The Prevalence of Histopathological Features of Pneumonia in Goats with Symptomatic Caprine Arthritis-Encephalitis
Abstract
1. Introduction
2. Results
2.1. Serological Status
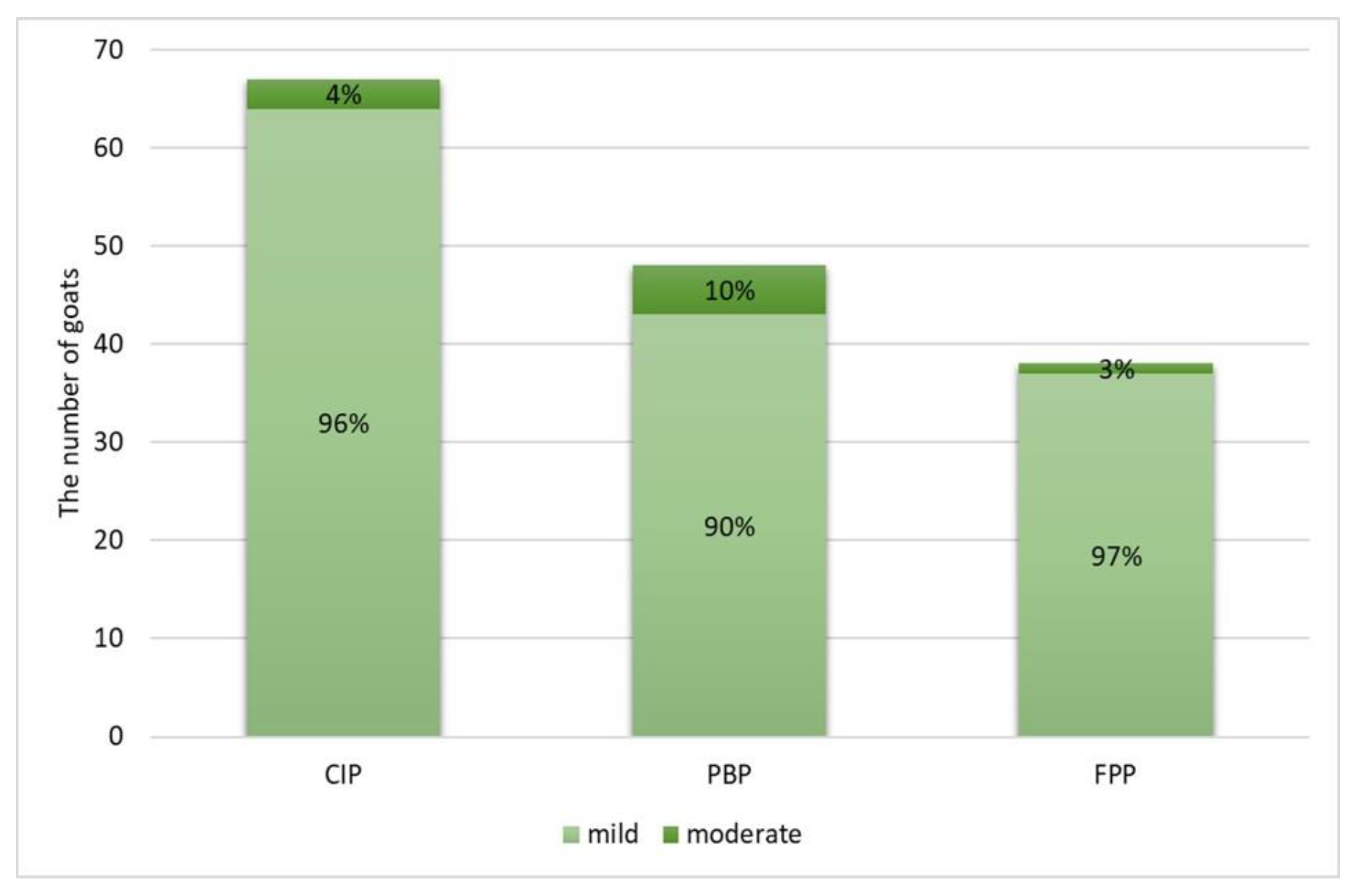
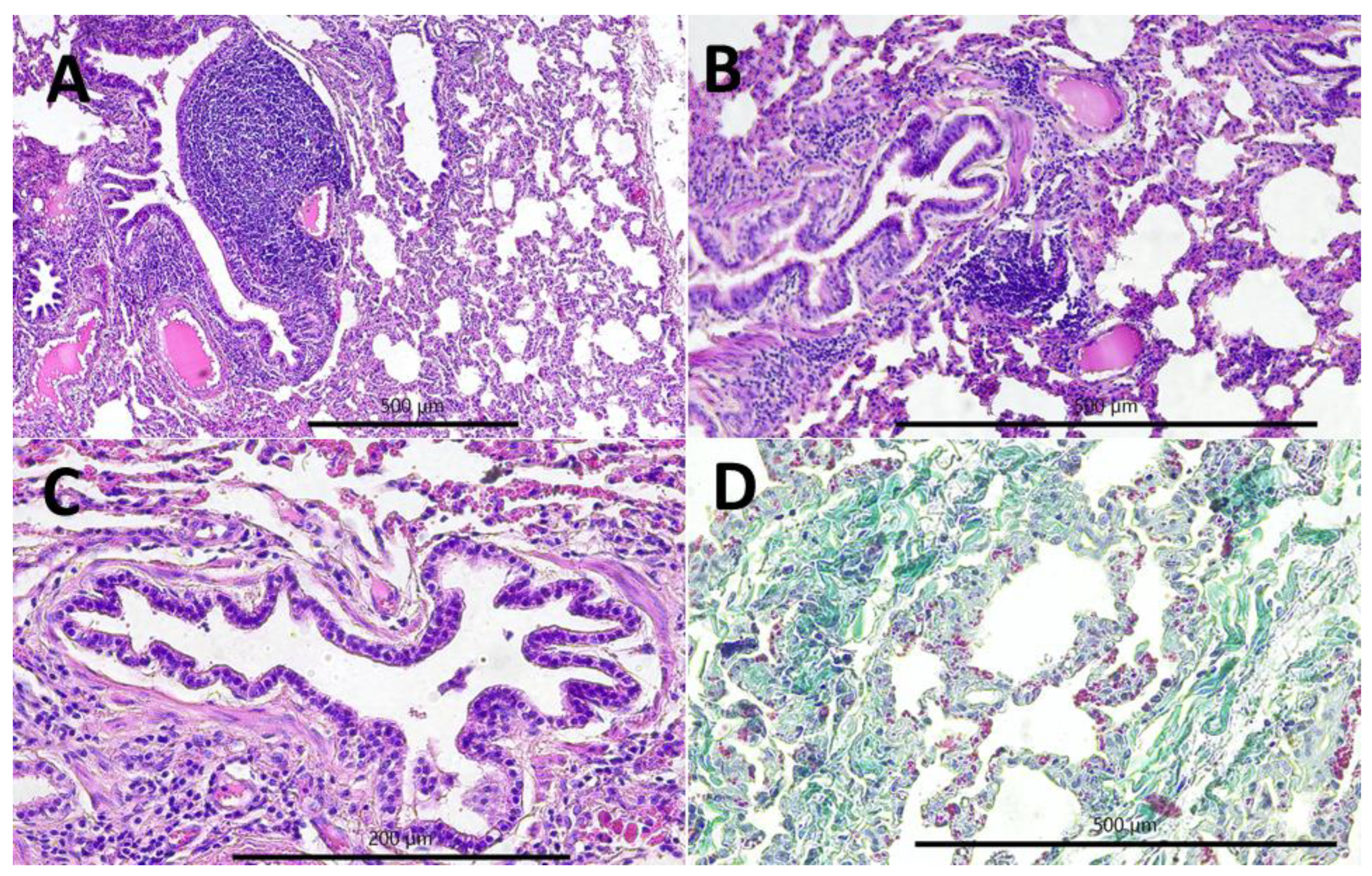
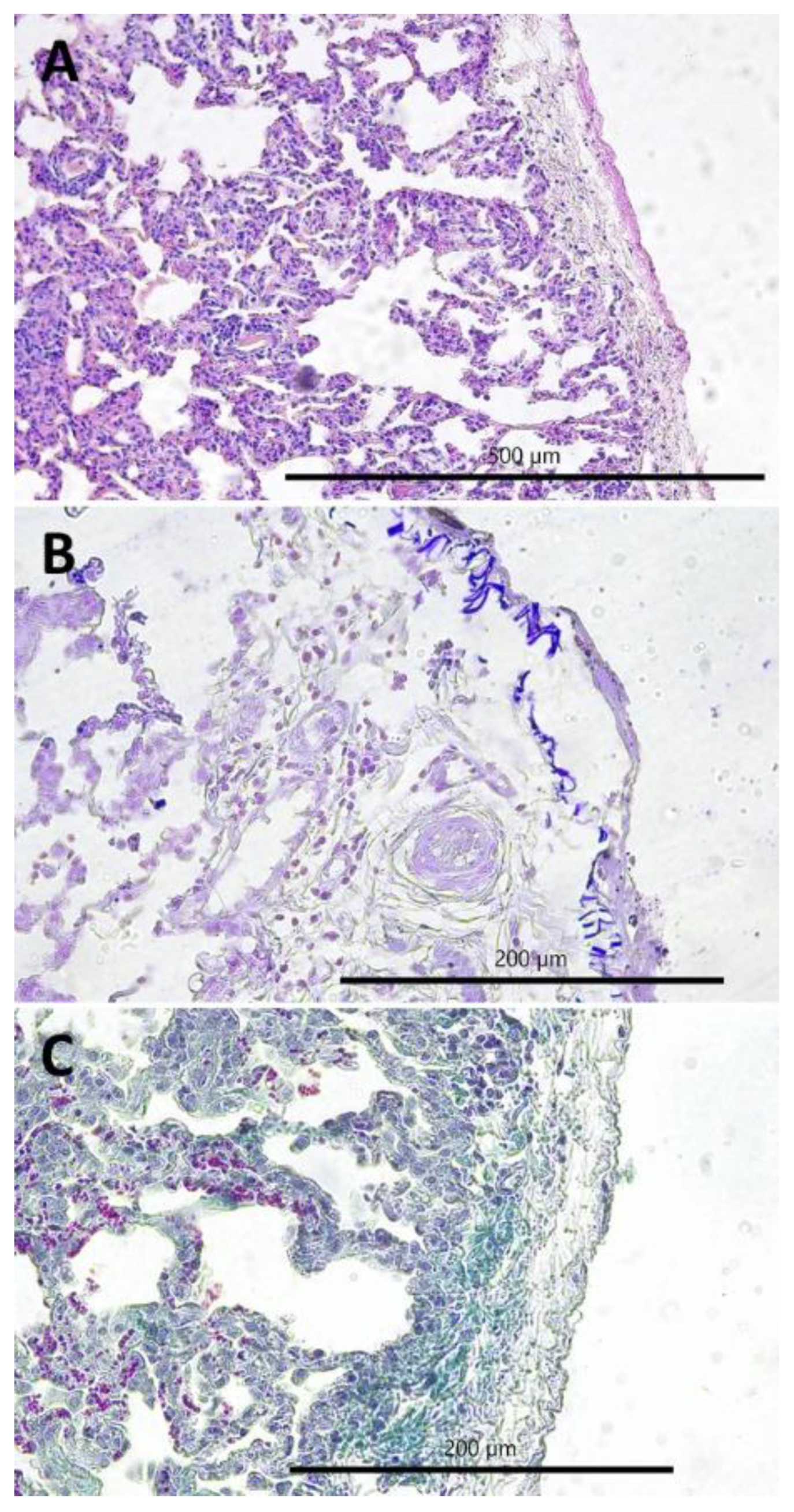
2.2. Gross and Histopathological Lesions
2.3. Bacterial Infections
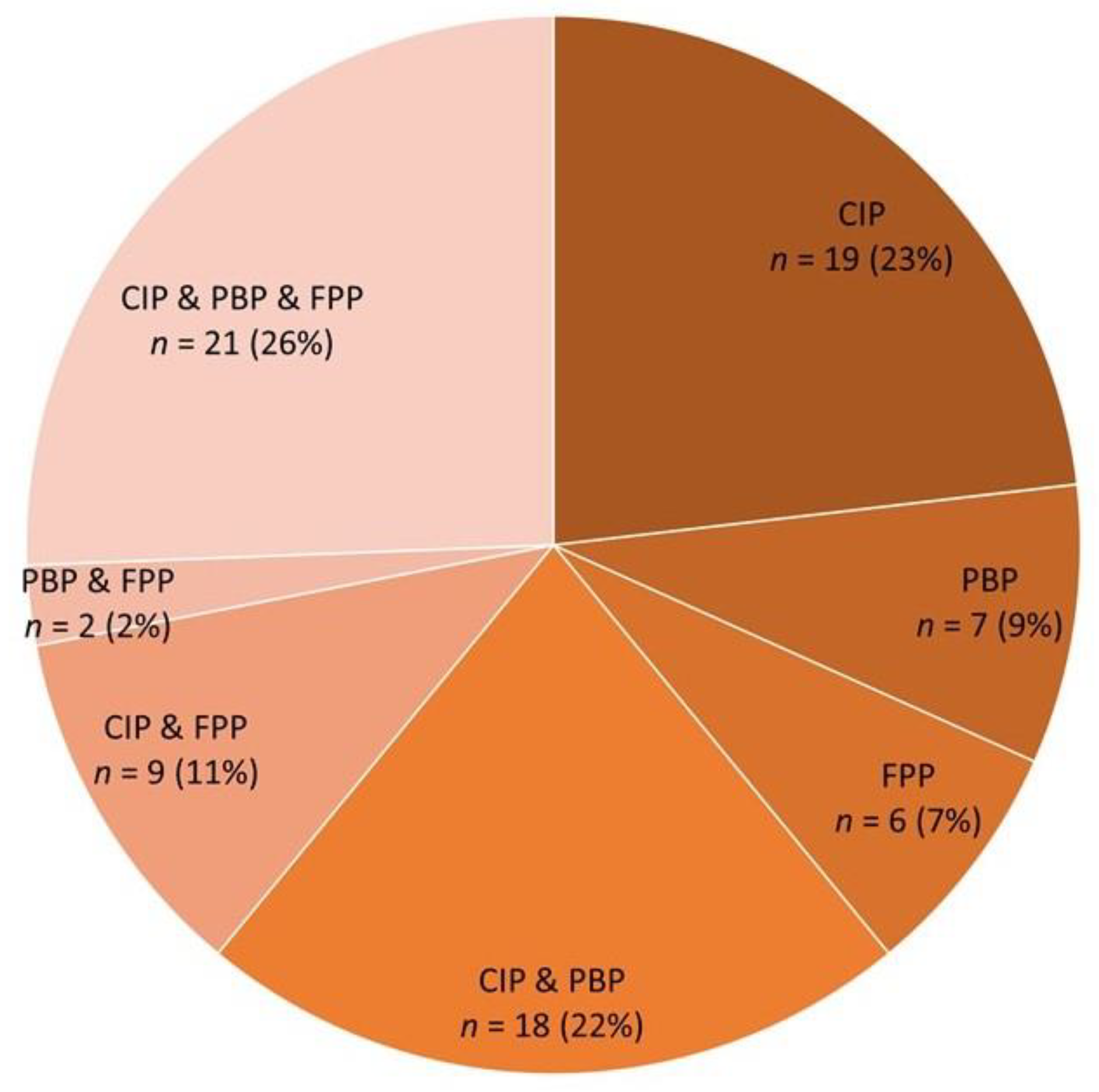
2.4. Relationship between Chronic Interstitial Pneumonia and other Respiratory System-Associated Conditions
3. Discussion
4. Materials and Methods
4.1. Goats and Serological Testing
4.2. Gross Examination
4.3. Histopathological Examination
- Chronic interstitial pneumonia (CIP) composed of six lesions [29,50,64,65,66]: (1) lymphatic nodules hyperplasia (peribronchial and perivascular); (2) interstitial (i.e., peribronchiolar and perialveolar) inflammatory infiltrates composed of lymphocytes, plasma cells, and macrophages; (3) interstitial connective tissue hyperplasia; (4) interalveolar septum thickening; (5) smooth muscle hyperplasia in the walls of the bronchioles; and (6) pneumocyte hyperplasia.
- Purulent bronchopneumonia (PBP) composed of six lesions: (1) peribronchial inflammatory infiltrates mostly composed of neutrophils and some lymphocytes and plasma cells; (2) perivascular inflammatory infiltrates mainly composed of neutrophils, some lymphocytes, and plasma cells; (3) exudate rich in the neutrophils in the lumen of alveoli; (4) focal emphysema; (5) focal atelectasis; and (6) focal necrosis of lung parenchyma.
- Fibrinous pleuropneumonia (FPP) composed of three lesions: (1) interstitial accumulation of fibrin, (2) subpleural accumulation of fibrin, and (3) subpleural connective tissue hyperplasia.
4.4. Bacteriological Examination
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramírez, H.; Reina, R.; Amorena, B.; de Andrés, D.; Martínez, H.A. Small ruminant lentiviruses: Genetic variability, tropism and diagnosis. Viruses 2013, 5, 1175–1207. [Google Scholar] [CrossRef] [PubMed]
- Banks, K.L.; Adams, D.S.; McGuire, T.C.; Carlson, J. Experimental infection of sheep by caprine arthritis-encephalitis virus and goats by progressive pneumonia virus. Am. J. Vet. Res. 1983, 44, 2307–2311. [Google Scholar] [PubMed]
- Pasick, J. Maedi-visna virus and caprine arthritis-encephalitis virus: Distinct species or quasispecies and its implications for laboratory diagnosis. Can. J. Vet. Res. 1998, 62, 241–244. [Google Scholar]
- Shah, C.; Boni, J.; Huder, J.B.; Vogt, H.R.; Muhlherr, J.; Zanoni, R.; Miserez, R.; Lutz, H.; Schupbach, J. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: Evidence for regular sheep-to-goat transmission and worldwide propagation through livestock trade. Virology 2004, 319, 12–26. [Google Scholar] [CrossRef]
- Pisoni, G.; Quasso, A.; Moroni, P. Phylogenetic analysis of small-ruminant lentivirus subtype B1 in mixed flocks: Evidence for natural transmission from goats to sheep. Virology 2005, 339, 147–152. [Google Scholar] [CrossRef]
- Pisoni, G.; Bertoni, G.; Puricelli, M.; Maccalli, M.; Moroni, P. Demonstration of coinfection with and recombination by caprine arthritis-encephalitis virus and maedi-visna virus in naturally infected goats. J. Virol. 2007, 81, 4948–4955. [Google Scholar] [CrossRef]
- Leroux, C.; Cruz, J.C.; Mornex, J.F. SRLVs: A genetic continuum of lentiviral species in sheep and goats with cumulative evidence of cross species transmission. Curr. HIV Res. 2010, 8, 94–100. [Google Scholar]
- Smith, M.C.; Sherman, D.M. Goat Medicine, 2nd ed.; Wiley: Blackwell, NJ, USA, 2009; pp. 348–359. [Google Scholar]
- Preziuso, S.; Taccini, E.; Rossi, G.; Renzoni, G.; Braca, G. Experimental Maedi Visna Virus Infection in sheep: A morphological, immunohistochemical and PCR study after three years of infection. Eur. J. Histochem. 2003, 47, 373–378. [Google Scholar] [CrossRef]
- de Miguel, R.; Arrieta, M.; Rodríguez-Largo, A.; Echeverría, I.; Resendiz, R.; Pérez, E.; Ruiz, H.; Pérez, M.; de Andrés, D.; Reina, R.; et al. Worldwide Prevalence of Small Ruminant Lentiviruses in Sheep: A Systematic Review and Meta-Analysis. Animals 2021, 11, 784. [Google Scholar] [CrossRef]
- Junkuszew, A.; Dudko, P.; Bojar, W.; Olech, M.; Osiński, Z.; Gruszecki, T.M.; Kania, M.G.; Kuźmak, J.; Czerski, G. Risk factors associated with small ruminant lentivirus infection in eastern Poland sheep flocks. Prev. Vet. Med. 2016, 127, 44–49. [Google Scholar] [CrossRef]
- Minguijón, E.; Reina, R.; Pérez, M.; Polledo, L.; Villoria, M.; Ramírez, H.; Leginagoikoa, I.; Badiola, J.J.; García-Marín, J.F.; de Andrés, D.; et al. Small ruminant lentivirus infections and diseases. Vet. Microbiol. 2015, 181, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Rachid, A.; Croisé, B.; Kuźmak, J.; Valas, S. Genetic and antigenic characterization of small ruminant lentiviruses circulating in Poland. Virus Res. 2012, 163, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Valas, S.; Kuźmak, J. Epidemiological survey in single-species flocks from Poland reveals expanded genetic and antigenic diversity of small ruminant lentiviruses. PLoS ONE 2018, 13, e0193892. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Murawski, M.; Kuźmak, J. Molecular analysis of small-ruminant lentiviruses in Polish flocks reveals the existence of a novel subtype in sheep. Arch. Virol. 2019, 164, 1193–1198. [Google Scholar] [CrossRef]
- Robinson, W.F. Chronic interstitial pneumonia in association with a granulomatous encephalitis in a goat. Aust. Vet. J. 1981, 57, 127–131. [Google Scholar] [CrossRef]
- Oliver, R.E.; Adams, D.S.; Gorham, J.R.; Julian, A.F.; McNiven, R.A.; Muir, J. Isolation of caprine arthritis-encephalitis virus from a goat. N. Z. Vet. J. 1982, 30, 147–149. [Google Scholar] [CrossRef]
- Sims, L.D.; Hale, C.J.; McCormick, B.M. Progressive interstitial pneumonia in goats. Aust. Vet. J. 1983, 60, 368–371. [Google Scholar] [CrossRef]
- Robinson, W.F.; Ellis, T.M. The pathological features of an interstitial pneumonia of goats. J. Comp. Pathol. 1984, 94, 55–64. [Google Scholar] [CrossRef]
- Hasegawa, M.Y.; Meira, E.B.S.; Lara, M.C.C.S.H.; Castro, R.S.; Rodrigues, J.N.M.; Araújo, J.; Keller, L.W.; Brandão, P.E.; Rizzo, H.; Barbosa, M.L.; et al. Small ruminant lentivirus variants and related clinical features in goats from southeastern Brazil. Small Rumin. Res. 2016, 140, 32–36. [Google Scholar] [CrossRef]
- Kaba, J.; Czopowicz, M.; Ganter, M.; Nowicki, M.; Witkowski, L.; Nowicka, D.; Szaluś-Jordanow, O. Risk factors associated with seropositivity to small ruminant lentiviruses in goat herds. Res. Vet. Sci. 2013, 94, 225–227. [Google Scholar] [CrossRef]
- Czopowicz, M.; Szaluś-Jordanow, O.; Mickiewicz, M.; Moroz, A.; Witkowski, L.; Bereznowski, A.; Markowska-Daniel, I.; Bagnicka, E.; Kaba, J. Relationship between the dissemination of small ruminant lentivirus infection in goat herds and opinion of farmers on the occurrence of arthritis. PLoS ONE 2018, 13, e0204134. [Google Scholar] [CrossRef] [PubMed]
- Kaba, J.; Bagnicka, E.; Czopowicz, M.; Nowicki, M.; Witkowski, L.; Szaluś-Jordanow, O. Long-term study on the spread of caprine arthritis-encephalitis in a goat herd. Cent. Eur. J. Immunol. 2011, 36, 170–173. [Google Scholar]
- Kaba, J.; Czopowicz, M.; Nowicki, M.; Nowicka, D.; Witkowski, L.; Szaluś-Jordanow, O. Role Caprine Arthritis Encephalitis Virus (CAEV) Infection in The Occurrence of Particular Clinical Symptoms in Adult Goats. Bull. USAMV 2012, 69, 30–32. [Google Scholar]
- Anzuino, K.; Knowles, T.G.; Lee, M.R.F.; Grogono-Thomas, R. Survey of husbandry and health on UK commercial dairy goat farms. Vet. Rec. 2019, 185, 267. [Google Scholar] [CrossRef] [PubMed]
- Mekibib, B.; Mikir, T.; Fekadu, A.; Abebe, R. Prevalence of Pneumonia in Sheep and Goats Slaughtered at Elfora Bishoftu Export Abattoir, Ethiopia: A Pathological Investigation. J. Vet. Med. 2019, 18, 5169040. [Google Scholar] [CrossRef] [PubMed]
- Ikheloa, J.O.; Ajuwape, A.T.P.; Adetosoye, A.I. Biochemical characterization and serological identification of mycoplasmas isolated from pneumonic lungs of goats slaughtered in abattoirs in Northern Nigeria. Small Rumin. Res. 2004, 52, 93–97. [Google Scholar] [CrossRef]
- Czopowicz, M.; Kaba, J.; Nowicki, M.; Nowicka, D.; Witkowski, L.; Szaluś-Jordanow, O. Long-Term Study on the Health Status of Polish Breeding Goat Population. Bull. USAMV 2012, 69, 329–334. [Google Scholar]
- Watt, N.J.; King, T.J.; Collie, D.; McIntyre, N.; Sargan, D.; McConnell, I. Clinicopathological investigation of primary, uncomplicated maedi-visna virus infection. Vet. Rec. 1992, 14, 455–461. [Google Scholar] [CrossRef]
- Herrmann-Hoesing, L.M.; Noh, S.M.; White, S.N.; Snekvik, K.R.; Truscott, T.; Knowles, D.P. Peripheral ovine progressive pneumonia provirus levels correlate with and predict histological tissue lesion severity in naturally infected sheep. Clin. Vaccine Immunol. 2009, 16, 551–557. [Google Scholar] [CrossRef][Green Version]
- Dolka, I.; Tomaszewski, M.; Wola, D.; Czopowicz, M.; Kaba, J. Lymphoepithelial Cyst of the Salivary Gland in a Small Ruminant Lentivirus-Positive Goat. Animals 2020, 10, 1545. [Google Scholar] [CrossRef]
- Ellis, T.M.; Robinson, W.F.; Wilcox, G.E. The pathology and aetiology of lung lesions in goats infected with caprine arthritis-encephalitis virus. Aust. Vet. J. 1988, 65, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Ayelet, G.; Roger, F.; Tibbo, M.; Tembely, S. Survey of maedi-visna (MV) in Ethiopian highland sheep. Vet. J. 2001, 161, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Woldemeskel, M.; Tibbo, M.; Potgieter, L.N. Ovine progressive pneumonia (Maedi-Visna): An emerging respiratory disease of sheep in Ethiopia. Dtsch. Tierarztl. Wochenschr. 2002, 109, 486–488. [Google Scholar] [PubMed]
- Management Entity. Ethiopia’s Livestock Systems: Overview and Areas of Inquiry; Feed the Future Innovation Lab for Livestock Systems: Gainesville, FL, USA, 2021; Available online: https://livestocklab.ifas.ufl.edu/media/livestocklabifasufledu/pdf-/LSIL_Livestock_Systems_Overview_Ethiopia_2021_08.pdf (accessed on 11 March 2022).
- Dousti, M.; Sayyari, M.; Esmailnejad, A. Histopathological, serological, molecular and electron microscopy detection of Maedi-Visna infection in sheep population in the West of Iran. Iran J. Vet. Res. 2020, 21, 103–108. [Google Scholar] [PubMed]
- Mohan, K.; Obwolo, M.J.; Hill, F.W. Mycoplasma ovipneumoniae infection in Zimbabwean goats and sheep. J. Comp. Pathol. 1992, 107, 73–79. [Google Scholar] [CrossRef]
- Orós, J.; Fernández, A.; Rodríguez, J.L.; Rodríguez, F.; Poveda, J.B. Bacteria associated with enzootic pneumonia in goats. Zentralbl. Veterinarmed. B 1997, 44, 99–104. [Google Scholar] [CrossRef]
- Rodríguez, F.; Fernández, A.; Orós, J.; Ramírez, A.S.; Luque, R.; Ball, H.J.; Sarradell, J. Changes in lymphocyte subsets in the bronchus-associated lymphoid tissue of goats naturally infected with different Mycoplasma species. J. Vet. Med. B Infect. Dis. Vet. Public Health 2001, 48, 259–266. [Google Scholar] [CrossRef]
- Di Provvido, A.; Averaimo, D.; Zilli, K.; Marruchella, G.; Scacchia, M. Mycoplasma pneumonia in small ruminants: A ten-year long retrospective survey. Small Rumin. Res. 2017, 153, 103–106. [Google Scholar] [CrossRef]
- Donachie, W. Pasteurellosis. In Diseases of Sheep, 4th ed.; Aitken, I.D., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 224–231. [Google Scholar]
- Jaÿ, M.; Ambroset, C.; Tricot, A.; Colin, A.; Tardy, F. Population structure and antimicrobial susceptibility of Mycoplasma ovipneumoniae isolates in France. Vet. Microbiol. 2020, 248, 108828. [Google Scholar] [CrossRef]
- Deeney, A.S.; Collins, R.; Ridley, A.M. Identification of Mycoplasma species and related organisms from ruminants in England and Wales during 2005–2019. BMC Vet. Res. 2021, 17, 325. [Google Scholar] [CrossRef]
- Chazel, M.; Tardy, F.; Le Grand, D.; Calavas, D.; Poumarat, F. Mycoplasmoses of ruminants in France: Recent data from the national surveillance network. BMC Vet. Res. 2010, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Bölske, G.; Msami, H.; Humlesjö, N.E.; Ernø, H.; Jönsson, L. Mycoplasma capricolum in an outbreak of polyarthritis and pneumonia in goats. Acta Vet. Scand. 1988, 29, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.; Ayling, R.; McAuliffe, L. Mycoplasma Diseases of Ruminants. In Respiratory Diseases of Small Ruminants, 1st ed.; CABI International: Oxforshire, UK, 2008; pp. 169–198. [Google Scholar]
- Bednarek, D.; Ayling, R.D.; Nicholas, R.A.; Dudek, K.; Szymańska-Czerwinska, M. Serological survey to determine the occurrence of respiratory Mycoplasma infections in the Polish cattle population. Vet. Rec. 2012, 171, 45. [Google Scholar] [CrossRef]
- Murphy, B.G.; Castillo, D.; Mete, A.; Vogel, H.; Goldsmith, D.; Barro, M.; Gonzales-Viera, O. Caprine Arthritis Encephalitis Virus Is Associated with Renal Lesions. Viruses 2021, 13, 1051. [Google Scholar] [CrossRef] [PubMed]
- Storset, A.K.; Evensen, O.; Rimstad, E. Immunohistochemical identification of caprine arthritis-encephalitis virus in paraffin-embedded specimens from naturally infected goats. Vet. Pathol. 1997, 34, 180–188. [Google Scholar] [CrossRef] [PubMed]
- de la Concha-Bermejillo, A. Maedi-Visna and ovine progressive pneumonia. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 13–33. [Google Scholar] [CrossRef]
- Pritchard, G.C.; McConnell, I. Maedi-visna. In Diseases of Sheep, 4th ed.; Aitken, I.D., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 217–223. [Google Scholar]
- Narayan, O. Immunopathology of lentiviral infections in ungulate animals. Curr. Opin. Immunol. 1990, 2, 399–402. [Google Scholar] [CrossRef]
- Campbell, R.S.; Robinson, W.F. The comparative pathology of the lentiviruses. J. Comp. Pathol. 1998, 119, 333–395. [Google Scholar] [CrossRef]
- Blacklaws, B.A. Small ruminant lentiviruses: Immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 259–269. [Google Scholar] [CrossRef]
- Stonos, N.; Wootton, S.K.; Karrow, N. Immunogenetics of small ruminant lentiviral infections. Viruses 2014, 22, 3311–3333. [Google Scholar] [CrossRef]
- Urbańska, D.; Puchała, R.; Jarczak, J.; Czopowicz, M.; Kaba, J.; Horbańczuk, K.; Bagnicka, E. Does Small Ruminant Lentivirus Infection in Goats Predispose to Bacterial Infection of the Mammary Gland? A Preliminary Study. Animals 2021, 22, 1851. [Google Scholar] [CrossRef] [PubMed]
- Thrusfield, M. Veterinary Epidemiology, 4th ed.; John Wiley & Sons: Chichester, UK, 2018; pp. 324, 430–443. [Google Scholar]
- Rowe, J.D.; East, N.E.; Franti, C.E.; Thurmond, M.C.; Pedersen, N.C.; Theilen, G.H. Risk factors associated with the incidence of seroconversion to caprine arthritis-encephalitis virus in goats on California dairies. Am. J. Vet. Res. 1992, 53, 2396–2403. [Google Scholar] [PubMed]
- Blacklaws, B.A.; Berriatua, E.; Torsteinsdottir, S.; Watt, N.J.; de Andres, D.; Klein, D.; Harkiss, G.D. Transmission of small ruminant lentiviruses. Vet. Microbiol. 2004, 14, 199–208. [Google Scholar] [CrossRef]
- Moroz, A.; Biernacka, K.; Czopowicz, M.; Mickiewicz, M.; Abril, C.; Bertoni, G.; Ózsvári, L.; Petkevicius, S.; Stuen, S.; Samojlenko, M.; et al. CAE-RAPID—Genetic diversity of small ruminant lentivirus in Polish goat population. In Proceedings of the XXI Middle European Buiatrics Congress, Stare Jabłonki, Poland, 19–22 May 2022; p. 184. [Google Scholar]
- Clavijo, A.; Thorsen, J. Serologic diagnosis of caprine arthritis-encephalitis by ELISA with two recombinant proteins in a parallel testing format. J. Immunoass. 1995, 16, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, D.; Czopowicz, M.; Mickiewicz, M.; Szaluś-Jordanow, O.; Witkowski, L.; Bagnicka, E.; Kaba, J. Diagnostic performance of ID screen MVV-CAEV Indirect Screening ELISA in identifying small ruminant lentiviruses-infected goats. Pol. J. Vet. Sci. 2014, 17, 501–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moroz, A.; Czopowicz, M.; Mickiewicz, M.; Witkowski, L.; Szaluś-Jordanow, O.; Nalbert, T.; Klimowicz-Bodys, M.D.; Markowska-Daniel, I.; Bagnicka, E.; Kaba, J. Antibodies to parainfluenza virus type 3 in goat population in Poland. Pol. J. Vet. Sci. 2021, 24, 235–241. [Google Scholar] [PubMed]
- Martin, W.B. Respiratory infections of sheep. Comp. Immunol. Microbiol. Infect. Dis. 1996, 19, 171–179. [Google Scholar] [CrossRef]
- Georgsson, G.; Pálsson, P.A. The histopathology of maedi a slow, viral pneumonia of sheep. Vet. Pathol. 1971, 8, 63–80. [Google Scholar] [CrossRef]
- Luján, L.; Begara, I.; Collie, D.; Watt, N.J. Ovine lentivirus (maedi-visna virus) protein expression in sheep alveolar macrophages. Vet. Pathol. 1994, 31, 695–703. [Google Scholar] [CrossRef]
- Feldman, D.B.; Gupta, B.N. Histopathologic changes in laboratory animals resulting from various methods of euthanasia. Lab. Anim. Sci. 1976, 26, 218–221. [Google Scholar]
- Brogden, K.A.; Lehmkuhl, H.D.; Cutlip, R.C. Pasteurella haemolytica complicated respiratory infections in sheep and goats. Vet. Res. 1998, 29, 233–254. [Google Scholar] [PubMed]
- Ribeiro, M.G.; Risseti, R.M.; Bolaños, C.A.; Caffaro, K.A.; de Morais, A.C.; Lara, G.H.; Zamprogna, T.O.; Paes, A.C.; Listoni, F.J.; Franco, M.M. Trueperella pyogenes multispecies infections in domestic animals: A retrospective study of 144 cases (2002 to 2012). Vet. Q. 2015, 35, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.; Machin, D.; Bryant, T.; Gardner, M. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed.; BMJ Books: Bristol, UK, 2000; pp. 46–47. [Google Scholar]


| Histopathological Lesion | N (%) of Goats with a Lesion | n (% of N) of Goats with a Lesion of Particular Severity | |||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| Chronic Interstitial Pneumonia (CIP) | |||||
| 1 | Interstitial inflammatory infiltrate composed of macrophages and lymphocytes | 101 (87.1) | 72 (71.3) | 17 (16.8) | 12 (11.9) |
| 2 | Interstitial connective tissue hyperplasia | 72 (62.1) | 48 (66.7) | 14 (19.4) | 10 (13.9) |
| 3 | Lymphoid follicles formation | 69 (59.5) | 51 (73.9) | 14 (20.3) | 4 (5.8) |
| 4 | Smooth muscle hyperplasia | 34 (29.3) | 32 (94.2) | 1 (2.9) | 1 (2.9) |
| 5 | Interstitial septum thickening | 16 (13.8) | 14 (87.5) | 0 (0) | 2 (12.5) |
| 6 | Pneumocyte hyperplasia | 6 (5.2) | 3 (50.0) | 2 (33.3) | 1 (16.7) |
| Purulent Bronchopneumonia (PBP) | |||||
| 1 | Peribronchial inflammatory infiltrate composed of neutrophils and lymphocytes | 114 (98.3) | 85 (74.6) | 16 (14.0) | 13 (11.4) |
| 2 | Perivascular inflammatory infiltrate composed of neutrophils and lymphocytes | 95 (81.9) | 77 (81.1) | 6 (6.3) | 12 (12.6) |
| 3 | Emphysema | 26 (22.4) | 26 (100) | 0 (0) | 0 (0) |
| 4 | Bronchial exudate | 17 (14.7) | 9 (52.9) | 1 (5.9) | 7 (41.2) |
| 5 | Atelectasis | 13 (11.2) | 12 (92.3) | 1 (7.7) | 0 (0) |
| 6 | Necrosis of lung parenchyma | 10 (8.6) | 9 (90.0) | 1 (10.0) | 0 (0) |
| Fibrinous Pleuropneumonia (FPP) | |||||
| 1 | Subpleural connective tissue hyperplasia | 68 (58.6) | 53 (77.9) | 8 (11.8) | 7 (10.3) |
| 2 | Subpleural fibrin accumulation | 27 (23.3) | 27 (100) | 0 (0) | 0 (0) |
| 3 | Interstitial fibrin accumulation | 15 (12.9) | 14 (93.3) | 0 (0) | 1 (6.7) |
| Bacterial Species | No. of Goats Infected | Prevalence (CI 95%) |
|---|---|---|
| Gram-Positive | ||
| Trueperella pyogenes | 16 | 13.8 (8.7–21.2) |
| Staphylococcus spp. | 9 | 7.8 (4.1–14.1) |
| Staphylococcus aureus | 8 | 6.9 (3.5–13.0) |
| Coagulase-negative staphylococci | 1 | 0.9 (0.2–4.7) |
| Others | ||
| Enterococcus sp. | 9 | 7.8 (4.1–14.1) |
| α-hemolytic Streptococcus sp. | 6 | 5.2 (2.4–10.8) |
| Corynebacterium pseudotuberculosis | 6 | 5.2 (2.4–10.8) |
| Gram-Negative | ||
| Enterobacterales | 24 | 20.7 (14.3–28.9) |
| Escherichia coli | 20 | 16.8 (11.2–24.5) |
| Proteus sp. | 3 | 2.6 (0.9–7.3) |
| Enterobacter sp. | 1 | 0.9 (0.2–4.7) |
| Pasteurellaceae | 14 | 11.8 (7.1–18.8) |
| Mannheimia hemolytica | 8 | 6.7 (3.4–12.7) |
| Pasteurella multocida | 6 | 5.2 (2.4–10.8) |
| Others | ||
| Pseudomonas aeruginosa | 3 | 2.6 (0.9–7.3) |
| Alcaligenes sp. | 1 | 0.9 (0.2–4.7) |
| Acinetobacter sp. | 1 | 0.9 (0.2–4.7) |
| Respiratory System-Associated Conditions | CIP (n (% of N)) | Prevalence Ratio (CI 95%) | p-Value | |
|---|---|---|---|---|
| Present (N = 67) | Absent (N = 49) | |||
| Purulent bronchopneumonia (PBP) | 39 (58.2) | 9 (18.4) | 3.17 (1.70–5.92) | <0.001 |
| Fibrinous pleuropneumonia (FPP) | 30 (44.8) | 8 (16.3) | 4.16 (1.69–10.2) | 0.001 |
| Infection with potential etiological agents of bacterial pneumonia | 28 (41.8) | 12 (24.5) | 1.71 (0.97–3.01) | 0.050 |
| Corynebacterium pseudotuberculosis infection | 3 (4.5) | 3 (6.1) | 0.73 (0.15–3.47) | 0.696 |
| Presence of accidental/commensal microorganisms | 18 (26.9) | 15 (30.6) | 0.88 (0.49–1.56) | 0.659 |
| Antibodies to Corynebacterium pseudotuberculosis phospholipase D antigen | 38 (56.7) | 32 (65.3) | 0.87 (0.65–1.16) | 0.349 |
| Concentration of antibodies to parainfluenza virus type 3 (PIV-3) (ng/mL) | 257, 173–540 (75–1494) | 296, 146–53 (39–1454) | – | 0.898 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroz, A.; Czopowicz, M.; Sobczak-Filipiak, M.; Dolka, I.; Rzewuska, M.; Kizerwetter-Świda, M.; Chrobak-Chmiel, D.; Mickiewicz, M.; Witkowski, L.; Szaluś-Jordanow, O.; et al. The Prevalence of Histopathological Features of Pneumonia in Goats with Symptomatic Caprine Arthritis-Encephalitis. Pathogens 2022, 11, 629. https://doi.org/10.3390/pathogens11060629
Moroz A, Czopowicz M, Sobczak-Filipiak M, Dolka I, Rzewuska M, Kizerwetter-Świda M, Chrobak-Chmiel D, Mickiewicz M, Witkowski L, Szaluś-Jordanow O, et al. The Prevalence of Histopathological Features of Pneumonia in Goats with Symptomatic Caprine Arthritis-Encephalitis. Pathogens. 2022; 11(6):629. https://doi.org/10.3390/pathogens11060629
Chicago/Turabian StyleMoroz, Agata, Michał Czopowicz, Małgorzata Sobczak-Filipiak, Izabella Dolka, Magdalena Rzewuska, Magdalena Kizerwetter-Świda, Dorota Chrobak-Chmiel, Marcin Mickiewicz, Lucjan Witkowski, Olga Szaluś-Jordanow, and et al. 2022. "The Prevalence of Histopathological Features of Pneumonia in Goats with Symptomatic Caprine Arthritis-Encephalitis" Pathogens 11, no. 6: 629. https://doi.org/10.3390/pathogens11060629
APA StyleMoroz, A., Czopowicz, M., Sobczak-Filipiak, M., Dolka, I., Rzewuska, M., Kizerwetter-Świda, M., Chrobak-Chmiel, D., Mickiewicz, M., Witkowski, L., Szaluś-Jordanow, O., Nalbert, T., Potârniche, A. V., Barszcz, K., Markowska-Daniel, I., Puchała, R., Bagnicka, E., & Kaba, J. (2022). The Prevalence of Histopathological Features of Pneumonia in Goats with Symptomatic Caprine Arthritis-Encephalitis. Pathogens, 11(6), 629. https://doi.org/10.3390/pathogens11060629








