Exploring Anastomosis of Hyphae and Mating-Type Compatibility of Pochonia chlamydosporia Isolates of the Meloidogyne, Heterodera and Globodera Biotypes
Abstract
:1. Introduction
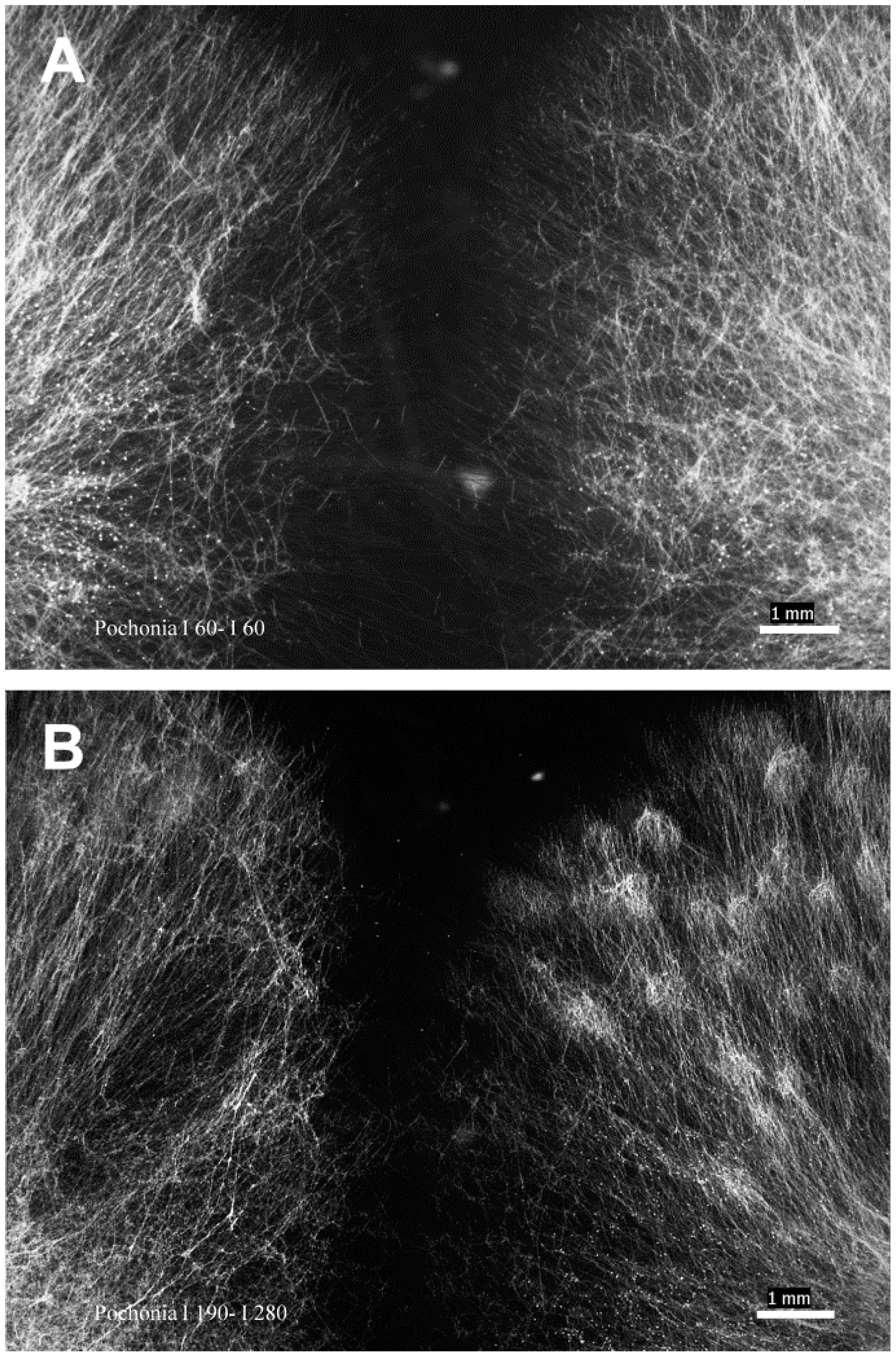
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolates
4.2. Monosporic Cultures and Medium-Coated Glass Slides
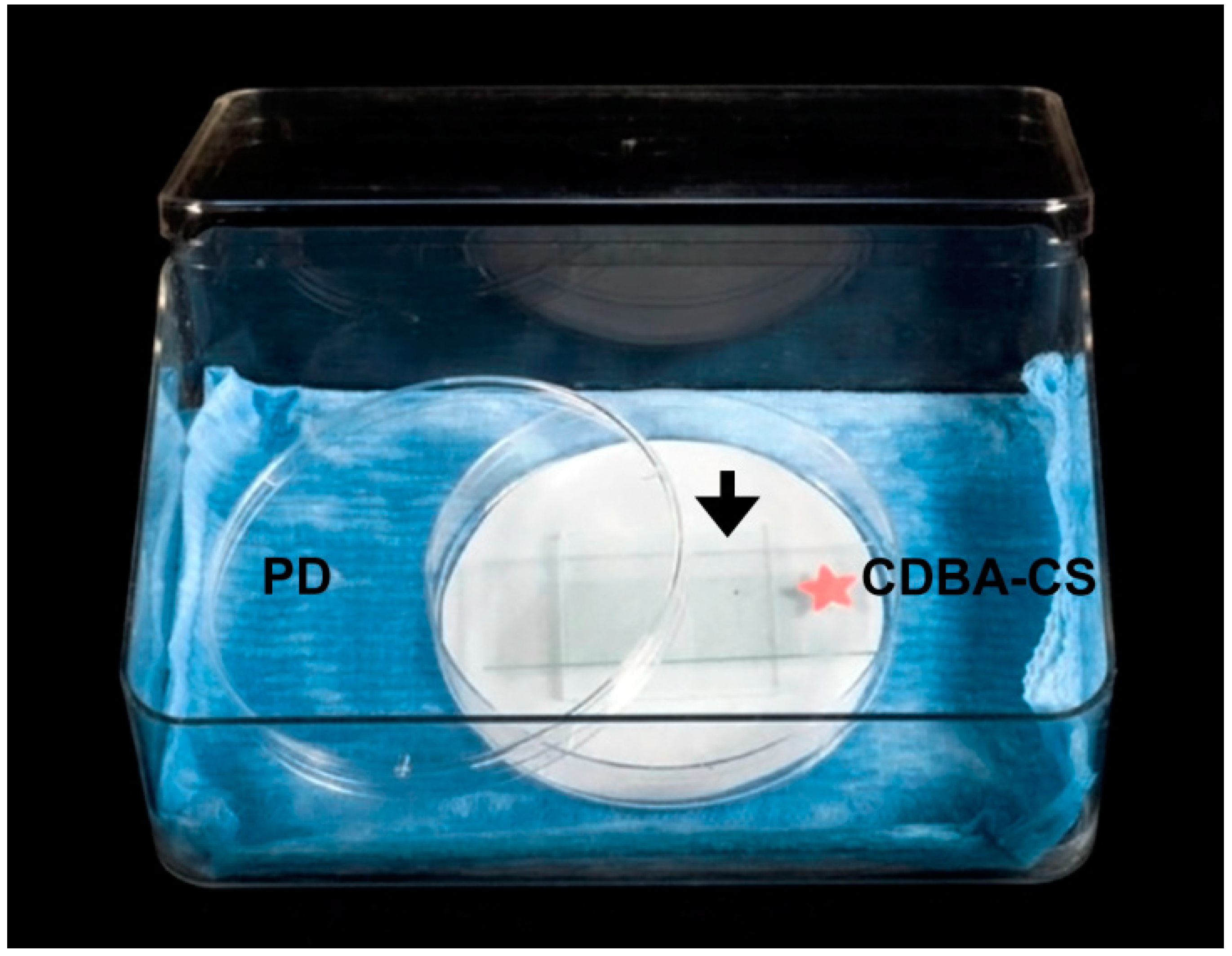
4.3. Anastomosis In Vitro Assay
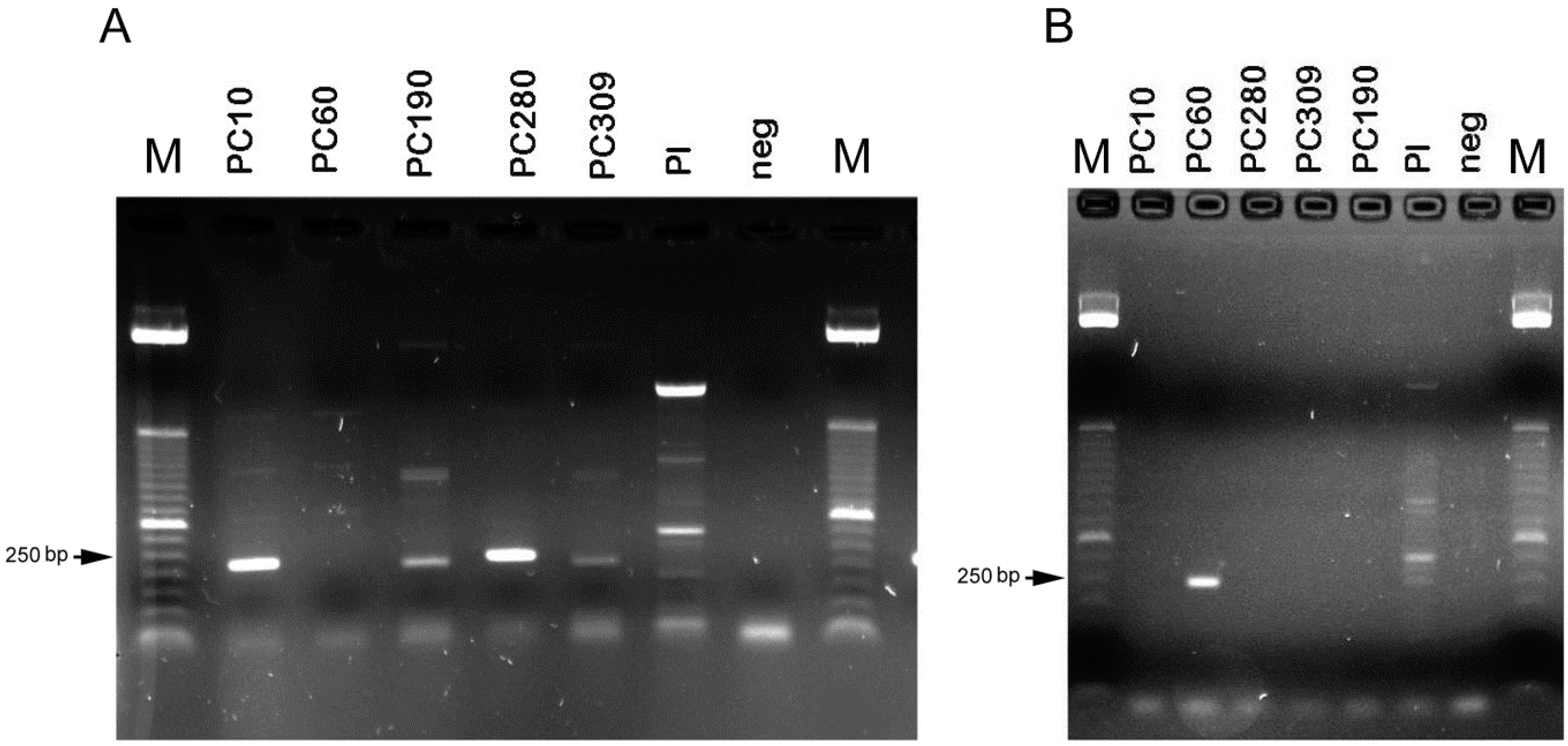
4.4. Molecular Mating-Type Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mesa-Valle, C.; Garrido-Cardenas, J.A.; Cebrian-Carmona, J.; Talavera, M.; Manzano-Agugliaro, F. Global research on plant nematodes. Agronomy 2020, 18, 1148. [Google Scholar] [CrossRef]
- Sung, G.H.; Spatafora, J.W.; Zare, R.; Hodge, K.; Gams, W. A revision of Verticillium sect. Prostrata. II. Phylogenetic analyses of SSU and LSU nuclear rDNA sequences from anamorphs and teleomorphs of the Clavicipitaceae. Nova. Hedwig. 2001, 72, 311–328. [Google Scholar] [CrossRef]
- Yokoyama, E.; Yamagishi, K.; Hara, A. Development of a PCR-based mating-type assay for Clavicipitaceae. FEMS Microbiol. Lett. 2004, 237, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leinhos, G.M.E.; Buchenauer, H. Inhibition of rust diseases of cereals by metabolic products of Verticillium chlamydosporium. J. Phytopathol. 1992, 136, 177–193. [Google Scholar] [CrossRef]
- Monfort, E.; Lopez-Llorca, L.V.; Jansson, H.B.; Salinas, J.; Park, J.O.; Sivasithamparam, K. Colonisation of seminal roots of wheat and barley by egg-parasitic nematophagous fungi and their effects on Gaeumannomyces graminis var. tritici & development of root-rot. Soil Biol. Biochem. 2005, 37, 1229–1235. [Google Scholar] [CrossRef]
- Evans, H.C.; Kirk, P. Systematics of Pochonia. In Perspectives in Sustainable Nematode Management Through Pochonia chlamydosporia Applications for Root and Rhizosphere Health; Manzanilla-López, R.H., Lopez-Llorca, L.V., Eds.; Springer: Cham, Switzerland, 2017; pp. 21–43. [Google Scholar]
- Siddiqui, I.A.; Atkins, S.D.; Kerry, B.R. Relationship between saprophytic growth in soil in different isolates of Pochonia chlamydosporia collected from cyst and root-knot nematodes and the infection of nematode eggs. Ann. Appl. Biol. 2009, 155, 131–141. [Google Scholar] [CrossRef]
- Morgan-Jones, G.; White, J.F.; Rodriguez-Kabana, R. Phytonematode pathology: Ultrastructural studies. I. Parasitism of Meloidogyne arenaria eggs by Verticillium chlamydosporium. Nematropica 1983, 13, 245–260. [Google Scholar]
- Flores-Camacho, R.; Atkins, S.D.; Manzanilla-López, R.H.; Cid del Prado-Vera, I.; Martínez Garza, A. Isolation and PCR of five Mexican strains of Pochonia chlamydosporia var. chlamydosporia, potential biological control agent of Nacobbus aberrans sensu lato. Rev. Mex. Fitopatol. 2008, 26, 93–104. [Google Scholar]
- Kerry, B.R.; Crump, D.H. Observations on fungal parasites of females and eggs of the cereal cyst-nematode, Heterodera avenae, and other cyst nematodes. Nematologica 1977, 23, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Freitas, L.G.; Dallemole-Giaretta, R.; Ferraz, S.; Zooca, R.J.F.; Podestá, G.S. Controle biológico de nematoides: Estudo de casos. In Controle Biológico de Pragas e Doenças: Exemplos Práticos; Zambolim, L., Picanço, M.C., Eds.; UFV/DFP: Viçosa, Brazil, 2009; pp. 41–82. [Google Scholar]
- Tolba, S.R.T.; Rosso, L.C.; Pentimone, I.; Colangiero, M.; Moustafa, M.M.A.; Elshawaf, I.I.S.; Bubici, G.; Prigigallo, M.I.; Ciancio, A. Root endophytism by Pochonia chlamydosporia affects defense-gene expression in leaves of Monocot and Dicot hosts under multiple biotic interactions. Plants 2021, 10, 78. [Google Scholar] [CrossRef]
- Kerry, B.R. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2000, 38, 423–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, S.D.; Peteira, B.; Clark, I.M.; Kerry, B.R. Use of real-time PCR to investigate root and gall colonization by co-inoculated isolates of the nematophagous fungus Pochonia chlamydosporia. Ann. Appl. Biol. 2009, 155, 143–152. [Google Scholar] [CrossRef]
- Manzanilla-López, R.H.; Clark, I.M.; Atkins, S.D.; Hirsch, P.R.; Kerry, B.R. Exploring competitiveness and variation in the nematophagous fungus Pochonia chlamydosporia var. chlamydosporia and its significance for biological control. Multitrophic Interact. Soil IOBC/Wprs Bull. 2011, 63, 43–47. [Google Scholar]
- Sorribas, F.J.; Ornat, C.; Galeano, M.; Verdejo-Lucas, S. Evaluation of a native and introduced isolate of Pochonia chlamydosporia against Meloidogyne. Incognita. Biocontrol Sci. Technol. 2003, 13, 707–714. [Google Scholar] [CrossRef]
- Magalhães, X.D.; Dallemole-Giaretta, R.; Grassi de Freitas, L.; Lopes, E.A.; Gonçalves, G.C.; Ferraz, S. Combination of isolates of Pochonia chlamydosporia for the control of Meloidogyne javanica in tomato. Chilean J. Agric. Anim. Sci. 2017, 33, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Mauchline, T.M.; Kerry, B.R.; Hirsch, P.R. The biocontrol fungus Pochonia chlamydosporia shows nematode host preference at the infraspecific level. Mycol. Res. 2004, 108, 161–169. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008; p. 668. [Google Scholar]
- Griffin, D.H. Fungal Physiology, 2nd ed.; Wiley-Liss: New York, NY, USA, 1994; pp. 301–336. [Google Scholar]
- Hickey, P.C.; Jacobson, N.D.; Glass, L.N. Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal. Genet. Biol. 2002, 37, 109–119. [Google Scholar] [CrossRef]
- Yokoyama, E.; Yamagishi, K.; Hara, A. Structures of the mating-type loci of Cordyceps takaomontana. Appl. Environ. Microbiol. 2003, 69, 5019–5022. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, E.; Yamagishi, K.; Hara, A. Heterothallism in Cordyceps takaomontana. FEMS Microbiol. Lett. 2005, 250, 145–150. [Google Scholar] [CrossRef]
- Yokoyama, E.; Arakawa, M.; Yamagishi, K.; Hara, A. Phylogenetic and structural analyses of the mating-type loci in Clavicipitaceae. FEMS Microbiol. Lett. 2006, 264, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Hua’an, Y.; Sivasithamparam, K.; O’Brien, P.A. An improved technique for fluorescence staining of fungal nuclei and septa. Australas. Plant Pathol. 1991, 20, 119–121. [Google Scholar] [CrossRef]
- Krnjaja, V.; Lević, J.; Stanković, S.; Vasić, T. The use of vegetative compatibility tests for identification of biodiversity of phytopatogenic fungi. Pestic. Phytomed. 2013, 28, 157–165. [Google Scholar] [CrossRef]
- Fleißner, A.; Starkar, S.; Jacobson, D.J.; Roca, M.G.; Reaad, N.D.; Glass, N.L. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot. Cell 2005, 4, 920–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zare, R.; Gams, W.; Evans, H.C. A revision of Verticillium section Prostrata V. The genus Pochonia, with notes on Rotiferophthora. Nova Hedgwigia 2001, 73, 51–58. [Google Scholar] [CrossRef]
- Short, D.P.G.; Gurung, S.; Hu, X.; Inderbitzin, P.; Subbarao, K.V. Maintenance of sex-related genes and the co-occurrence of both mating types in Verticillium dahliae. PLoS ONE 2014, 9, e112145. [Google Scholar]
- Kim, H.; Wright, S.J.; Park, G.; Ouyang, S.; Kristofova, S.; Borkpvich, K. Roles for receptors, pheromones and mating type genes during sexual reproduction in Neurospora crassa. Genetics 2012, 190, 1389–1404. [Google Scholar] [CrossRef] [Green Version]
- Morton, C.O.; Mauchline, T.H.; Kerry, B.R.; Hirsch, P.R. PCR-based DNA fingerprinting indicates host related genetic variation in the nematophagous fungus Pochonia chlamydosporia. Mycol. Res. 2003, 107, 198–205. [Google Scholar] [CrossRef]
- Morton, C.O.; Hirsch, P.R.; Peberdy, J.P.; Kerry, B.R. Cloning and genetic variation in protease VCP1 from the nematophagous fungus Pochonia chlamydosporia. Mycol. Res. 2003, 107, 38–46. [Google Scholar] [CrossRef]
- Turgeon, B.G. Application of mating type gene technology to problems in fungal biology. Annu. Rev. Phytopathol. 1998, 36, 115–137. [Google Scholar] [CrossRef]
- St Leger, R.J.; Wand, J.B. Metarhizium: Jack of all trades, master of many. Open Biol. 2020, 10, 200307. [Google Scholar] [CrossRef]
- Maciá-Vicente, J.G.; Jansson, H.B.; Talbot, N.J.; Lopez-Llorca, L.V. Real-time PCR quantification and live-cell imaging of endophytic colonization of barley (Hordeum vulgare) roots by Fusarium equiseti and Pochonia chlamydosporia. New Phytol. 2009, 182, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D. The Growth and Sporulation of Verticillium Chlamydosporium. Master’s Thesis, Faculty of Science, University of Manchester, Manchester, UK, 1994; pp. 33–37. [Google Scholar]
- Castro-Urrutia, I.; Andrade-Soto, N.; Valenzuela-Flores, E.; Contreras-Méndez, A. Identification of anastomosis groups in Rhizoctonia solani Kühn isolated from potato in the Décima Region of Chile. Fitopatología 2006, 41, 10–14. [Google Scholar]
- Navarrete-Maya, R.; Esteves, I.; Manzanilla-López, R.H. Pochonia spp. in vitro culturing: Media, strain maintenance and deposition. In Perspectives in Sustainable Nematode Management Through Pochonia chlamydosporia Applications for Root and Rhizosphere Health; Manzanilla-López, R.H., Lopez-Llorca, L.V., Eds.; Springer: Cham, Switzerland, 2017; pp. 211–233. [Google Scholar]
- Esteves, I.; Navarrete-Maya, R.; Manzanilla-López, R.H. Pochonia spp.: Screening and isolate selection for managing plant-parasitic nematodes. In Perspectives in Sustainable Nematode Management through Pochonia chlamydosporia Applications for Root and Rhizosphere Health; Manzanilla-López, R.H., Lopez-Llorca, L.V., Eds.; Springer: Cham, Switzerland, 2017; pp. 235–270. [Google Scholar]
- Esteves, I. Factors Affecting the Performance of Pochonia chlamydosporia as a Biological Control Agent for Nematodes. Ph.D. Thesis, Cranfield University, Bedford, UK, 2007. [Google Scholar]
- Rodríguez-Guerra, R.; Ramírez-Rueda, M.T.; de la Vega, O.M.; Simpson, J. Variation in genotype, pathotype and anastomosis groups of Colletotrichum lindemuthianum isolates from Mexico. Plant Pathol. 2002, 52, 228–235. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]


| Primer Name/Amplified Locus | Sequence (5′–3′) * |
|---|---|
| MAT1-F1 (MAT1-1-1) | CG(A/G)GC(A/T)AA(A/G)CG(A/G)CCATT(G/T)AA(C/T)GC |
| MAT1-R1 (MAT1-1-1) | TT(G/T)CCCATCTC(A/G)TC(A/G)CGGA(C/T)(A/G)AA(A/G)GA |
| MAT1-F2 (MAT1-1-1) | CCAAGCCGGTATCAGTGAATGC |
| MAT1-R2 (MAT1-1-1) | CGACCTGTTGTCGAACAAAGGT |
| MAT2-F1 (MAT1-2-1) | GC(A/G)TATATTCT(A/G)TACCGCAG |
| MAT2-R1 (MAT1-2-1) | CGAGGTTGATA(T/C)TGATA(T/C)TG |
| MAT2-F2 (MAT1-2-1) | ACGCATATATT(T/C)TGTACCG(T/C)AA |
| MAT2-R2 (MAT1-2-1) | GAAGGCTTTCG(A/T)GGT(T/C)TGTAC |
| Species | Isolate | Biotype | Mating-Type PCR-Based Assay | ||
|---|---|---|---|---|---|
| MAT1-1-1 | MAT1-2-1 | Genotype | |||
| Pochonia chlamydosporia | Pc10 | Meloidogyne | ON075837 a | NA | MAT1-1 |
| Pochonia chlamydosporia | Pc280 | Globodera | ON075838 a | NA | MAT1-1 |
| Pochonia chlamydosporia | Pc309 | Meloidogyne | ON075839 a | NA | MAT1-1 |
| Pochonia chlamydosporia | Pc190 | Meloidogyne | ON075840 a | NA | MAT1-1 |
| Pochonia chlamydosporia | Pc60 | Heterodera | NA | ON075841 a | MAT1-2 |
| Purpureocillium lilacinum | NA | NA | NA | NA | NA |
| Fungus Species | Isolate | Country | Biotype |
|---|---|---|---|
| Pochonia chlamydosporia var. chlamydosporia | Pc10 ** | Brazil | Meloidogyne |
| P. chlamydosporia var. chlamydosporia | Pc60 * | UK | Heterodera |
| P. chlamydosporia var. chlamydosporia | Pc190 * | Kenya | Meloidogyne |
| P. chlamydosporia var. chlamydosporia | Pc280 * | Jersey | Globodera |
| P. chlamydosporia var. chlamydosporia | Pc309 * | Zimbabwe | Meloidogyne |
| Purpureocillium lilacinum | ** | South Africa | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finetti-Sialer, M.M.; Manzanilla-López, R.H. Exploring Anastomosis of Hyphae and Mating-Type Compatibility of Pochonia chlamydosporia Isolates of the Meloidogyne, Heterodera and Globodera Biotypes. Pathogens 2022, 11, 619. https://doi.org/10.3390/pathogens11060619
Finetti-Sialer MM, Manzanilla-López RH. Exploring Anastomosis of Hyphae and Mating-Type Compatibility of Pochonia chlamydosporia Isolates of the Meloidogyne, Heterodera and Globodera Biotypes. Pathogens. 2022; 11(6):619. https://doi.org/10.3390/pathogens11060619
Chicago/Turabian StyleFinetti-Sialer, Mariella Matilde, and Rosa Helena Manzanilla-López. 2022. "Exploring Anastomosis of Hyphae and Mating-Type Compatibility of Pochonia chlamydosporia Isolates of the Meloidogyne, Heterodera and Globodera Biotypes" Pathogens 11, no. 6: 619. https://doi.org/10.3390/pathogens11060619
APA StyleFinetti-Sialer, M. M., & Manzanilla-López, R. H. (2022). Exploring Anastomosis of Hyphae and Mating-Type Compatibility of Pochonia chlamydosporia Isolates of the Meloidogyne, Heterodera and Globodera Biotypes. Pathogens, 11(6), 619. https://doi.org/10.3390/pathogens11060619






