First Record of Clonostachys rosea (Ascomycota: Hypocreales) Entomopathogenic Fungus in the Mango Hopper Amritodus atkinsoni (Hemiptera: Cicadellidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection, Isolation, and Culture Conditions of C. rosea
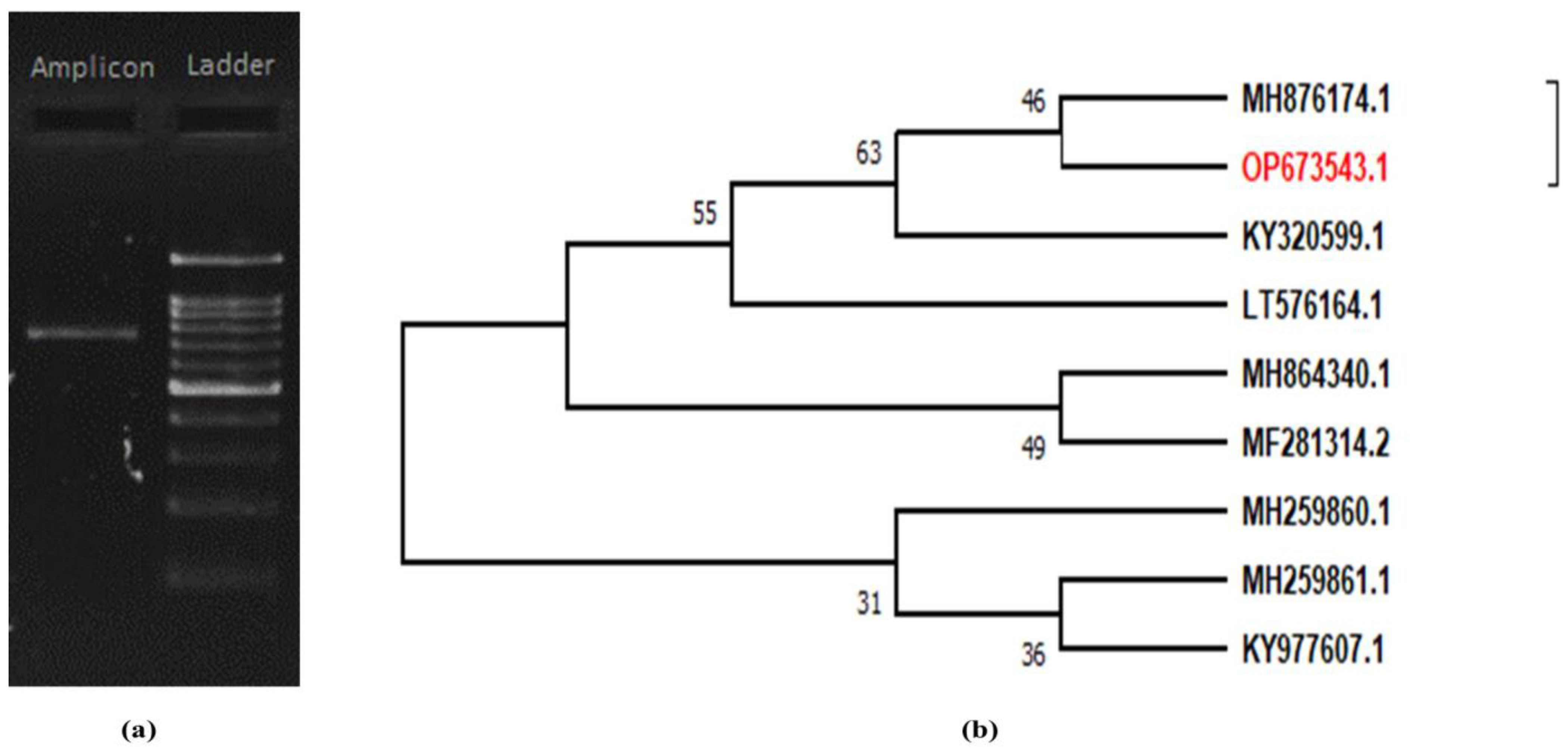
2.2. Morpho-Molecular Characterization of C. rosea
2.3. Media Optimization for Mass Culturing of C. rosea
2.4. Preparation of C. rosea Spore Suspension for Pathogenicity Assays
2.5. Bioassay of C. rosea
3. Results
3.1. Collection, Isolation, and Culture Conditions of C. rosea
3.2. Molecular Identification of Local Isolates
3.3. Media Optimization for Mass Culturing
3.4. Efficacy of C. rosea against A. atkinsoni
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, P.V.R.; Gundappa, B.; Chakravarthy, A.K. Pests of Mango. In Pests and Their Management; Omkar, Ed.; Springer: Singapore, 2018; pp. 415–440. [Google Scholar] [CrossRef]
- Dietrich, C.H. Phylogeny of the leafhopper subfamily Evacanthinae with a review of Neotropical species and notes on related groups (Hemiptera: Membracoidea: Cicadellidae). Syst. Entomol. 2004, 29, 455–487. [Google Scholar] [CrossRef]
- Visalakshy, P.G.; Mani, M.; Krishnamoorthy, A.; Pillai, K.G. Epizootics of Entomophthora sp. on mango inflorescence hopper, Idioscopus nitidulus (Walker). J. Biol. Control 2010, 24, 274–275. [Google Scholar]
- Rahman, S.M. Mango hopper: Bioecology and management–A review. Agric. Rev. 2007, 28, 49–55. [Google Scholar]
- Varshneya, A.; Rana, K.S. Effect of some abiotic factors on population buildup of Idioscopus clypealis (Lethierry) in western Uttar Pradesh. J. Environ. Biol. 2008, 29, 811–812. [Google Scholar]
- Anant, A.K.; Awasthi, A.; Pandi, G. Evaluation of insecticides against mango hoppers Amritodus atkinsoni and Idioscopus clypealis. Indian J. Entomol. 2019, 81, 340–342. [Google Scholar] [CrossRef]
- Donkor, A.; Osei-Fosu, P.; Dubey, B.; Kingsford-Adaboh, R.; Ziwu, C.; Asante, I. Pesticide residues in fruits and vegetables in Ghana: A review. Environ. Sci. Pollut. Res. 2016, 23, 18966–18987. [Google Scholar] [CrossRef]
- Schroers, H.J.; Samuels, G.J.; Seifert, K.A.; Gams, W. Classification of the mycoparasite Gliocladium roseum in Clonostachys as C. rosea, its relationship to Bionectria ochroleuca, and notes on other Gliocladium-like fungi. Mycologia 1999, 91, 365–385. [Google Scholar] [CrossRef]
- Schroers, H.J. A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs. Stud. Mycol. 2001, 46, 1–214. [Google Scholar]
- Toledo, A.V.; Virla, E.; Humber, R.A.; Paradell, S.L.; Lastra, C.L. First record of Clonostachys rosea (Ascomycota: Hypocreales) as an entomopathogenic fungus of Oncometopia tucumana and Sonesimia grossa (Hemiptera: Cicadellidae) in Argentina. J. Invertebr. Pathol. 2006, 92, 7–10. [Google Scholar] [CrossRef]
- Sutton, J.C.; Li, D.W.; Peng, G.; Yu, H.; Zhang, P.; Valdebenito-Sanhueza, R.M. Gliocladium roseum a versatile adversary of Botrytis cinerea in crops. Plant Dis. 1997, 81, 316–328. [Google Scholar] [CrossRef]
- Prabhu, D.K.; Kumar, A. Gliocladium roseum as a microbial control agent of malaria vector Anopheles stephensi Liston and filaria vector Culex quinquefasciatus Say. Kavaka 2008, 36, 53–56. [Google Scholar]
- Mustafa, R.A.; Assaf, L.H.; Abdullah, S.K. Comparative pathogenicity of Beauveria bassiana, Clonostachys rosea, Metarhizium anisopliae, and Lecanicillium lecanii to adult alfalfa weevil Hypera postica Gyllenhal (Coleoptera: Curculionidae). In Proceedings of the 3rd International Conference on Applied Life Sciences, Bali, Indonesia, 23–25 September 2014; pp. 11–14. [Google Scholar]
- Mahmoudi, H.; Amini, A.; Mirzaee, M.R.; Sadeghi, H.; Tavakkoli, G.R. Clonostachys rosea, a new and promising entomopathogenic fungus infecting pupa of jujube fruit fly. Carpomya vesuviana. Mycol. Iran 2018, 5, 43–49. [Google Scholar]
- Anwar, W.; Ali, S.; Nawaz, K.; Iftikhar, S.; Javed, M.A.; Hashem, A.; Akhter, A. Entomopathogenic fungus Clonostachys rosea as a biocontrol agent against whitefly (Bemisia tabaci). Biocontrol. Sci. Technol. 2018, 28, 750–760. [Google Scholar] [CrossRef]
- Sun, Z.B.; Li, S.D.; Ren, Q.; Xu, J.L.; Lu, X.; Sun, M.H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef]
- Gupte, M.; Kulkarni, P. A study of antifungal antibiotic production by Thermomonospora sp. MTCC 3340 using full factorial design. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2003, 78, 605–610. [Google Scholar]
- Franco-Lara, E.; Link, H.; Weuster-Botz, D. Evaluation of artificial neural networks for modelling and optimization of medium composition with a genetic algorithm. Process Biochem. 2006, 41, 2200–2206. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, X.; An, F.; Wang, G.; Zhang, X. Improvement of antibiotic activity of Xenorhabdus bovienii by medium optimization using response surface methodology. Microb. Cell Factories 2011, 10, 98. [Google Scholar] [CrossRef]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Bainier, G. Gliocladium roseum sp. nov. et Cephalosporium acremonium (Corda). Bull. Soc. Mycol. Fr. 1907, 23, 111–114. [Google Scholar]
- Foëx, E. Une pouriture verticillienne du tubercle de pomme de terre. Ann. EPiphyt’ 1923, 9, 121–133. [Google Scholar]
- Tehon, L.R.; Jacobs, H.L. A Verticillium root disease of American Elm; The Davey Tree Expert Co.: Kent, OH, USA, 1936; Volume 12, pp. 529–546. [Google Scholar]
- Isaac, I. Gliocladium roseum Bain. and its synonyms. Trans. Br. Mycol. Soc. 1954, 37, 193–208. [Google Scholar] [CrossRef]
- Alvindia, D.G.; Hirooka, Y. Identification of Clonostachys and Trichoderma spp. from banana fruit surfaces by cultural, morphological and molecular methods. Mycology 2011, 2, 109–115. [Google Scholar] [CrossRef]
- Humber, R.A. Identification of entomopathogenic fungi. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 151–187. [Google Scholar]
- Zhang, Y.J.; Zhang, S.; Liu, X.Z.; Wen, H.A.; Wang, M. A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett. Appl. Microb. 2010, 51, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Edelstein, J.D.; Trumper, E.V.; Lecuona, R.E. Temperature-dependent development of the entomopathogenic fungus Nomuraea rileyi (Farlow) Samson in Anticarsia gemmatalis (Hübner) larvae (Lepidoptera: Noctuidae). Neotrop. Entomol. 2005, 34, 593–599. [Google Scholar] [CrossRef]
- Mamarabadi, M.; Jensen, B.; Jensen, D.F.; Lübeck, M. Real-time RT-PCR expression analysis of chitinase and endoglucanase genes in the three-way interaction between the biocontrol strain Clonostachys rosea IK726, Botrytis cinerea and strawberry. FEMS Microbiol. Lett. 2008, 285, 101–110. [Google Scholar] [CrossRef]
- Costa, L.B.; Rangel, D.E.; Morandi, M.A.; Bettiol, W. Impact of UV-B radiation on Clonostachys rosea germination and growth. World J. Microbiol. Biotechnol. 2012, 28, 2497–2504. [Google Scholar] [CrossRef]
- Verdejo-Lucas, S.; Ornat, C.; Sorribas, F.J.; Stchiegel, A. Species of root-knot nematodes and fungal egg parasites recovered from vegetables in Almería and Barcelona, Spain. J. Nematol. 2002, 34, 405. [Google Scholar]
- Viccini, G.; Martinelli, T.R.; Cognialli, R.C.R.; de Faria, R.O.; Carbonero, E.R.; Sassaki, G.L.; Mitchell, D.A. Exopolysaccharide from surface-liquid culture of Clonostachys rosea originates from autolysis of the biomass. Arch. Microbiol. 2009, 191, 369–378. [Google Scholar] [CrossRef]
- de Andrade Carvalho, A.L.; de Rezende, L.C.; Costa, L.B.; de Almeida Halfeld-Vieira, B.; Pinto, Z.V.; Morandi, M.A.; de Medeiros, F.H.; Bettiol, W. Optimizing the mass production of Clonostachys rosea by liquid-state fermentation. Biol. Control 2018, 118, 16–25. [Google Scholar] [CrossRef]
- Sun, B.D.; Liu, X.Z. Occurrence and diversity of insect-associated fungi in natural soils in China. Appl. Soil Ecol. 2008, 39, 100–108. [Google Scholar] [CrossRef]
- Sreekanth, D.; Sushim, G.K.; Syed, A.; Khan, B.M.; Ahmad, A. Molecular and morphological characterization of a taxol-producing endophytic fungus, Gliocladium sp., from Taxus baccata. Mycobiology 2011, 39, 151–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perdikis, D.C.; Lykouressis, D.P. Thermal requirements for development of the polyphagous predator Macrolophus pygmaeus (Hemiptera: Miridae). Environ. Entomol. 2002, 31, 661–667. [Google Scholar] [CrossRef]
- Shah, F.A.; Wang, C.S.; Butt, T.M. Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett. 2005, 251, 259–266. [Google Scholar] [CrossRef]


| 15 °C | 20 °C | 25 °C | 30 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Media | Radial Growth (cm) | Conidia/Bit | Germination (% ) | Radial Growth (cm) | Conidia/Bit | Germination (% ) | Radial Growth (cm) | Conidia/Bit | Germination (% ) | Radial Growth (cm) | Conidia/Bit | Germination (% ) |
| −106 | (106) | (106) | (106) | |||||||||
| SDAY | 2.67 | 2.37 | 78.52 | 5.84 | 4.71 | 76.64 | 5.29 | 0.5 | 82.64 | 3.18 | 0.73 | 78.6 |
| SMAY | 3.55 | 0.37 | 76.16 | 7.53 | 3.65 | 74.32 | 7.2 | 0.31 | 78.84 | 2.66 | 0.45 | 80.45 |
| PDA | 1.7 | 0.27 | 75.34 | 3.83 | 1.91 | 79.16 | 5.66 | 1.63 | 74.67 | 1.4 | 0.53 | 80.38 |
| OMA | 4.65 | 2.79 | 86.77 | 8.21 | 9.81 | 92.25 | 7.74 | 4.4 | 86.94 | 4.12 | 1.73 | 83.66 |
| CZA | 2.41 | 2.35 | 72.79 | 6.56 | 3.83 | 75.67 | 6.59 | 0.85 | 74.86 | 2.79 | 0.13 | 74.81 |
| SE | 0.54 | 0.37 | 1.32 | 0.56 | 0.53 | 1.21 | 0.74 | 0.53 | 1.61 | 0.56 | 0.18 | 1.19 |
| CD at 5% | 0.16 | 0.11 | 3.983 | 0.17 | 0.16 | 3.68 | 0.22 | 0.16 | 4.86 | 0.17 | 0.53 | 3.58 |
| CV | 3.58 | 4.57 | 3.39 | 1.74 | 2.23 | 3.05 | 2.29 | 2.23 | 4.04 | 3.94 | 4.96 | 2.99 |
| 15 °C | 20 °C | 25 °C | 30 °C | |||||
|---|---|---|---|---|---|---|---|---|
| Media | Fresh | Dry | Fresh | Dry | Fresh | Dry | Fresh | Dry |
| SMAY | 5.55 | 0.87 | 5.58 | 1.04 | 7.37 | 1.13 | 0.85 | 0.69 |
| SDYB | 5.39 | 0.9 | 4.67 | 1.02 | 7.66 | 1.03 | 3.17 | 0.88 |
| PDB | 4.36 | 0.8 | 5.22 | 0.83 | 4.76 | 0.73 | 1.65 | 0.75 |
| OMB | 7.56 | 1.07 | 8.87 | 1.28 | 7.95 | 1.39 | 4.66 | 0.96 |
| CZB | 2.13 | 0.64 | 2.26 | 0.75 | 6.56 | 0.28 | 4.41 | 0.77 |
| SE | 0.49 | 0.23 | 0.6 | 0.26 | 0.56 | 0.12 | 0.49 | 0.12 |
| CD at 5% | 0.15 | 0.74 | 0.19 | 0.81 | 0.18 | 0.4 | 0.15 | 0.4 |
| CV | 1.7 | 4.8 | 1.96 | 4.54 | 1.41 | 2.43 | 2.87 | 2.73 |
| Concentration (Conidia/mL) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|---|
| T1 (3 × 108) | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 13.3 ± 5.7 b | 33.3 ± 15.2 b | 56.6 ± 11.5 c | 76.6 ± 5.7 d | 96.6 ± 5.7 e |
| T2 (2.5 × 107) | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 3.3 ± 5.7 a | 13.3 ± 15.2 a | 33.3 ± 20.8 b | 46.6 ± 15.2 c | 70.0 ± 10.0 d |
| T3 (2 × 106) | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 3.3 ± 5.7 a | 13.3 ± 15.2 ab | 33.3 ± 15.2 bc | 53.3 ± 5.7 c |
| T4 (1.5 × 105) | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 3.3 ± 5.7 a | 13.3 ± 11.5 ab | 20.0 ± 17.3 ab | 16.6 ± 11.5 b |
| T5 (1 × 104) | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 3.3 ± 5.7 a | 6.6 ± 5.7 a | 6.6 ± 5.7 ab |
| Control | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamta, A.K.; Pandey, R.; Sharma, J.R.; Rai, R.; Barman, M.; M. G., D.; Mitra, D.; Mohapatra, P.K.D.; Sami, R.; Al-Mushhin, A.A.M.; et al. First Record of Clonostachys rosea (Ascomycota: Hypocreales) Entomopathogenic Fungus in the Mango Hopper Amritodus atkinsoni (Hemiptera: Cicadellidae). Pathogens 2022, 11, 1447. https://doi.org/10.3390/pathogens11121447
Tamta AK, Pandey R, Sharma JR, Rai R, Barman M, M. G. D, Mitra D, Mohapatra PKD, Sami R, Al-Mushhin AAM, et al. First Record of Clonostachys rosea (Ascomycota: Hypocreales) Entomopathogenic Fungus in the Mango Hopper Amritodus atkinsoni (Hemiptera: Cicadellidae). Pathogens. 2022; 11(12):1447. https://doi.org/10.3390/pathogens11121447
Chicago/Turabian StyleTamta, Abhishek Kumar, Renu Pandey, Jiten R. Sharma, Rajnish Rai, Mritunjoy Barman, Deeksha M. G., Debasis Mitra, Pradeep Kumar Das Mohapatra, Rokayya Sami, Amina A. M. Al-Mushhin, and et al. 2022. "First Record of Clonostachys rosea (Ascomycota: Hypocreales) Entomopathogenic Fungus in the Mango Hopper Amritodus atkinsoni (Hemiptera: Cicadellidae)" Pathogens 11, no. 12: 1447. https://doi.org/10.3390/pathogens11121447
APA StyleTamta, A. K., Pandey, R., Sharma, J. R., Rai, R., Barman, M., M. G., D., Mitra, D., Mohapatra, P. K. D., Sami, R., Al-Mushhin, A. A. M., Baakdah, F., Mostafa, Y. S., Alrumman, S. A., & Helal, M. (2022). First Record of Clonostachys rosea (Ascomycota: Hypocreales) Entomopathogenic Fungus in the Mango Hopper Amritodus atkinsoni (Hemiptera: Cicadellidae). Pathogens, 11(12), 1447. https://doi.org/10.3390/pathogens11121447








