Abstract
Wolbachia is an endosymbiotic bacterium that can restrict the transmission of human pathogenic viruses by Aedes aegypti mosquitoes. Recent field trials have shown that dengue incidence is significantly reduced when Wolbachia is introgressed into the local Ae. aegypti population. Female Ae. aegypti are anautogenous and feed on human blood to produce viable eggs. Herein, we tested whether people who reside on Tri Nguyen Island (TNI), Vietnam developed antibodies to Wolbachia Surface Protein (WSP) following release of Wolbachia-infected Ae. aegypti, as a measure of exposure to Wolbachia. Paired blood samples were collected from 105 participants before and after mosquito releases and anti-WSP titres were measured by ELISA. We determined no change in anti-WSP titres after ~30 weeks of high levels of Wolbachia-Ae. aegypti on TNI. These data suggest that humans are not exposed to the major Wolbachia surface antigen, WSP, following introgression of Wolbachia-infected Ae. aegypti mosquitoes.
1. Introduction
Aedes aegypti mosquitoes are the major vector for many human pathogenic viruses, including dengue, Zika, and chikungunya. Dengue virus prevalence has risen sharply in recent decades, driven in part by the adaptation of these mosquitoes to thrive in densely populated mega-cities [1,2]. Despite the global burden of dengue, there is no approved antiviral therapeutic to treat infections, and uptake of the one approved vaccine has been poor [3]. As such, vector control methods remain the major defence employed to limit the spread of dengue virus.
Wolbachia pipientis is an intracellular, endosymbiotic bacterium that has been developed as a biocontrol tool to reduce the vector competence of Ae. aegypti. Several Wolbachia strains have been transinfected into Ae. aegypti and shown to reduce the risk of transmission of flavi- and alphaviruses [4,5,6,7,8,9]. Introgression of the Wolbachia strain wMel (from Drosophila melanogaster) into Ae. aegypti populations significantly reduces the incidence of dengue and chikungunya in humans [10,11,12,13,14].
Ae. aegypti are anautogenous, strictly requiring a blood meal in order to lay viable eggs, and humans are predominantly their meal source [15]. This direct interaction, whereby mosquitoes expel saliva as they probe for a blood vessel, could potentially provide an opportunity for humans to be exposed to Wolbachia antigens. Wolbachia surface protein (WSP) is a major surface membrane protein exposed to its host and therefore may represent the main antigenic target. Previous studies have attempted to measure Wolbachia in the saliva of Ae. aegypti with a Wolbachia infection [16] and antibodies to Wolbachia in the plasma of people who routinely feed lab colonies of Wolbachia-Ae. aegypti [17]. However, no study to date has directly investigated the potential for Wolbachia exposure to residents at field sites where these mosquitoes are introgressed.
In 2013, the Wolbachia strain wMelPop-CLA (originally from D. melanogaster, then adapted to the mosquito host by long-term passage through two Ae. albopictus cell lines [18]; referred to herein as wMelPop) was assessed for its ability to introgress into Ae. aegypti populations at sites in Northern Australia and central Vietnam [19]. Herein, we examine whether this introgression of Wolbachia-Ae. aegypti into local mosquito populations caused residents in Vietnam to develop antibodies to WSP as a measure of Wolbachia exposure. Paired blood samples were collected pre- and post-release of wMelPop-Ae. aegypti from 105 residents of Tri Nguyen Island (TNI) in Khanh Hoa Province, Vietnam. We measured the IgA/G/M antibodies to Wolbachia WSP in plasma from these participants using a new enzyme-linked immunosorbent assay (ELISA). Our data support the hypothesis that humans are not exposed to Wolbachia antigens when bitten by Ae. aegypti infected with wMelPop.
2. Results
2.1. Enrolment of Participants on Tri Nguyen Island
As reported previously [19], in 2013, wMelPop-Ae. aegypti were released for 23 consecutive weeks across three hamlets on TNI, Khanh Hoa Province, Vietnam [19] (Figure 1A,B shaded area). At its peak, this Wolbachia strain was found in approximately 90% of the Ae. aegypti population (Figure 1B). However, when releases ceased, wMelPop frequency declined rapidly, most likely due to fitness costs incurred by the mosquito [19]. Despite this, residents of TNI experienced nearly 7 months when wMelPop-Ae. aegypti frequency was > 50% of the total Ae. aegypti population. To determine whether residents are exposed to Wolbachia when these biting mosquitoes are established in the local environment, baseline plasma samples were collected one month prior to commencement of mosquito releases, and then again 22 months later (Figure 1B, red arrows). Plasma samples from 105 participants were assessed in this study, including 51 from hamlet 1, 24 from hamlet 2, and 30 from hamlet 3 (Figure 1C). Participants’ ages ranged from 1 to 76 years at the time of enrolment, and a total of 38 male participants and 67 female participants were studied (Figure 1D).
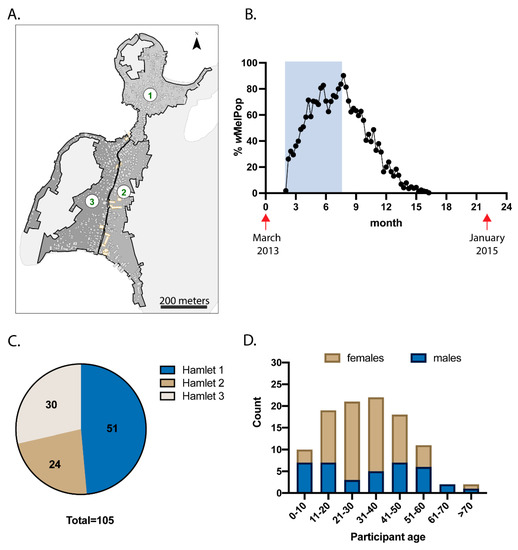
Figure 1.
Participant demographics. (A) Releases of Ae. aegypti with wMelPop strain of Wolbachia were performed across 3 hamlets on TNI, Vietnam. (B) wMelPop-Ae. aegypti were released weekly for 23 weeks (shaded rectangle), and routine trapping of mosquitoes was performed using Biogents Sentinel traps to monitor the proportion of Ae. aegypti with wMelPop infection (plotted in black). Plasma was collected from 105 participants prior to mosquito releases, then collected again 22 months later (collection times indicated by red arrows). Participant breakdown by hamlet location (C) and by age and gender (D) is shown.
2.2. Establishment of an Enzyme-Linked Immunosorbent Assay (ELISA) to Detect Plasma Antibodies with Reactivity to WSP
We developed an ELISA to measure plasma IgA/G/M against WSP to assess whether individuals are exposed to Wolbachia following establishment of these mosquitoes in the local environment. Recombinant WSP residues 33-201 (WSP33-201) representing soluble wMelPop WSP (identical in sequence to wMel WSP, Figure S1A and [20]) were expressed in E. coli with a 10× His tag to facilitate purification. The purified protein (Figure S1B) was used to raise polyclonal antibodies to WSP in rabbits. Anti-WSP polyclonal antibodies were shown to bind to both wMel and wMelPop WSP from Ae. aegypti-derived Aag2 cells (Figure S1C,D).
A direct ELISA was established using WSP33-201 to coat 96-well immunosorbent plates. Plasma from blood donors located in southern regions of Australia, where Wolbachia-Ae. aegypti are not found, was used to determine the background reactivity of human plasma to WSP. Reactivity was shown to be very low compared to plasma spiked with anti-WSP (Figure 2A). To validate the integrity of the plasma samples, we also ran a parallel ELISA using tetanus toxoid protein to confirm the presence of anti-tetanus toxoid IgA/G/M (Figure 2B). This antigen was selected due to the high global vaccination rate against tetanus, including in Vietnam and Australia [21,22]. Each of the three donor plasmas demonstrated strong reactivity to this control antigen.
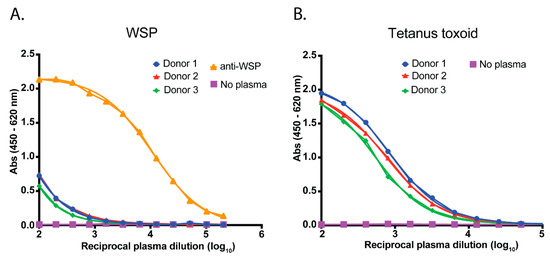
Figure 2.
Assay validation. (A) A direct ELISA was established to measure human plasma IgA/G/M reactivity to WSP. Plasma from donors located in southern regions of Australia (where Ae. aegypti with Wolbachia are not found) were used as negative controls. These plasmas were pooled and then spiked with polyclonal anti-WSP to generate a positive serological signal in the assay. (B) In a parallel ELISA, plasma samples were tested for antibody reactivity to tetanus toxoid protein to demonstrate the integrity of the plasma samples for serological purposes. Data have been fitted with a sigmoidal dose-response (variable slope) curve using GraphPad Prism® software (PRISM 7 version, GraphPad Software Inc., San Diego, CA, USA).
2.3. Release of wMelPop-Ae. aegypti Does Not Alter Plasma Immunoreactivity to WSP
To determine whether individuals developed antibodies to Wolbachia after exposure to wMelPop-Ae. aegypti, paired plasma samples from 105 TNI residents were titrated across immunosorbent plates that had been coated with WSP33-201. Plasma samples from 29 Red Cross blood donors located in southern regions of Australia were included as individual negative controls for Wolbachia serology. In parallel, each plasma sample was also tested for reactivity to tetanus toxoid.
Using a paired t-test, we measured no significant difference in the anti-WSP titres in plasma from individuals before or after exposure to wMelPop-Ae. aegypti (Figure 3A). The median anti-WSP titres for these groups were also not significantly different from the negative control plasmas (Kruskal-Wallis test with Dunn’s correction). As expected, the median anti-WSP-spiked plasma titre was significantly higher than the negative control plasmas (p < 0.0001), demonstrating that the assay had the capacity to measure antibodies to WSP. Plasma from the TNI participants also did not significantly change in reactivity to tetanus toxoid between the pre- and post-release plasma samples (paired t-test; Figure 3B). The Australian Red Cross donor control plasmas had significantly higher reactivity to tetanus toxoid compared to a PBS negative control (Kruskal-Wallis test with Dunn’s correction, p < 0.0001). The median titre of anti-tetanus toxoid was also slightly but significantly higher in the Red Cross control plasmas compared to the TNI pre- and post-release groups (p < 0.0001).
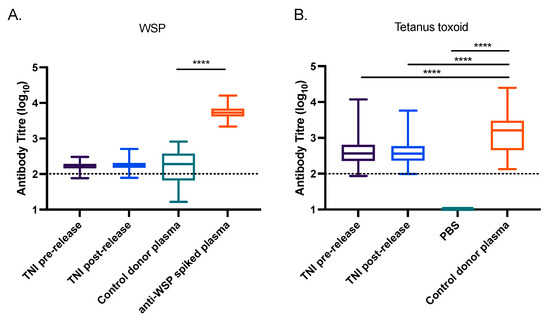
Figure 3.
Introgression of Wolbachia into Ae. aegypti mosquito populations does not induce a detectable anti-Wolbachia immune response in humans. (A,B) Plasma from 105 donors living on TNI, Vietnam, pre- and post-release of wMelPop-Ae. aegypti were screened for antibodies to WSP (A), or tetanus toxoid (B) by ELISA. Australian plasma collected from 29 Red Cross donors in regions where Wolbachia-Ae. aegypti are not found were also assessed (control donor plasma). This served as a negative control for the WSP ELISA, and a positive control for the tetanus toxoid ELISA. A pool of 5 negative control donor plasmas spiked with polyclonal anti-WSP was used to indicate a positive serological signal for the WSP ELISA, while PBS was included as a negative control for the tetanus toxoid ELISA. The antibody titre for each sample is expressed as the reciprocal of the highest dilution of plasma required to achieve an absorbance value of 0.4, determined by nonlinear regression analysis performed using GraphPad Prism® software (PRISM 7 version, GraphPad Software Inc., San Diego, CA, USA). Data are the median, interquartile range, minimum, and maximum antibody titres for each cohort. Asterisks indicate significance, determined by a Kruskal-Wallis test with Dunn’s correction, where **** indicates p < 0.0001. The minimum measurable antibody titre for each antigen is indicated by a dotted line.
Breakdown of the data from Figure 3A to directly compare the TNI pre- and post-release plasma samples for each individual participant identified no clear trend in increasing or decreasing anti-WSP IgG/A/M titres following release of wMelPop-Ae. aegypti (Figure S2). Together, these data suggest that environmental exposure to wMelPop-Ae. aegypti does not induce a detectable level of antibodies to the Wolbachia antigen WSP in local residents.
3. Discussion
Wolbachia naturally resides as a non-pathogenic endosymbiont in a wide range of invertebrates [23,24,25], including in human-biting mosquito species Ae. albopictus (wAlbA and wAlbB) [26,27] and Culex quinquefasciatus (wPip) [23]. Wolbachia strains found in insect species are not considered pathogens of vertebrate animals. However, distantly related Wolbachia species from nematodes may contribute to filariasis pathogenesis [28,29,30,31,32]. With the recent development of population replacement methods introgressing Ae. aegypti with Wolbachia into mosquito populations, here we ask whether residents become seropositive to the antigenic Wolbachia protein, WSP, following their introgression.
Our findings build on the safety profile for the use of Wolbachia as a biocontrol tool in Ae. aegypti [16,17,33,34]. The results indicate that residents of TNI did not develop a measurable antibody response to WSP following the transient establishment of wMelPop-Ae. aegypti in their local area. This is consistent with evidence that Wolbachia is not detectably transferred through mosquito saliva. For example, Moreira et al. (2009) collected saliva expectorated from wMelPop-Ae. aegypti and attempted to amplify Wolbachia genes WSP and the multi-copy transposable element IS5, but were unable to detect either Wolbachia gene [16]. Popovici et al. (2010) investigated whether human volunteers that routinely allowed Wolbachia-Ae. aegypti colonies to directly feed on their arms developed antibodies against wMelPop [17]. To do this, they analysed sera from 17 volunteers who had fed mosquito colonies over a 4-year period, using Western blots and ELISAs with Wolbachia-infected or -uninfected cell extracts used as antigen. IgG reactivity to Wolbachia was not found to be different compared to volunteers who had not previously fed Wolbachia-infected Ae. aegypti mosquitoes, whilst blood-feeder volunteers (who may be exposed to tens of thousands of mosquito bites over 4 years) developed antibodies to a 45 kDa Ae. aegypti protein. While our findings are consistent with these previous studies, we acknowledge that the collection of plasma from residents occurred ~5 months after wMelPop was no longer detectable in the local Ae. aegypti population. We therefore cannot exclude the possibility that a short-lived antibody response to Wolbachia was induced.
Mosquito salivary proteins are well-known to induce an IgG response in humans [35,36,37], which occurs following salivation of mosquitoes as they probe the dermal layer for blood vessels. This probing and salivation can lead to the transfer of pathogenic viruses from infected mosquitoes into humans or other animals [38]. However, since Wolbachia is an obligate intracellular bacterium, this would require it to be released from host mosquito cells, secreted into the lumen of the salivary gland duct, and transferred through the proboscis of the mosquito. If Wolbachia were released from host cells, transfer may be further restricted by the diameter of the salivary gland ducts (1–2 μm [39]), which may not readily allow passage of Wolbachia (up to 1.5 μm in diameter for wMel, wAlbB, and wMelPop [40,41,42,43]).
Most of the studies investigating the risk of Wolbachia transfer from mosquitoes to humans have assessed wMelPop, while current field trials are primarily using wMel or wAlbB Wolbachia strains. While wMelPop has been introgressed into Ae. aegypti populations at field sites in both Vietnam and Northern Queensland (Australia), it has not remained stable, eventually dropping out [19]. By contrast, wMel and wAlbB have remained at high frequency following introgression into mosquito populations at multiple sites, potentially exposing residents to these strains of Wolbachia for much longer periods of time [11,13,44,45]. It is therefore important to consider whether these strains similarly present the same low risk. All three strains are distributed throughout a range of mosquito tissues, including the ovaries, midgut, malpighian tubules, and salivary glands [9,46]. However, wMelPop resides at a higher overall density in whole mosquitoes than wMel and wAlbB [47], which might suggest that the risk of transfer for these strains is lower.
Whilst we were unable to detect human Ig responses to WSP, it is possible that other Wolbachia antigens do enter the mosquito saliva, perhaps following their secretion into host cells via the bacterial type IV secretion system [48,49]. We therefore cannot exclude the possibility that these products induce an immune response in humans. In regions endemic for the nematode Brugia malayi (which parasitises human hosts), residents develop anti-WSP antibodies as well as antibodies that recognise the Wolbachia secreted protein Wolbachia Translation Initiation Factor-1 [50]. During Onchocerca volvulus (another human-parasitic nematode) infection, Wolbachia lipopeptide can induce the production of Neutrophil Extracellular Traps (an anti-microbial defence mechanism) [51]. Additionally, release of Wolbachia into patients, following Ivermectin treatment to clear O. volvulus infections, correlates with post-treatment inflammation events [52]. These studies indicate that various Wolbachia products may be able to induce an immune response. However, dozens of adult nematodes and thousands of microfilariae are present in human infections [53,54], with each nematode cell carrying multiple Wolbachia [55]. Therefore, the human exposure to Wolbachia components from nematodes may be magnitudes higher than the exposure that may hypothetically occur from Wolbachia components present in the saliva of Wolbachia-infected Ae. aegypti.
Interestingly, Punkodsy et al. (2003) reported that 15 of 67 serum samples from individuals from North America with no history of lymphatic filariasis were seropositive to WSP [32]. These antibodies recognized a highly conserved region of the second transmembrane domain of WSP, perhaps suggesting that these people were exposed to Wolbachia from other non-nematode species. Notably, Wolbachia strains from nematodes cluster phylogenetically to supergroups C and D, and are mostly obligate mutualists [56]; curing nematodes of their Wolbachia infection by antibiotic treatment leads to nematode death [57]. By contrast, supergroup A and B Wolbachia strains, including wMel, wMelPop, and wAlbB, are usually found in arthropods, and their relationship is not required for the survival of the host [58]. Additionally, Wolbachia species from nematodes survive at their host temperature of 37 °C compared to the cooler temperatures habited by Wolbachia from arthropods. This clear distinction in phylogeny and host relationship for Wolbachia strains found in nematodes compared to arthropods could predict that arthropod-derived Wolbachia strains may be unable to establish infection in humans, even if exposure events do occur.
It should be acknowledged that a 2015 study detected Wolbachia DNA in the blood of a patient subsequently diagnosed with non-Hodgkin’s lymphoma, with the authors proposing a possible novel pathogenic infection by Wolbachia alone [59]. Wolbachia 16S and fbpA genes were amplified from the infected patient’s blood and found to cluster most closely with Wolbachia supergroup B strains. This is consistent with Wolbachia species from insects. No causative agent was isolated, and there have been no further reports of possible direct human infections with Wolbachia, so the significance of this study remains unclear.
With several recent publications demonstrating the efficacy of Wolbachia-Ae. aegypti introgression programs in reducing vector-borne disease, it is likely that a global expansion of these programs will occur in coming years [10,11,12,13,14]. Our evidence indicates that transient introgression of wMelPop into Ae. aegypti populations did not seroconvert residents to the major Wolbachia antigen, WSP. These data add to the body of evidence that Wolbachia is a safe biocontrol tool to limit the spread of mosquito-borne viruses.
4. Materials and Methods
4.1. Recruitment of Blood Donors on Tri Nguyen Island, Vietnam
The release of Ae. aegypti with wMelPop at Tri Nguyen Island (TNI) and recruitment of volunteers for plasma donations was approved by the institutional review board (IRB) of the National Institute of Hygiene and Epidemiology (Approval reference number: 32/HDD 15/12/2011) and the IRB of Vietnam Ministry of Health (Approval reference number: 615/CN-BYT 19/7/2012).
Blood samples were collected from residents in each of the 3 hamlets of TNI as part of general health surveys before and after the release of Ae. aegypti with wMelPop. These samples were used to measure changes in plasma reactivity to Wolbachia before and after intervention (i.e., release of Wolbachia-Ae. aegypti), similar to an interrupted time series study design. Informed consent to participate was obtained from all participants aged over 18 and from parents/guardians for those under 18. Baseline plasma samples were collected from residents in March 2013, before release of any wMelPop-Ae. aegypti. Beginning in April 2013, mosquitoes were released weekly for 23 weeks, as described previously [19]. The proportion of Ae. aegypti infected with wMelPop increased throughout the release period, peaking at ~90% of the total Ae. aegypti population by week 23. However, immediately after releases stopped, the frequency of mosquitoes with wMelPop declined rapidly, with fewer than 10% of Ae. aegypti carrying wMelPop by week 47 [19]. Overall, it is expected that residents had ~30 weeks of exposure to wMelPop-Ae. aegypti where these mosquitoes comprised > 50% of the Ae. aegypti population. In January 2015, repeat plasma collections were taken from the same individuals. Overall, 142 paired plasma samples were collected from residents (pre- and post-release of wMelPop-Ae. aegypti), and 105 of these were randomly chosen to be assayed here. Participant ages ranged from 1 to 76 years at the time of the first collection.
Plasma was extracted by centrifuging whole blood at 290× g for 10 min. The upper plasma layer was collected and heat inactivated at 56 °C for 30 min.
4.2. Control Plasmas
Red Cross blood from donors in Australian states where Ae. aegypti mosquitoes are not found (Victoria and South Australia) were used as negative controls for the ELISA assay that measured Wolbachia immunogenicity. Plasma from 29 donors was extracted from whole blood and heat inactivated, as described above.
4.3. WSP Overexpression and Purification
The WSP sequence from wMel and wMelPop are identical (Figure S1A). wMelPop-CLA is a variant of wMelPop that was generated by passage of wMelPop through mosquito cell lines for ~3.5 years [18]. This variant acquired a suite of genomic changes outside of the WSP gene (WD_1063) and was then transinfected into Ae. aegypti, where the genome remained quite stable [20]. The sequence of WSP from wMelPop-CLA is identical to both wMel and wMelPop. Note that wMelPop-CLA from transinfected Ae. aegypti has previously been referred to as wMelPop-PGYP in other publications [19,20]. For simplicity, we refer to wMelPop-CLA throughout this manuscript as wMelPop.
WSP from wMel was expressed and purified by The Commonwealth Scientific and Industrial Research Organisation (CSIRO; Parkville, Melbourne, Australia) in a truncated form; the N-terminal 33 amino acids and C-terminal 36 amino acids were removed to promote stability and solubilization. WSP residues 33–201 were expressed with an N-terminal 10× His tag to facilitate purification.
Briefly, the truncated WSP nucleotide sequence was cloned into the pD434-SR E. coli expression vector (ATUM, Newark, CA, USA). BL21(DE3) cells were transformed with pD434-SR-WSP and grown in 1.0 L Terrific Broth media containing 100 μg/mL ampicillin at 37 °C and 200 rpm until an OD600 of 0.8. Protein expression was induced by 0.5 mM IPTG at 18 °C for 24 h. The bacterial cell pellet was lysed in phosphate-buffered saline (PBS) containing an additional 150 mM NaCl, 10% glycerol, 2 mM MgCl2, 0.5 mg/mL lysozyme, 2.0 μL Benzonase (250 U/μL), 1 mM PMSF, and 2× protease inhibitor tablets (EDTA-free; Roche). Cells were homogenized and bacterial inclusion bodies were harvested by centrifugation. Inclusion bodies were washed 3 times in chilled PBS containing 1% Triton X-100 (v/v), then resuspended in PBS containing 8 M urea/10 mM imidazole and filtered through a 0.22-μm low protein binding syringe filter (Acrodisc, Pall Corporation, Port Washington, NY, USA). Protein purification was performed by standard denaturing IMAC (Bio-Rad, Hercules, CA, USA), using a 5-mL HisTRAP fast flow column (Sigma Aldrich, St. Louis, MO, USA) connected to a Bio-Rad Profinia chromatography workstation (Bio-Rad, Hercules, CA, USA). The denatured IMAC elution fraction was refolded by dialysis against 500 mL of PBS + 4 M urea (6 h, 4 °C), PBS + 2 M urea (overnight at 4 °C), and then PBS + 1 M urea (6 h, 4 °C). The final purified WSP33-201 protein concentration was 2.54 mg/mL.
The tetanus toxoid protein (Pfizer Animal Health, Geelong, Australia) used as a control antigen was a kind gift from Dr. Brendon Chua (University of Melbourne).
4.4. Anti-WSP Antibody
Polyclonal anti-WSP was generated and purified by the Walter and Eliza Hall Antibody Facility (WEHI; Melbourne, Australia). Briefly, 2 rabbits (R1881 and R1882) were immunized with 200 μg wMel WSP33-201 + complete Freund’s adjuvant, followed by 5 × monthly immunisations with 200 μg wMel WSP33-201 + incomplete Freund’s adjuvant. Serum was collected and tested by ELISA pre-immunization, and after the 3rd, 4th, 5th, and 6th immunisations. Polyclonal antibodies were affinity purified using Protein A sepharose. Anti-WSP polyclonal antibody from the final bleed of R1882 was shown to have a strong reactivity to wMel WSP33-201 and was therefore selected for use as a positive serological signal control in our experimental ELISAs. All animal experiments were performed in accordance with ethics criteria as set out by the WEHI Animal Ethics Committee.
4.5. Wolbachia Detection by Western Blot and qPCR
Aag2 cells with wMel or wMelPop, or without Wolbachia [60,61], were lysed directly in reducing Laemmeli buffer (Bio-Rad, Hercules, CA, USA) and run on a 12% SDS-PAGE. Proteins were transferred to PVDF membrane, blocked with 5% skim milk in PBS, and then probed with rabbit polyclonal anti-WSP (R1882, 1:1000) and mouse anti-alpha tubulin (Sigma Aldrich, St. Louis, MO, USA; 1:5000). HRP-conjugated anti-rabbit and anti-mouse secondary antibodies (Promega, Madison, WI, USA; 1:2000) were applied, and the blots imaged by enhanced chemiluminescence.
Concurrently, triplicate cell samples were taken from the same flask and analysed by qPCR to quantify the Wolbachia density (number of Wolbachia per Aag2 cell) using the single copy gene WSP. This was done as previously described [62], except qPCR was performed with 3 μL of supernatant, using LightCycler 480 Probes Master kit (Roche, Basel, Switzerland). Probe and primer sequences: WSP detection—WSP F (5′-CATTGGTGTTGGTGTTGGTG) WSP R (5′-ACACCAGCTTTTACTTGACCAG), and WSP LC640 probe (5′-TCCTTTGGAACCCGCTGTGAATGA-IowaBlackRQ). Housekeeping gene, RPS17—RPS17 F (5′-TCCGTGGTATCTCCATCAAGCT), RPS17 R (5′-CACTTCCGGCACGTAGTTGTC), and RPS17 FAM probe (5′-CAGGAGGAGGAACGTGAGCGCAG-BHQ1) [63]. Relative quantification of Wolbachia number per cell was determined using the delta CT method (2CT(reference)/2CT(target)).
4.6. Wolbachia Detection by Immunofluoresence
Aag2 cells with wMel or wMelPop, or without Wolbachia, were seeded on poly-L-Lysine coated coverslips (ProSciTech, Queensland, Australia) in a 12-well plate. Cells were left to settle for 2 days. Media was removed, and the cells were washed 1 × with PBS, then fixed in 4% PFA/PBS for 10 min. Cells were washed 3 × with PBS, then permeabilized with 0.1% triton-x 100 for 5 min. Cells were washed 3 × with PBS, then blocked with 1% BSA/PBS for 1 h. Coverslips were incubated with rabbit anti-WSP (1:1000) in 1% BSA/PBS for 1 h in a humidified chamber. Cells were washed 3 × with PBS, then incubated with Alexafluor anti-rabbit 594 (1:1000) in 1% BSA/PBS for 1 h in a humidified chamber. Cells were washed 3 × with PBS, then 1 × with milliQ water. Coverslips were mounted in a drop of ProLong Gold antifade mountant with DAPI. Images were captured on a fluorescence Zeiss Imager A1 microscope (ZEISS, Göttingen, Germany) using a 40 × objective and prepared using Fiji software (version 1.52i, Fiji, Madison, WI, USA).
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
All plasma samples were tested for immunoreactivity towards WSP as well as a control antigen, tetanus toxoid protein. Vietnam has a strong vaccination program that includes vaccinating against tetanus from 1 year [21]. We reasoned that all study participants had been previously immunized with this antigen at some time, and that the detection of antibodies against tetanus toxoid would demonstrate the integrity of each plasma sample.
Flat-bottom 96-well Nunc Maxisorb plates (Thermo Fisher Scientific, Waltham, MA, USA) were coated with either recombinant WSP or tetanus toxoid protein (5 μg/mL) in PBS overnight at 4 °C. Plates were washed three times with PBS containing 0.05% Tween-20 (PBST), then blocked with 10% BSA in PBS for 1 h at room temperature. Blocking solution was removed, and plates were washed twice with PBST and once with PBS. Plasmas were diluted 1:100 in 5% BSA in PBST, then serially diluted 7 times at 0.5log10 dilutions (in 5% BSA in PBST).
Each WSP-coated plate included paired plasmas from 5 individual participants (taken pre- and post-release of Wolbachia-Ae. aegypti), a negative control plasma pool (a pool of 5 × Australian Red Cross donors from southern regions of Australia where Ae. aegypti are not found), and a positive serological signal control (negative control plasma pool spiked with polyclonal anti-WSP, 1:1000). Tetanus-toxoid-coated plates were similarly assayed with the same paired plasma samples, a negative control (PBS), and a positive signal control (the same pool of 5 × Australian Red Cross donors from southern regions of Australia used above).
In addition to the 105 TNI participants, plasma samples from 29 Red Cross blood donors located in the Australian states of Victoria or South Australia were included as individual negative controls for Wolbachia immunoreactivity. These were also assayed in parallel for reactivity to tetanus toxoid.
All plasmas were incubated at room temperature for 2 h and then washed (×6) with PBST. Bound antibodies were detected using HRP-conjugated goat anti-human IgA/G/M (Thermo Fisher Scientific, Waltham, MA, USA) or goat anti-rabbit IgG (for the positive anti-WSP control wells; Promega, Madison, WI, USA). Secondary antibodies were diluted 1:1000 in 5% BSA in PBST and incubated for 1 h at room temperature. Plates were washed (×6) with PBST, then incubated with 3,3′,5,5′-Tetramethylbenzidine (TMB) for ~2 min. The reaction was stopped by addition of 0.1 M HCl. The absorbance of each well was measured at 450 nm (signal) and 620 nm (background) using a BioTek Gen5 microplate reader (Agilent, Santa Clara, CA, USA).
The background-subtracted absorbance values were normalised to the maximum signal of the positive control on that plate (i.e., the pool of 5 × Australian Red Cross plasma samples spiked with rabbit anti-WSP polyclonal antibody for the WSP-coated plates, and unspiked pooled Australian Red Cross plasmas for the tetanus toxoid-coated plates). These normalised values were then graphed against the reciprocal of the dilution for each plasma sample. The antibody titre for each sample was expressed as the reciprocal of the dilution of plasma required to achieve an absorbance value of 0.4 (determined by nonlinear regression analysis performed using GraphPad Prism® software PRISM 7 version, GraphPad Software, Inc., San Diego, CA, USA) [64]. All plasma samples achieved a normalised absorbance value of >0.4 when assayed against tetanus toxoid, and therefore all samples were included in the analyses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11050535/s1, Figure S1: Expression of recombinant WSP, and anti-WSP reactivity to wMel and wMelPop-CLA WSP. Figure S2: Anti-WSP plasma titres from individuals pre- and post-release of wMelPop-CLA-Ae. aegypti.
Author Contributions
Conceptualization, S.L.O. and C.P.S.; resources, I.I.-O., H.A.F., T.H.N., T.Y.N., S.N.V., N.D.T., L.T.N., Q.M.V., T.D.N. and D.A.D.; data curation, E.L., R.K.L. and J.E.F.; writing—original draft preparation, E.L. and J.E.F.; writing—review and editing, I.I.-O., H.A.F. and C.P.S.; supervision—C.P.S., S.L.O., D.A.D. and J.E.F.; funding acquisition—S.L.O. and C.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bill and Melinda Gates Foundation [OPP1180815], the Vector-Based Control of Transmission: Discovery Research (VCTR) program of the Grand Challenges in Global Health initiative [OPP1153619] managed by the Foundation for the National Institutes of Health, Welcome Trust [102591], and the Macquarie Group Foundation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the National Institute of Hygiene and Epidemiology (Approval reference number: 32/HDD 15/12/2011) and the IRB of Vietnam Ministry of Health (Approval reference number: 615/CN-BYT 19/7/2012).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Where participants were aged <18 years, consent was obtained from their parent/guardian.
Data Availability Statement
The raw data generated in this study are available on request from the corresponding author.
Acknowledgments
We thank Brendon Chua (University of Melbourne, Australia) for gifting the tetanus toxoid protein. We acknowledge the Walter and Eliza Hall Institute Antibody Facility (Parkville, Melbourne, Australia) for the development of the rabbit polyclonal antibodies. Production and purification of Wolbachia Surface Protein was enabled by the National Biologics Facilities at the Commonwealth Scientific and Industrial Research Organisation (CSIRO; Parkville, Melbourne, Australia), which is supported by the National Collaborative Research Infrastructure Strategy and Therapeutic Innovation Australia.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2016, 166, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Yoon, I.-K. A review of Dengvaxia®: Development to deployment. Hum. Vaccines Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Walker, E.C.; Yepes, A.U.; Velez, I.D.; Christensen, B.M.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef]
- Carrington, L.B.; Tran, B.C.N.; Le, N.T.H.; Luong, T.T.H.; Nguyen, T.T.; Nguyen, P.T.; Nguyen, C.V.V.; Nguyen, H.T.C.; Vu, T.T.; Vo, L.T.; et al. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA 2017, 115, 361–366. [Google Scholar] [CrossRef]
- Chouin-Carneiro, T.; Ant, T.H.; Herd, C.; Louis, F.; Failloux, A.B.; Sinkins, S.P. Wolbachia strain w AlbA blocks Zika virus transmission in Aedes aegypti. Med. Veter.-Èntomol. 2019, 34, 116–119. [Google Scholar] [CrossRef]
- Flores, H.A.; De Bruyne, J.T.; O’Donnell, T.B.; Nhu, V.T.; Giang, N.T.; Trang, H.T.X.; Van, H.T.T.; Long, V.T.; Dui, L.T.; Huy, H.L.A.; et al. Multiple Wolbachia strains provide comparative levels of protection against dengue virus infection in Aedes aegypti. PLoS Pathog. 2020, 16, e1008433. [Google Scholar] [CrossRef]
- Fraser, J.E.; O’Donnell, T.B.; Duyvestyn, J.M.; O’Neill, S.L.; Simmons, C.P.; Flores, H.A. Novel phenotype of Wolbachia strain wPip in Aedes aegypti challenges assumptions on mechanisms of Wolbachia-mediated dengue virus inhibition. PLoS Pathog. 2020, 16, e1008410. [Google Scholar] [CrossRef]
- Indriani, C.; Tantowijoyo, W.; Rancès, E.; Andari, B.; Prabowo, E.; Yusdi, D.; Ansari, M.R.; Wardana, D.S.; Supriyati, E.; Nurhayati, I.; et al. Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: A quasi-experimental trial using controlled interrupted time series analysis. Gates Open Res. 2020, 4, 50. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Ryan, P.A.; Turley, A.P.; Wilson, G.; Retzki, K.; Iturbe-Ormaetxe, I.; Dong, Y.; Kenny, N.; Paton, C.J.; Ritchie, S.A.; et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res 2019, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.B.; Riback, T.I.S.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.S.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A.; et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: A quasi-experimental study. PLoS Negl. Trop. Dis. 2021, 15, e0009556. [Google Scholar] [CrossRef]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2020, 3, 1547. [Google Scholar] [CrossRef] [PubMed]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Saig, E.; Turley, A.P.; Ribeiro, J.M.C.; O’Neill, S.L.; McGraw, E.A. Human Probing Behavior of Aedes aegypti when Infected with a Life-Shortening Strain of Wolbachia. PLoS Negl. Trop. Dis. 2009, 3, e568. [Google Scholar] [CrossRef] [PubMed]
- Popovici, J.; A Moreira, L.; Poinsignon, A.; Iturbe-Ormaetxe, I.; McNaughton, D.; O’Neill, S.L. Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem. Inst. Oswaldo Cruz 2010, 105, 957–964. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Lane, A.M.; Fong, A.W.C.; Voronin, D.A.; Iturbe-Ormaetxe, I.; Yamada, R.; McGraw, E.A.; O’Neill, S.L. Host Adaptation of a Wolbachia Strain after Long-Term Serial Passage in Mosquito Cell Lines. Appl. Environ. Microbiol. 2008, 74, 6963–6969. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Le Nguyen, H.; Nguyen, T.Y.; Vu, S.N.; Tran, N.D.; Le, T.N.; Vien, Q.M.; Bui, T.C.; Le, H.T.; Kutcher, S.; et al. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors 2015, 8, 563. [Google Scholar] [CrossRef]
- Woolfit, M.; Iturbe-Ormaetxe, I.; Brownlie, J.C.; Walker, T.; Riegler, M.; Seleznev, A.; Popovici, J.; Rancès, E.; Wee, B.A.; Pavlides, J.W.; et al. Genomic Evolution of the Pathogenic Wolbachia Strain, wMelPop. Genome Biol. Evol. 2013, 5, 2189–2204. [Google Scholar] [CrossRef]
- Jit, M.; Huyen, D.T.T.; Friberg, I.; Van Minh, H.; Kiet, P.H.T.; Walker, N.; Van Cuong, N.; Duong, T.N.; Toda, K.; Hutubessy, R.; et al. Thirty years of vaccination in Vietnam: Impact and cost-effectiveness of the national Expanded Programme on Immunization. Vaccine 2015, 33, A233–A239. [Google Scholar] [CrossRef]
- Available online: https://www.health.gov.au/health-topics/immunisation/childhood-immunisation-coverage/current-coverage-data-tables-for-all-children (accessed on 30 March 2022).
- Hertig, M. The Rickettsia, Wolbachia pipientis (gen. et sp.n.) and Associated Inclusions of the Mosquito, Culex pipiens. Parasitology 1936, 28, 453–486. [Google Scholar] [CrossRef]
- Hoffmann, A.; Hercus, M.; Dagher, H. Population Dynamics of the Wolbachia Infection Causing Cytoplasmic Incompatibility in Drosophila melanogaster. Genetics 1998, 148, 221–231. [Google Scholar] [CrossRef]
- Gavotte, L.; Mercer, D.R.; Stoeckle, J.J.; Dobson, S.L. Costs and benefits of Wolbachia infection in immature Aedes albopictus depend upon sex and competition level. J. Invertebr. Pathol. 2010, 105, 341–346. [Google Scholar] [CrossRef]
- Wright, J.D.; Barr, A.R. The ultrastructure and symbiotic relationships of Wolbachia of mosquitoes of the Aedes scutellaris group. J. Ultrastruct. Res. 1980, 72, 52–64. [Google Scholar] [CrossRef]
- Kambhampati, S.; Rai, K.S.; Burgun, S.J. Unidirectional cytoplasmic incompatibility in the mosquito, Aedes albopictus. Evolution 1993, 47, 673. [Google Scholar] [CrossRef]
- Punkosdy, G.A.; Dennis, V.A.; Lasater, B.L.; Tzertzinis, G.; Foster, J.M.; Lammie, P.J. Detection of Serum IgG Antibodies Specific for Wolbachia Surface Protein in Rhesus Monkeys Infected with Brugia malayi. J. Infect. Dis. 2001, 184, 385–389. [Google Scholar] [CrossRef]
- Suba, N.; Shiny, C.; Taylor, M.; Narayanan, R. Brugia malayi Wolbachia hsp60 IgG antibody and isotype reactivity in different clinical groups infected or exposed to human bancroftian lymphatic filariasis. Exp. Parasitol. 2007, 116, 291–295. [Google Scholar] [CrossRef]
- Jha, R.; Gangwar, M.; Chahar, D.; Balakrishnan, A.S.; Negi, M.P.S.; Misra-Bhattacharya, S. Humans from Wuchereria bancrofti endemic area elicit substantial immune response to proteins of the filarial parasite Brugia malayi and its endosymbiont Wolbachia. Parasites Vectors 2017, 10, 40. [Google Scholar] [CrossRef]
- Brattig, N.W.; Büttner, D.W.; Hoerauf, A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 2001, 3, 439–446. [Google Scholar] [CrossRef]
- Punkosdy, G.A.; Addiss, D.G.; Lammie, P.J. Characterization of Antibody Responses to Wolbachia Surface Protein in Humans with Lymphatic Filariasis. Infect. Immun. 2003, 71, 5104–5114. [Google Scholar] [CrossRef]
- Edenborough, K.M.; Flores, H.A.; Simmons, C.P.; Fraser, J.E. Using Wolbachia to Eliminate Dengue: Will the Virus Fight Back? J. Virol. 2021, 95, e0220320. [Google Scholar] [CrossRef]
- Murray, J.V.; Jansen, C.C.; De Barro, P. Risk Associated with the Release of Wolbachia-Infected Aedes aegypti Mosquitoes into the Environment in an Effort to Control Dengue. Front. Public Health 2016, 4, 43. [Google Scholar] [CrossRef]
- Orlandi-Pradines, E.; Almeras, L.; de Senneville, L.D.; Barbe, S.; Remoué, F.; Villard, C.; Cornelie, S.; Penhoat, K.; Pascual, A.; Bourgouin, C.; et al. Antibody response against saliva antigens of Anopheles gambiae and Aedes aegypti in travellers in tropical Africa. Microbes Infect. 2007, 9, 1454–1462. [Google Scholar] [CrossRef]
- Ndille, E.E.; Dubot-Pérès, A.; Doucoure, S.; Mouchet, F.; Cornelie, S.; Sidavong, B.; Fournet, F.; Remoue, F. Human IgG antibody response to Aedes aegypti Nterm-34 kDa salivary peptide as an indicator to identify areas at high risk for dengue transmission: A retrospective study in urban settings of Vientiane city, Lao PDR. Trop. Med. Int. Health 2014, 19, 576–580. [Google Scholar] [CrossRef]
- Doucoure, S.; Misse, D.; Roca, Y.; Flores, J.V.; Cournil, A.; Le Goff, G.; Giraldez, M.G.; Cornelie, S.; Herve, J.P.; Mouchet, F.; et al. Human Antibody Response to Aedes aegypti Saliva in an Urban Population in Bolivia: A New Biomarker of Exposure to Dengue Vector Bites. Am. J. Trop. Med. Hyg. 2012, 87, 504–510. [Google Scholar] [CrossRef]
- Styer, L.M.; Kent, K.A.; Albright, R.G.; Bennett, C.J.; Kramer, L.D.; Bernard, K.A. Mosquitoes Inoculate High Doses of West Nile Virus as They Probe and Feed on Live Hosts. PLoS Pathog. 2007, 3, e132. [Google Scholar] [CrossRef]
- Janzen, H.G.; Wright, K.A. The salivary glands of Aedes aegypti (L.): An electron microscope study. Can. J. Zoöl 1971, 49, 1343–1345. [Google Scholar] [CrossRef]
- Li, S.-J.; Ahmed, M.Z.; Lv, N.; Shi, P.-Q.; Wang, X.-M.; Huang, J.-L.; Qiu, B.-L. Plantmediated horizontal transmission of Wolbachia between whiteflies. ISME J. 2016, 11, 1019–1028. [Google Scholar] [CrossRef]
- Min, K.-T.; Benzer, S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 1997, 94, 10792–10796. [Google Scholar] [CrossRef]
- Fattouh, N.; Cazevieille, C.; Landmann, F. Wolbachia endosymbionts subvert the endoplasmic reticulum to acquire host membranes without triggering ER stress. PLoS Negl. Trop. Dis. 2019, 13, e0007218. [Google Scholar] [CrossRef]
- Raquin, V.; Moro, C.V.; Saucereau, Y.; Tran, F.-H.; Potier, P.; Mavingui, P. Native Wolbachia from Aedes albopictus Blocks Chikungunya Virus Infection In Cellulo. PLoS ONE 2015, 10, e0125066. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Mancini, M.-V.; Ant, T.H.; Martinez, J.; Kamarul, G.M.R.; Nazni, W.A.; Hoffmann, A.A.; Sinkins, S.P. Wolbachia strain w AlbB maintains high density and dengue inhibition following introduction into a field population of Aedes aegypti. Philos. Trans. R. Soc. B Biol. Sci. 2020, 376, 20190809. [Google Scholar] [CrossRef]
- Tantowijoyo, W.; Andari, B.; Arguni, E.; Budiwati, N.; Nurhayati, I.; Fitriana, I.; Ernesia, I.; Daniwijaya, E.W.; Supriyati, E.; Yusdiana, D.H.; et al. Stable establishment of wMel Wolbachia in Aedes aegypti populations in Yogyakarta, Indonesia. PLoS Negl. Trop. Dis. 2020, 14, e0008157. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Joubert, D.A.; Walker, T.; Carrington, L.B.; De Bruyne, J.T.; Kien, D.H.T.; Hoang, N.L.T.; Chau, N.V.V.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016, 12, e1005434. [Google Scholar] [CrossRef]
- Pichon, S.; Bouchon, D.; Cordaux, R.; Chen, L.; Garrett, R.A.; Grève, P. Conservation of the Type IV Secretion System throughout Wolbachia evolution. Biochem. Biophys. Res. Commun. 2009, 385, 557–562. [Google Scholar] [CrossRef]
- Rancès, E.; Voronin, D.; Tran-Van, V.; Mavingui, P. Genetic and Functional Characterization of the Type IV Secretion System in Wolbachia. J. Bacteriol. 2008, 190, 5020–5030. [Google Scholar] [CrossRef]
- Nag, J.K.; Shrivastava, N.; Gupta, J.; Misra-Bhattacharya, S. Recombinant translation initiation factor-1 of Wolbachia is an immunogenic excretory secretory protein that elicits Th2 mediated immune protection against Brugia malayi. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 25–38. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Turner, J.; Pionnier, N.; Midgley, A.; Guimaraes, A.F.; Johnston, K.L.; Edwards, S.W.; Taylor, M.J. Wolbachia endosymbionts induce neutrophil extracellular trap formation in human onchocerciasis. Sci. Rep. 2016, 6, 35559. [Google Scholar] [CrossRef]
- Keiser, P.B.; Reynolds, S.M.; Awadzi, K.; Ottesen, E.A.; Taylor, M.J.; Nutman, T.B. Bacterial Endosymbionts ofOnchocerca volvulusin the Pathogenesis of Posttreatment Reactions. J. Infect. Dis. 2002, 185, 805–811. [Google Scholar] [CrossRef][Green Version]
- O Duke, B. The population dynamics of Onchocerca volvulus in the human host. Trop. Med. Parasitol. Off. Organ Dtsch. Trop. Ges. Dtsch. Ges. fur Tech. Zs. (GTZ) 1993, 44, 61–68. [Google Scholar]
- Basáñez, M.G.; Boussinesq, M.; Prod’Hon, J.; Frontado, H.; Villamizar, N.J.; Medley, G.F.; Anderson, R.M. Density-dependent processes in the transmission of human onchocerciasis: Intensity of microfilariae in the skin and their uptake by the simuliid host. Parasitology 1994, 108, 115–127. [Google Scholar] [CrossRef]
- Fenn, K.; Blaxter, M. Quantification of Wolbachia bacteria in Brugia malayi through the nematode lifecycle. Mol. Biochem. Parasitol. 2004, 137, 361–364. [Google Scholar] [CrossRef]
- Taylor, M.J.; Voronin, D.; Johnston, K.L.; Ford, L. Wolbachia filarial interactions. Cell. Microbiol. 2012, 15, 520–526. [Google Scholar] [CrossRef]
- Bandi, C.; Trees, A.; Brattig, N.W. Wolbachia in filarial nematodes: Evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases. Veter.-Parasitol. 2001, 98, 215–238. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Genet. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Chen, X.-P.; Dong, Y.-J.; Guo, W.-P.; Wang, W.; Li, M.-H.; Xu, J.; Dumler, J.; Zhang, Y.-Z. Detection of Wolbachia genes in a patient with non-Hodgkin’s lymphoma. Clin. Microbiol. Infect. 2015, 21, 182.e1–182.e4. [Google Scholar] [CrossRef][Green Version]
- McLean, B.J.; Dainty, K.R.; Flores, H.A.; O’Neill, S.L. Differential suppression of persistent insect specific viruses in trans-infected wMel and wMelPop-CLA Aedes-derived mosquito lines. Virology 2018, 527, 141–145. [Google Scholar] [CrossRef]
- Thomas, S.; Verma, J.; Woolfit, M.; O’Neill, S.L. Wolbachia-mediated virus blocking in mosquito cells is dependent on XRN1-mediated viral RNA degradation and influenced by viral replication rate. PLoS Pathog. 2018, 14, e1006879. [Google Scholar] [CrossRef]
- Manokaran, G.; Flores, H.A.; Dickson, C.T.; Narayana, V.K.; Kanojia, K.; Dayalan, S.; Tull, D.; McConville, M.J.; MacKenzie, J.M.; Simmons, C.P. Modulation of acyl-carnitines, the broad mechanism behind Wolbachia-mediated inhibition of medically important flaviviruses in Aedes aegypti. Proc. Natl. Acad. Sci. USA 2020, 117, 24475–24483. [Google Scholar] [CrossRef]
- Frentiu, F.D.; Zakir, T.; Walker, T.; Popovici, J.; Pyke, A.T.; Hurk, A.V.D.; McGraw, E.A.; O’Neill, S.L. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLoS Negl. Trop. Dis. 2014, 8, e2688. [Google Scholar] [CrossRef]
- Chua, B.Y.; Al Kobaisi, M.; Zeng, W.; Mainwaring, D.; Jackson, D.C. Chitosan Microparticles and Nanoparticles as Biocompatible Delivery Vehicles for Peptide and Protein-Based Immunocontraceptive Vaccines. Mol. Pharm. 2011, 9, 81–90. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).