (PH1) in Zwartbles Sheep. Genes 2020, 11, 1147. [Google Scholar] [CrossRef]
- Prins, S.; Junker, K.; Lievaart-Peterson, K.; Sargison, N.D.; Vellema, P. Colibacillary Arthritis and Severe Osteomyelitis in Lame Goat Kids Due to Management Procedures. Vet. Rec. Case Rep. 2021, 9, 6. [Google Scholar] [CrossRef]
- Dijkstra, E.; Harkema, L.; Vellema, P. First Case of Pithomycotoxicosis in Sheep in the Netherlands. Vet. Rec. Case Rep. 2022, 10, e268. [Google Scholar] [CrossRef]
- Schreuder, B.E.C.; ter Laak, E.A.; Griesen, H.W. An Outbreak of Caseous Lymphadenitis in Dairy Goats: First Report of the Disease in the Netherlands. Vet. Q. 1986, 8, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Dercksen, D.P.; ter Laak, E.A.; Schreuder, B.E.C. Eradication Programme for Caseous Lymphadenitis in Goats in The Netherlands. Vet. Rec. 1996, 138, 237. [Google Scholar] [CrossRef] [PubMed]
- Strugnell, B.W.; Gaudie, C.M.; Wessels, M.; Schock, A.; Davies, I. Sheep: Severe Oxalate Nephropathy in Zwartbles Sheep. Vet. Rec. 2011, 169, 81. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Incorrect Vaccine Administration Leads to Spinal Cord Compression in Sheep. Vet. Rec. 2022, 190, 153–155. [Google Scholar] [CrossRef]
- Ortín, A.; Pérez de Villarreal, M.; Minguijón, E.; Cousens, C.; Sharp, J.M.; De las Heras, M. Coexistence of Enzootic Nasal Adenocarcinoma and Jaagsiekte Retrovirus Infection in Sheep. J. Comp. Pathol. 2004, 131, 253–258. [Google Scholar] [CrossRef]
- Bleul, U.; Schwantag, S.; Stocker, H.; Corboz, L.; Grimm, F.; Engels, M.; Borel, N.; Lutz, H.; Schonmann, M.; Kähn, W. Floppy Kid Syndrome Caused by D-Lactic Acidosis in Goat Kids. J. Vet. Intern. Med. 2006, 20, 1003–1008. [Google Scholar] [CrossRef]
- Elbers, A.R.W.W.; Backx, A.; Meroc, E.; Gerbier, G.; Staubach, C.; Hendrickx, G.; van der Spek, A.; Mintiens, K. Field Observations during the Bluetongue Serotype 8 Epidemic in 2006: I. Detection of First Outbreaks and Clinical Signs in Sheep and Cattle in Belgium, France and the Netherlands. Prev. Vet. Med. 2008, 87, 21–30. [Google Scholar] [CrossRef]
- Gerbier, G.; Baldet, T.; Tran, A.; Hendrickx, G.; Guis, H.; Mintiens, K.; Elbers, A.R.W.; Staubach, C. Modelling Local Dispersal of Bluetongue Virus Serotype 8 Using Random Walk. Prev. Vet. Med. 2008, 87, 119–130. [Google Scholar] [CrossRef]
- Saegerman, C.; Mellor, P.; Uyttenhoef, A.; Hanon, J.B.; Kirschvink, N.; Haubruge, E.; Delcroix, P.; Houtain, J.Y.; Pourquier, P.; Vandenbussche, F.; et al. The Most Likely Time and Place of Introduction of BTV8 into Belgian Ruminants. PLoS ONE 2010, 5, e9405. [Google Scholar] [CrossRef] [PubMed]
- Santman-Berends, I.M.G.A.; Bartels, C.J.M.; van Schaik, G.; Stegeman, J.A.; Vellema, P. The Increase in Seroprevalence of Bluetongue Virus (BTV) Serotype 8 Infections and Associated Risk Factors in Dutch Dairy Herds, in 2007. Vet. Microbiol. 2010, 142, 268–275. [Google Scholar] [CrossRef]
- Vellema, P. Practical Experiences of Bluetongue in the Netherlands. In Proceedings of the Sheep Veterinary Society Spring Meeting, Darlington, UK, 12–14 May 2008. [Google Scholar]
- Elbers, A.R.W.; de Koeijer, A.A.; Scolamacchia, F.; van Rijn, P.A. Questionnaire Survey about the Motives of Commercial Livestock Farmers and Hobby Holders to Vaccinate Their Animals against Bluetongue Virus Serotype 8 in 2008–2009 in the Netherlands. Vaccine 2010, 28, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Santman-Berends, I.; Hage, J.J.; van Rijn, P.A.; Stegeman, J.A.; van Schaik, G. BTV-8 in Dutch Cows in 2008; Fertility Performance and the Infection Status of the Offspring. Theriogenology 2010, 74, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Santman-Berends, I.M.G.A.; Olde Riekerink, R.G.M.; Sampimon, O.C.; van Schaik, G.; Lam, T.J.G.M. Incidence of Subclinical Mastitis in Dutch Dairy Heifers in the First 100 Days in Lactation and Associated Risk Factors. J. Dairy Sci. 2012, 95, 2476–2484. [Google Scholar] [CrossRef]
- Peperkamp, N.H.; Luttikholt, S.J.; Dijkman, R.; Vos, J.H.; Junker, K.; Greijdanus, S.; Roumen, M.P.; van Garderen, E.; Meertens, N.; van Maanen, C.; et al. Ovine and Bovine Congenital Abnormalities Associated With Intrauterine Infection With Schmallenberg Virus. Vet. Pathol. 2015, 52, 1057–1066. [Google Scholar] [CrossRef]
- Beer, M.; Conraths, F.J.; Van Der Poel, W.H.M. ‘Schmallenberg Virus’—A Novel Orthobunyavirus Emerging in Europe. Epidemiol. Infect. 2013, 141, 1–8. [Google Scholar] [CrossRef]
- Westra, S.A.; Cardozo, E.L.; Berg, J.A.G. De Eerste Gevallen van Q-Koorts in Nederland. Ned. T. Geneesk. 1958, 102, 69–72. [Google Scholar]
- Van der Hoek, W.; van de Kassteele, J.; Bom, B.; de Bruin, A.; Dijkstra, F.; Schimmer, B.; Vellema, P.; ter Schegget, R.; Schneeberger, P.M. Smooth Incidence Maps Give Valuable Insight into Q Fever Outbreaks in The Netherlands. Geospat. Health 2012, 7, 127–134. [Google Scholar] [CrossRef]
- Vellema, P.; Santman-Berends, I.; Dijkstra, F.; van Engelen, E.; Aalberts, M.; Ter Bogt-Kappert, C.; van den Brom, R. Dairy Sheep Played a Minor Role in the 2005–2010 Human Q Fever Outbreak in The Netherlands Compared to Dairy Goats. Pathogens 2021, 10, 1579. [Google Scholar] [CrossRef]
- Strikwerda, R. Gezonde Dieren Een Nationaal Belang 1919–2019 Een Eeuw Gezondheidsdienst Voor Dieren, 1st ed.; BV Gezondheidsdienst voor Dieren te Deventer: Deventer, The Netherlands, 2019. [Google Scholar]
- Royal GD. Ahead in Animal Health. Available online: https://gdanimalhealth.com/ (accessed on 9 March 2022).
- Hoinville, L.J.; Alban, L.; Drewe, J.A.; Gibbens, J.C.; Gustafson, L.; Häsler, B.; Saegerman, C.; Salman, M.; Stärk, K.D.C. Proposed Terms and Concepts for Describing and Evaluating Animal-Health Surveillance Systems. Prev. Vet. Med. 2013, 112, 1–12. [Google Scholar] [CrossRef]
- Dijkstra, E.; Gonggrijp, M.; van den Brom, R.; van der Heijden, M.; Vellema, P. Data Analysis as a Tool to Monitor Trends and Developments in Goat Health in the Netherlands. In Proceedings of the ISVA Virtual Meeting, Virtual. 23–25 November 2021; p. 26. [Google Scholar]
- Van der Heijden, M.; Gonggrijp, M.; Dijkstra, E.; van den Brom, R.; Vellema, P. Data Analysis as a Tool to Monitor Trends and Developments in Sheep Health in the Netherlands. In Proceedings of the ISVA Virtual Meeting, Virtual. 23–25 November 2021; p. 27. [Google Scholar]
- Salopek, S.F.; Nelson, D.F.; McConnel, C.F.; Moore, D.A. Factors Influencing Dairy Veterinarian Necropsy Practices and Their Use of Diagnostic Laboratories. Bov. Pract. 2017, 51, 140–152. [Google Scholar] [CrossRef]
- Sawford, K.; Vollman, A.R.; Stephen, C. A Focused Ethnographic Study of Alberta Cattle Veterinarians’ Decision Making about Diagnostic Laboratory Submissions and Perceptions of Surveillance Programs. PLoS ONE 2013, 8, e64811. [Google Scholar] [CrossRef]
- McFarland, L.; Macken-Walsh, Á.; Claydon, G.; Casey, M.; Douglass, A.; McGrath, G.; McAloon, C.G. Irish Dairy Farmers’ Engagement with Animal Health Surveillance Services: Factors Influencing Sample Submission. J. Dairy Sci. 2020, 103, 10614–10627. [Google Scholar] [CrossRef] [PubMed]
- Santman-Berends, I.M.G.A.; Stegeman, J.A.; Vellema, P.; van Schaik, G. Estimation of the Reproduction Ratio (R0) of Bluetongue Based on Serological Field Data and Comparison with Other BTV Transmission Models. Prev. Vet. Med. 2013, 108, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Middelesch, H.; Moll, L.; Bartels, C.; van Schaik, G. Data Analysis of Small Ruminants in 2009; Royal GD: Deventer, The Netherlands, 2009. [Google Scholar]
- Santman-Berends, I.M.G.A.; van Schaik, G.; Bartels, C.J.M.; Stegeman, J.A.; Vellema, P. Mortality Attributable to Bluetongue Virus Serotype 8 Infection in Dutch Dairy Cows. Vet. Microbiol. 2011, 148, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Van Steenbergen, J.E.; Morroy, G.; Groot, C.A.R.; Ruikes, F.G.H.; Marcelis, J.H.; Speelman, P. An Outbreak of Q Fever in The Netherlands—Possible Link to Goats. Ned. Tijdschr. Geneeskd. 2007, 151, 1998–2003. [Google Scholar]
- Dijkstra, F.; van der Hoek, W.; Wijers, N.; Schimmer, B.; Rietveld, A.; Wijkmans, C.J.; Vellema, P.; Schneeberger, P.M. The 2007–2010 Q Fever Epidemic in the Netherlands: Characteristics of Notified Acute Q Fever Patients and the Association with Dairy Goat Farming. FEMS Immunol. Med. Microbiol. 2012, 64, 3–12. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; van den Brom, R.; van Schaik, G.; Vellema, P. Data Analysis of Small Ruminants in 2012; Royal GD: Deventer, The Netherlands, 2013. [Google Scholar]
- Van den Brom, R.; Santman-Berends, I.; Dijkman, R.; Vellema, P.; Dijkman, R.; van Engelen, E. An Accessible Diagnostic Toolbox to Detect Bacterial Causes of Ovine and Caprine Abortion. Pathogens 2021, 10, 1147. [Google Scholar] [CrossRef]
- Vellema, P. Opbouw Nederlandse Schapen- En Geitenstapel; Royal GD: Deventer, The Netherlands, 2003. [Google Scholar]
- Commission Regulation (EC) No. 21/2004. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0021&from=SV (accessed on 29 April 2022).
- Holstege, M.; Gonggrijp, M.; van den Brom, R.; Dijkstra, E.; Ter Bogt-Kappert, C.; Van Schaik, G.; Vellema, P. Data Analysis of Small Ruminants in 2020; Royal GD: Deventer, The Netherlands, 2021. [Google Scholar]
- National Institute for Public Health and the Environment. Flyer Signalling and Risk Assessment of Emerging Zoonoses. A One Health Approach in the Netherlands. Available online: https://www.rivm.nl/en/documenten/flyer-signalling-and-risk-assessment-of-emerging-zoonoses-one-health-approach-in (accessed on 3 March 2022).

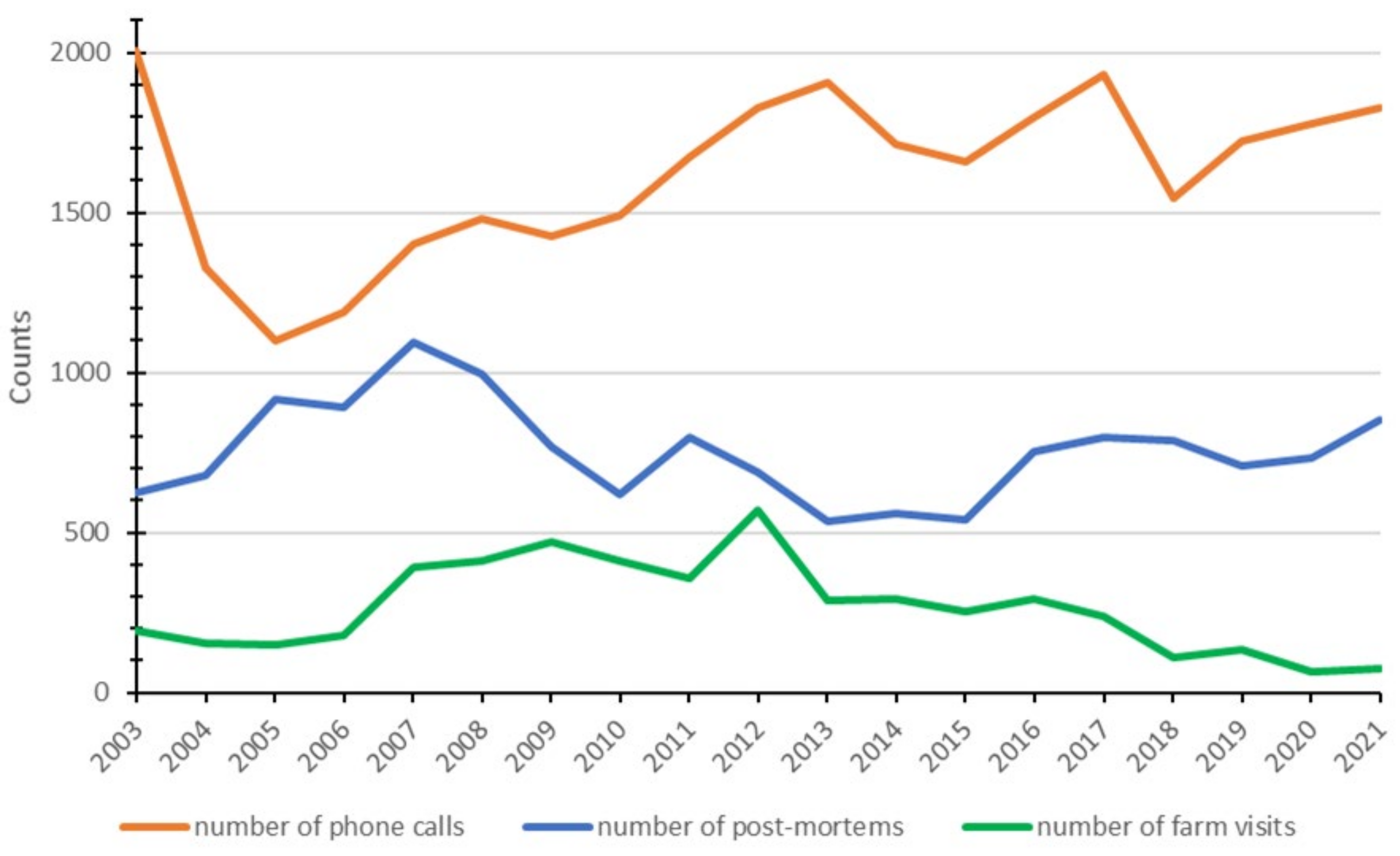

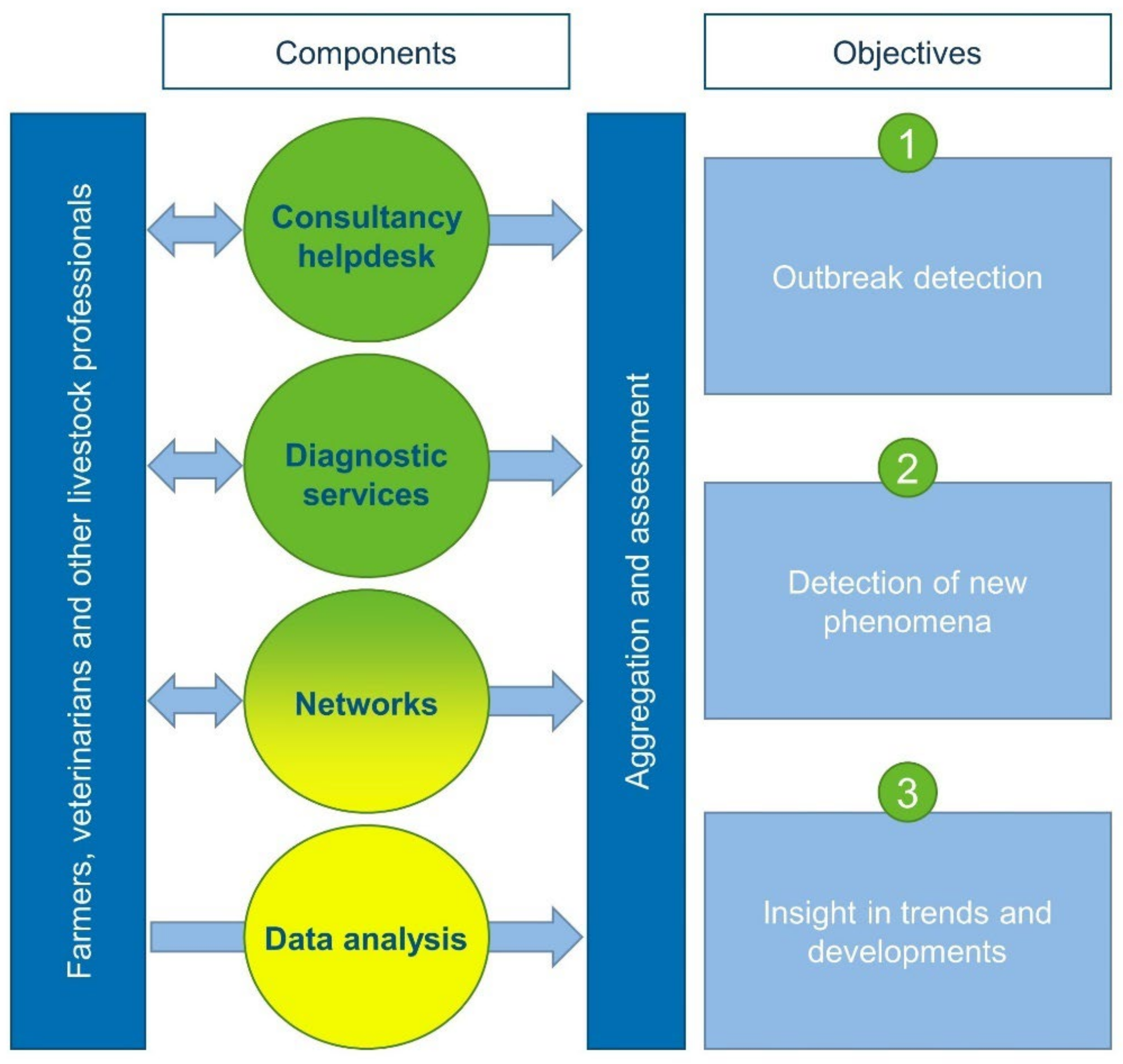
| Disease/Year | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Monitoring Component (s) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fasciolosis | A, B, C | [8,9,10,14] | |||||||||||||||||||
| abortion | C, B | [8,15,16,17] | |||||||||||||||||||
| copper poisoning | * 1 | * 1 | A, B, C | [8] | |||||||||||||||||
| anthelmintic resistance | * 2 | * 3 | * 4 | * 5 | A, C, B | [8,9,11,12,13,18] | |||||||||||||||
| osteogenesis imperfecta lambs | A, B | [8] | |||||||||||||||||||
| coxiellosis | B, C, A | [8,19,20,21,22,23,24,25,26,27,28,29,30,31] | |||||||||||||||||||
| severe (clostridial) metritis in goats | B, A | [8] | |||||||||||||||||||
| bluetongue | * 6 | A, C, B | [8,32,33,34,35,36,37] | ||||||||||||||||||
| caseous lymphadenitis | * 7 | * 8 | * 8 | * 9 | * 10 | * 11 | * 12 | * 13 | * 14 | A, B, C | [8,38] | ||||||||||
| Dictyocaulus filaria in lambs | B, C | [8] | |||||||||||||||||||
| Schmallenberg virus disease | A, B | [8,39,40,41,42,43,44,45] | |||||||||||||||||||
| hyperoxaluria in Zwartbles sheep | B, A | [8,46] | |||||||||||||||||||
| cerebrocortical necrosis in kids | B, A | [8] | |||||||||||||||||||
| paresis/paralysis after Footvax® vaccination | A, B | [8] | |||||||||||||||||||
| salmonellosis dairy goat kids | B, A, C | [8] | |||||||||||||||||||
| malignant catarrhal fever in goat | B, C, A | [8] | |||||||||||||||||||
| enzootic nasal tumor virus in sheep | A, B, C | [8] | |||||||||||||||||||
| osteomyelitis in kids | A, B | [8,47] | |||||||||||||||||||
| dysbacteriosis associated diarrhoea in dairy goats | A, B | [8] | |||||||||||||||||||
| pithomycotoxicosis in sheep | A, B | [8,48] | |||||||||||||||||||
| jaagsiekte or ovine pulmonary adenocarcinoma | A, B | [8] | |||||||||||||||||||
| severe outbreak of floppy kid syndrome | A, B | [8] |
| Sensitivity (%) | ||||
|---|---|---|---|---|
| Prevalence (%) | 25 | 50 | 75 | 90 |
| 0.1 | 18,000 | 9000 | 6000 | 5000 |
| 0.5 | 3280 | 1640 | 1230 | 1020 |
| 1.0 | 1840 | 920 | 613 | 511 |
| 5.0 | 360 | 180 | 120 | 100 |
| 10.0 | 176 | 88 | 59 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dijkstra, E.; Vellema, P.; Peterson, K.; Bogt-Kappert, C.t.; Dijkman, R.; Harkema, L.; van Engelen, E.; Aalberts, M.; Santman-Berends, I.; van den Brom, R. Monitoring and Surveillance of Small Ruminant Health in The Netherlands. Pathogens 2022, 11, 635. https://doi.org/10.3390/pathogens11060635
Dijkstra E, Vellema P, Peterson K, Bogt-Kappert Ct, Dijkman R, Harkema L, van Engelen E, Aalberts M, Santman-Berends I, van den Brom R. Monitoring and Surveillance of Small Ruminant Health in The Netherlands. Pathogens. 2022; 11(6):635. https://doi.org/10.3390/pathogens11060635
Chicago/Turabian StyleDijkstra, Eveline, Piet Vellema, Karianne Peterson, Carlijn ter Bogt-Kappert, Reinie Dijkman, Liesbeth Harkema, Erik van Engelen, Marian Aalberts, Inge Santman-Berends, and René van den Brom. 2022. "Monitoring and Surveillance of Small Ruminant Health in The Netherlands" Pathogens 11, no. 6: 635. https://doi.org/10.3390/pathogens11060635
APA StyleDijkstra, E., Vellema, P., Peterson, K., Bogt-Kappert, C. t., Dijkman, R., Harkema, L., van Engelen, E., Aalberts, M., Santman-Berends, I., & van den Brom, R. (2022). Monitoring and Surveillance of Small Ruminant Health in The Netherlands. Pathogens, 11(6), 635. https://doi.org/10.3390/pathogens11060635






