Abstract
Diseases caused by tick-transmitted pathogens including bacteria, viruses, and protozoa are of veterinary and medical importance, especially in tropical and subtropical regions including Turkey. Hence, molecular surveillance of tick-borne diseases will improve the understanding of their distribution towards effective control. This study aimed to investigate the presence and perform molecular characterization of Babesia sp., Theileria sp., Anaplasma sp., Ehrlichia sp., and Rickettsia sp. in tick species collected from cattle in five provinces of Turkey. A total of 277 adult ticks (males and females) were collected. After microscopic identification, tick pools were generated according to tick species, host animal, and sampling sites prior to DNA extraction. Molecular identification of the tick species was conducted through PCR assays. Out of 90 DNA pools, 57.8% (52/90) were detected to harbor at least 1 pathogen. The most frequently-detected pathogens were Babesia bovis, with a minimum detection rate of 7.9%, followed by Ehrlichia sp. (7.2%), Theileria annulata (5.8%), Coxiella sp. (3.3%), Anaplasma marginale (2.5%), Rickettsia sp. (2.5%), and B. occultans (0.7%). Rickettsia sp. identified in this study include Candidatus Rickettsia barbariae, R. aeschlimannii, and Rickettsia sp. Chad. All sequences obtained from this study showed 99.05–100% nucleotide identity with those deposited in GenBank (query cover range: 89–100%). This is the first molecular detection of Rickettsia sp. Chad, a variant of Astrakhan fever rickettsia, in Turkey. Results from this survey provide a reference for the distribution of ticks and tick-borne pathogens in cattle and expand the knowledge of tick-borne diseases in Turkey.
1. Introduction
Ticks and tick-borne diseases (TBDs) significantly affect livestock production in many countries of the world. They are considered second only to mosquitoes in importance as vectors of disease agents causing great impact on animal and human health [1]. The global distribution of ticks and TBDs have led to considerable economic losses, such as loss of productivity, decreased meat and milk production, and death of livestock [2,3,4,5]. To date, 731 species of Ixodidae (hard ticks), 216 species of Argasidae (soft ticks), and 1 species of Nuttalliellidae (Nuttalliella namaqua) have been described worldwide [6]. Due to suitable climatic conditions and the large amounts of wild and domestic animals, a total number of 51 tick species have been identified in Turkey (43 from the family Ixodidae and 8 from the family Argasidae). Dermacentor, Haemaphysalis, Hyalomma, Ixodes, and Rhipicephalus ticks are widely present throughout Turkey [7,8,9,10].
In Turkey, 27% of farm animals are cattle, with approximately 90% of milk and meat produced from bovines [11]. However, TBDs have a significant impact on the economy and animal health. The epidemiology of TBDs in Turkey had been reported previously [12,13,14], but there is still limited data about pathogens carried or transmitted by ticks. Therefore, the aims of this study were to investigate the presence of and genetically characterize the pathogens harbored by ticks collected from cattle to improve understanding of their distribution towards effective control in Turkey.
2. Results
2.1. Tick Species Identification
In total, 277 ticks were collected from 22 severely infested cattle in provinces of Diyarbakır (n = 2), Gaziantep (n = 4), Kahramanmaraş (n = 4), Karaman (n = 9), and Şanlıurfa (n = 3) (Figure 1). Morphological observation-based identification revealed that the 277 ticks belong to two tick genera, namely Hyalomma and Rhipicephalus. The five species were identified as Hyalomma marginatum (n = 2), H. excavatum (n = 203), H. anatolicum (n = 5), Rhipicephalus turanicus (n = 60), and Rh. bursa (n = 7) (Figure 1). The length of the obtained tick mitochondrial 16S rDNA sequences varied from 448 to 453 bp and the BLASTn analysis of the H. anatolicum, H. excavatum, H. marginatum, Rh. turanicus, and Rh. bursa sequences in this study showed percent identity ranging from 99.33 to 100% (query cover range: 89–100%) with deposited sequences from GenBank (Table S1).
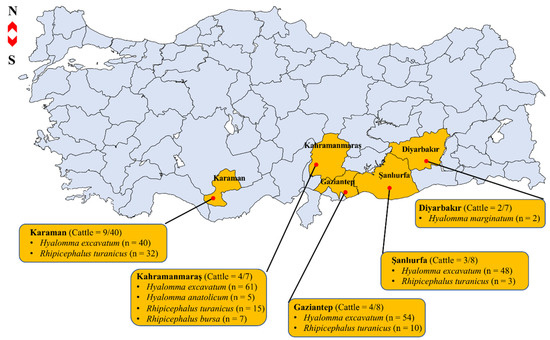
Figure 1.
Map of sample collection sites in Turkey showing numbers of cattle examined (no. infested/total) and numbers of ticks of each species collected.
2.2. Microorganisms Detected in Ticks and Minimum Detection Rates
A total of 57.8% of tick pools (52/90) harbored at least one pathogen (Table 1). Seven pathogens, namely Anaplasma marginale (7), Babesia bovis (22), B. occultans (2), Coxiella sp. (9), Ehrlichia sp. (20), Rickettsia sp. (7), and Theileria annulata (16) were detected in the tick pools (Table 1). The most frequently-detected pathogens in this study (Table 1) were B. bovis (minimum detection rate 7.9%, 22 positive pools), followed by Ehrlichia sp. (minimum detection rate 7.2%,20 positive pools), T. annulata (minimum detection rate 5.8%, 16 positive pools), Coxiella sp. (minimum detection rate 3.3%, 9 positive pools), A. marginale and Rickettsia spp. (minimum detection rate 2.5%, 7 positive pools each), and B. occultans (minimum detection rate 0.7%, 2 positive pools).

Table 1.
Detection of microorganisms in tick pools based on provinces and total minimum detection rates.
For Rickettsia sp., 7 pools were positive when screened using the 16S rRNA assay, whereas 5 of 7 were positive when screened for gltA and ompA genes. Three samples positive for all genes of Rickettsia and two samples positive for 16S rRNA only were selected for sequencing. In the case of Anaplasma/Ehrlichia, all samples that showed an amplicon using the 16S rRNA assay were positive using A. marginale msp4 and/or E. ruminantium pCS20 assays, thus, amplicons from the latter assays only were sequenced.
2.3. Analyses of Tick-Borne Pathogen DNA Sequences
Positive samples of each pathogen were randomly selected as representative for sequencing and analysis. The NCBI BLASTn analysis of A. marginale (OL408894; H. excavatum), B. bovis (OL408893; H. excavatum), and B. occultans (OL377855; H. excavatum) sequences obtained from this study showed 100% identity with the reference sequences MF377455 (Turkey cattle), MN870661 (Egypt cattle), and KP745626 (Turkey cattle), respectively. The Coxiella sp. sequence (OL413002; Rh. turanicus) identified in this study showed 99.66% identity with Candidatus Coxiella mudrowiae (CP011126), which was detected in a Rh. turanicus tick in Israel. BLASTn analysis hits of Rickettsia sp. (16S rRNA, gltA, and ompA) included three main species including R. aeschlimannii (OL377895, OM541406, OM717963 and OM717960), Candidatus Rickettsia barbariae (OL377896, OM541407, OM717964, and OM717961), and Rickettsia sp. Chad (OM541405, OM717962, and OM717959). OL377895, and OM541406 were detected in different H. excavatum pools which were collected from different cattle in Kahramanmaraş. Meanwhile, OL377896 was detected in H. excavatum in Kahramanmaraş, while OM541407 was detected in Rh. turanicus in Karaman. The BLASTn analysis showed 99.29–100% (query cover range: 95–100%) with reference strains (Table 2). Furthermore, T. annulata (OL408892) shared the highest percent identity (99.07%) with a dog isolate from Tunisia (KX130956) (Table 2).

Table 2.
Pathogens identified in tick pools and GenBank accession numbers in this study.
2.4. Phylogenetic Analyses of Rickettsial Agents
The A. marginale msp4 (OL408894) sequence from this study was found in the same clade with cattle isolates from Turkey (MF377455), Italy (KF739428), Algeria (KX179906), and Tunisia (KJ512170) (Figure 2). Meanwhile, the Ehrlichia sp. pCS20 sequence obtained in this study clustered with KT362172, an isolate from Rh. (Boophilus) microplus in China (Figure 3). Phylogenetic trees inferred from 16S rRNA, gltA, and ompA genes of Rickettsia sp. are shown in Figure 4. The Rickettsia 16S rRNA sequences were grouped in two clades (Figure 4A). Two sequences (OM541406 and OL377895) created a polyphyletic group with other R. aeschlimannii and R. massiliae sequences, while the remaining sequences (OM541405, OM541407, and OL377896) were grouped into R. conorii complex clade. Interestingly, OM541405 formed a well-supported branch with Rickettsia sp. isolated from a human with clinical Astrakhan fever in Chad. Phylogenetic analysis of gltA (Figure 4B) and ompA (Figure 4C) sequences further confirmed that OM717962 and OM717959 were most closely related to Rickettsia sp. Chad. Meanwhile, OM717963 and OM717960 were in the same group of R. aeschlimannii, whereas OM717964 and OM717961 were in the same group of Candidatus Rickettsia barbariae, respectively (Figure 4).
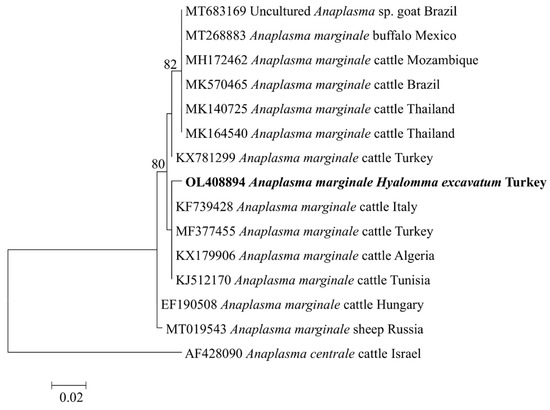
Figure 2.
Phylogenetic analysis of Anaplasma marginale based on msp4. The sequences determined in this study are shown in bold font. Numbers at the nodes represent the percentage of occurrence of clades in 1000 bootstrap replicates of the taxa.
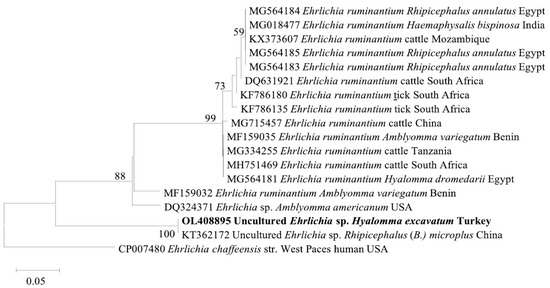
Figure 3.
Phylogenetic analysis of Ehrlichia sp. based on pCS20. The sequences in bold font are from this study. The numbers at the nodes represent the percentage of occurrence of clades in 1000 bootstrap replicates of the taxa.
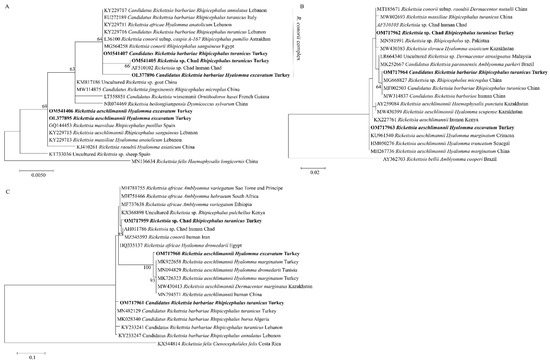
Figure 4.
Phylogenetic analysis of Rickettsia sp. based on 16S rRNA (A), gltA (B) and ompA (C) detected in ticks collected from Turkey. The sequences determined in this study are shown in bold font. The numbers at the nodes represent the percentage of occurrence of clades in 1000 bootstrap replicates of the taxa.
3. Discussion
Tick-borne protozoan and rickettsial diseases are major health problems for cattle and cause economic losses worldwide [4,14,15]. In recent years, studies related to ticks and TBDs have increased in Turkey. Understanding the complex relationships between ticks and pathogens is important to public health in mitigating tick-borne pathogen (TBP) transmission. In this study, we performed a molecular survey of tick-transmitted pathogens in ticks obtained from cattle in Diyarbakır, Gaziantep, Kahramanmaraş, Karaman, and Şanlıurfa provinces located in the south of Turkey. Overall, seven tick-associated microorganisms, including A. marginale, B. bovis, B. occultans, Coxiella sp., Ehrlichia sp., Rickettsia sp. (Candidatus R. barbariae, R. aeschlimannii, and Rickettsia sp. Chad), and T. annulata were found in four tick species identified, namely H. excavatum, H. anatolicum, Rh. turanicus, and Rh. bursa.
Six Anaplasma sp., including A. bovis, A. centrale, A. marginale, A. ovis, A. platys, and A. phagocytophilum, cause a range of diseases in humans and other vertebrates [16]. The most common agent for bovine anaplasmosis is A. marginale, which is highly pathogenic and can be fatal in susceptible cattle [17]. Major surface protein 4 (MSP4) is an important protein, which is considered as a stable marker for genetic characterization of A. marginale strains [18,19]. In this study, a molecular survey based on the PCR amplification of the target gene msp4 was performed and A. marginale was detected in H. excavatum and Rh. turanicus ticks with a minimum detection rate of 2.5%. Previously, A. marginale has been reported in many regions of Turkey, which has been confirmed as the most prevalent tick-borne pathogen in cattle [10,15,20,21,22,23,24]. However, A. marginale infection in ticks was rarely reported in Turkey. The phylogenetic analysis of A. marginale revealed that the msp4 sequence obtained from this study was highly similar to isolates from other Mediterranean countries. This result demonstrates the utility of A. marginale msp4 as a marker for phylogeographic characterization of A. marginale isolates from the field [18].
Bovine babesiosis is one of the most important TBDs of cattle in Turkey and has been reported in all Turkish provinces [11]. The most important causative agents of bovine babesiosis are B. bovis and B. bigemina, which are transmitted by Rhipicephalus ticks. The minimum detection rate of B. bovis in ticks was 7.9% and was only detected in ticks from Gaziantep and Kahramanmaraş. Interestingly, we did not identify ticks harboring B. bigemina. In Turkey, B. bigemina is the most frequent Babesia species and is transmitted by Rh. annulatus ticks [10]. Such results may be explained by the limited number of collected tick species and the number of samples in this study. Meanwhile, B. occultans was first described in South Africa and Nigeria and has been identified in Hyalomma ticks [25,26]. Of the 90 pools, 2 (minimum detection rate 0.7%) pools from Şanlıurfa and Karaman were found positive for B. occultans, which was carried by H. excavatum in this study. Previous reports have proved the presence of B. occultans in Turkey [27] and found that it was not only carried by Hyalomma ticks, but was also observed in Rh. turanicus ticks [22].
Coxiella burnetii causes Q fever, a zoonotic disease which threatens human health and commonly causes abortions in cattle and sheep. In the present study, the Coxiella sp. positive tick species were H. excavatum, Rh. turanicus, and Rh. bursa. The presence of Coxiella sp. in ticks has been reported previously in Turkey wherein it was detected in H. marginatum, H. anatolicum excavatum, H. detritum, and Rh. annulatus ticks [28]. In another study, Coxiella sp. DNA was detected in H. excavatum, Rh. turanicus, and Rh. bursa ticks [29]. This suggests that Coxiella sp. is widespread in a variety of Hyalomma and Rhipicephalus species in Turkey. Additional studies are warranted to determine the presence of C. burnetii in the tick samples from this study.
Ehrlichia ruminantium causes heartwater disease in cattle and the main vector is the Amblyomma tick, particularly A. hebraeum and A. variegatum [30]. The partial sequences of E. ruminantium were reported previously in Rh. evertsi evertsi, H. truncatum, and H. marginatum ticks [31]. In this study, we detected Ehrlichia sp. in different tick species, including H. anatolicum, H. excavatum, Rh. turanicus, and Rh. bursa, suggesting that Ehrlichia sp. may be carried by Hyalomma and Rhipicephalus ticks. So far, only a few studies have documented the detection of Ehrlichia in ticks. Although Ehrlichia sp. was detected in H. excavatum and Rh. bursa in studies conducted in Turkey, that Ehrlichia sp. sequence found was similar to E. canis and Ehrlichia sp. Omatjenne strain [21,22]. The present study used an E. ruminantium-specific PCR assay, but the obtained Ehrlichia sp. pCS20 gene sequence has a 99.28% identity with the Ehrlichia sp. (KT362172) sequence, previously reported in Hunan province, China [32]. Further studies are needed to confirm the species of the detected Ehrlichia, to investigate its presence in the blood samples of bovines where the positive ticks were collected from, and assess its significance for animal health.
Three species of Rickettsia were confirmed by phylogenetic analysis based on the 16S rRNA, gltA, and ompA genes in this study, including Candidatus Rickettsia barbariae (OM541407, OM717964, and OM717961 from one sample), R. aeschlimannii (OM541406, OM717963 and OM717960 from one sample), and Rickettsia sp. Chad (OM541405, OM717962, and OM717959 from one sample). Our findings confirmed the presence of Candidatus Rickettsia barbariae in Turkey, which is in concordance with a previous report [33]. This rickettsial agent’s DNA was firstly detected in Rh. turanicus ticks collected from European hare (Lepus europaeus) in 2020 [34]. The present study revealed the presence of Candidatus Rickettsia barbariae in ticks and reported the presence of this agent in Turkey for the second time. Moreover, the Rickettsia sp. Chad isolated from a patient was reported previously in a clinical Astrakhan fever case [35]. There is no information about the vector of Rickettsia sp. Chad. We have detected Rickettsia sp. Chad in Rh. turanicus in this study. Although there are only a few reports of Rickettsia sp. Chad infections in humans, our result suggests that Rickettsia sp. Chad infection may be a potential threat for humans, which manifests as fever and maculopapulous rash after infection [35]. As it was detected in ticks of cattle, further studies regarding its infectivity on cattle should be conducted.
Theileriosis is another tick-borne protozoan disease of cattle. The causative agents are transmitted from an infected animal to others by transstadial transmission via ticks. Theileria annulata transmitted by Hyalomma is the main TBP affecting cattle in Turkey [10]. The current study detected T. annulata infection in H. excavatum, H. anatolicum, and Rh. turanicus with a minimum detection rate of 5.8%. The main vectors of T. annulata are some species in the genus Hyalomma, and there are studies conducted in Turkey reporting that T. annulata was detected in H. excavatum [22,33]. Rhipicephalus turanicus harboring T. annulata was also reported in a previous study [36]. Therefore, it is necessary to evaluate whether Rh. turanicus will be a risk for T. annulata transmission in Turkey.
The detection of Coxiella sp. from ticks in this study despite using specific PCR primers, coupled with the results in BLASTn analysis, indicates that this species is an endosymbiont. Endosymbionts are non-pathogenic microorganisms that have evolved in the carriers or hosts either as obligate or facultative symbionts [37]. Ticks were reported previously to be highly infected with endosymbionts [38,39]. In this study, an isolate highly similar to Candidatus Coxiella mudrowiae, a known Coxiella endosymbiont, was detected in Rh. turanicus ticks, as it was similarly described in a previous study [40]. The extent to which the endosymbionts of ticks in Turkey affect transmission of other TBPs needs to be studied in the future.
In the study, a large number of single or multiple pathogenic microorganisms’ DNA was detected in cattle tick pools. It is difficult to determine whether these microorganisms originated from ticks or from hosts blood since all ticks were collected while sucking blood from cattle. Karim et al. [38] reported that the characterization of multiple infections in ticks poses a major scientific challenge for understanding the epidemiology of tick-borne infectious diseases. However, since the tick DNA samples in this study were not extracted from each individual ticks but from 1–10 ticks collected in pools, this limits the discussion of single and coinfection status. Additionally, our study does not discuss the co-transmission of many microorganisms from ticks to cattle, and further studies are needed to obtain information about potential tick-borne pathogens and the dynamics of tick-borne infections in a region [38,39].
The limitation of this study was that it was carried out on a restricted number of cattle. The collection of ticks at a certain time of the year and the lack of periodic tick collection according to the seasons are considered as other limiting factors of this study. The study is also limited in choosing sampling locations and, thus, could not cover the whole of Turkey to provide a better representation of tick and tick-borne pathogens. Another limiting factor is the inability to obtain blood from infested cattle. Although we were aware of the importance of this, it was outside the scope of the present study.
In conclusion, the results obtained from the present study will contribute to the present knowledge on the distribution of ticks and the possible TBPs they may carry. Further investigation for the vector competence of tick species in transmitting these pathogens and more research with a greater number of ticks, blood samples, and sampling locations should be conducted in order to fully assess the risk factors for tick-transmitted livestock diseases in Turkey.
4. Materials and Methods
4.1. Ethical Statements
The owners of the cattle were informed about the study, and their approvals were obtained for tick collection. Tick sampling, as well as sample processing, were carried out according to the ethical guidelines permitted by Obihiro University of Agriculture and Veterinary Medicine (Permit for DNA experiment: 1723-4 and 1724-4; Pathogen: 201712-5).
4.2. Tick Samples and DNA Extraction
Tick samples were collected from March to June 2013. Seventy apparently healthy cattle were examined. Tick infestations (1–45 ticks per animal) were determined in 22 (31.43%) of these cattle. The study consisted of 277 adult ticks, which were collected in 5 provinces (Diyarbakır, Gaziantep, Karaman, Kahramanmaraş, Şanlıurfa) of Turkey. All ticks were removed from cattle skin with the consent of the livestock owner, and care was taken to minimize animal discomfort. The tick samples were kept in 70% ethanol prior to identification using a binocular microscope (Olympus SZX16, Tokyo, Japan) based on standard taxonomic keys [41,42]. Tick identification was done at the National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Japan. After microscopic identification, the tick samples were pooled according to species, gender, and sampling site. Ninety pools were generated based on sample size (1 to 10 ticks per pool). Tick DNA extraction was done as previously described [43] by using a QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The extracted DNA was eluted with 50 μL of double-distilled water and stored at −30 °C until use.
4.3. Molecular Characterization of Ticks
To further confirm the tick species, molecular identification of the tick species was conducted through PCR assays. A primer set targeting the 16S rDNA of ticks was used, followed by cloning and sequencing of amplicons (Table S2). DNA amplification was done with a 10 μL PCR reaction volume containing 1 μL of 10 × Thermopol® Buffer (New England Biolabs, Ipswich, MA, USA), 0.2 μL of 10 mM dNTP mix (New England Biolabs, Ipswich, MA, USA), 0.2 μL of 10 μM forward and reverse primers, 0.05 μL of Taq polymerase (New England Biolabs, Ipswich, MA, USA), 1 μL of DNA sample, and 7.35 μL of double distilled water. The thermocycling conditions consisted of an initial denaturation (95 °C for 2 min); 30 cycles of denaturation (95 °C for 30 s); annealing (52 °C for 30 s) and extension (68 °C for 1 min), then final extension (68 °C for 5 min) [44]. PCR products were checked by electrophoresis on 1.5% agarose gels, stained with ethidium bromide, and visualized under UV light. All amplicons were extracted from gels using a Gel Extraction Kit (QIAGEN, Hilden, Germany) and sequenced.
4.4. Detection and Characterization of Tick-Borne Pathogens
A total of 90 tick DNA pools were generated in this study. All tick pool samples were primarily screened using primers targeting the genes 18S rRNA (V4 hypervariable region) for Babesia and Theileria [45], 16S rRNA for Anaplasma and Ehrlichia [46], and 16S rRNA for Rickettsia [47], as listed in Table S2. The positive samples based on these TBP assays were selected for species-specific detection. Partial sequences of B. bovis spherical body protein 4 (sbp4) [48], B. bigemina rhoptry-associated protein 1a (RAP1a) [48], T. orientalis major piroplasm surface protein (MPSP) [49], T. annulata merozoite surface antigen 1 (Tams-1) [50], Anaplasma marginale major surface protein 4 (msp4) [51], Ehrlichia pCS20 [52], C. burnetii 16S rRNA [53], and Rickettsia citrate synthase gltA and ompA [54,55] genes were amplified. The primers used are shown in Table S2. The DNA amplification and PCR cycling for all pathogens followed the same conditions as aforementioned, except for the annealing temperatures, wherein those documented in referenced publications were used. PCR products were checked and purified as described above. The samples were further confirmed by sequencing.
4.5. Cloning and Sequencing
The amplicons (tick 16S rDNA or pathogen DNA) were cloned in pGEM-T Easy Vector and sequenced as described [56] using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) and the ABI PRISM 3100 genetic analyzer (Applied Biosystems). The GenBank accession numbers are shown in Table 2 and Table S1.
4.6. Sequence Alignment and Phylogenetic Analyses
The phylogenetic analyses of the sequences obtained from the PCRs specific for A. marginale, Ehrlichia sp., and Rickettsia sp. were performed subsequently, as rickettsial pathogens are relatively rarely studied in Turkey. Sequenced DNA samples were analyzed using the BLASTn tool of NCBI GenBank database and Clustal X program. In addition, phylogenetic analyses were inferred using MEGA version 7.0 software. The maximum likelihood method was employed as the method for tree construction because of its use of complex models to simulate biological reality and infer sequence evolution [57]. The sequences included in the phylogenetic analysis were chosen based on the BLASTn search results and geographical origin. Bootstrap analysis was performed with 1000 replicates to estimate the confidence of branching patterns of the tree.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11050500/s1, Table S1. Tick species identification based on the mitochondrial 16S rDNA gene. Table S2. Primer sets used for the detection of tick-borne pathogens.
Author Contributions
Conceptualization, O.C. collected the ticks. S.J., O.C., F.S. and X.X. designed the study. S.J. and Z.M. carried out the experiments. E.M.G., I.Z., H.L. and Y.H. contributed reagents/materials preparation. S.J., M.S., T.M., A.I., O.K., R.U.-S. and M.A. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (18H02336 and 18KK0188) and Japan Society for the Promotion of Science Core-to-Core program, both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a grant from the Strategic International Collaborative Research Project (JPJ008837) promoted by the Ministry of Agriculture, Forestry and Fisheries of Japan.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Obihiro University of Agriculture and Veterinary Medicine (Permit for DNA experiment: 1723-4 and 1724-4; Pathogen: 201712-5).
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Peña, A.; Jongejan, F. Ticks feeding on humans: A review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 1999, 23, 685–715. [Google Scholar] [CrossRef] [PubMed]
- Sparagano, O.A.; Allsopp, M.T.; Mank, R.A.; Rijpkema, S.G.; Figueroa, J.V.; Jongejan, F. Molecular detection of pathogen DNA in ticks (Acari: Ixodidae): A review. Exp. Appl. Acarol. 1999, 23, 929–960. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends. Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Wang, T.; Sun, W.; Yang, X.; Liu, J. Tick-borne pathogens and the vector potential of ticks in China. Parasites Vectors 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantas-Torres, F. Species Concepts: What about Ticks? Trends. Parasitol. 2018, 34, 1017–1026. [Google Scholar] [CrossRef]
- Sayin, F.; Dinçer, S.; Karaer, Z.; Cakmak, A.; Inci, A.; Yukari, B.A.; Eren, H.; Vatansever, Z.; Nalbantoglu, S. Studies on the epidemiology of tropical theileriosis (Theileria annulata infection) in cattle in Central Anatolia, Turkey. Trop. Anim. Health. Prod. 2003, 35, 521–539. [Google Scholar] [CrossRef]
- Vatansever, Z.; Gargili, A.; Aysul, N.S.; Sengoz, G.; Estrada-Peña, A. Ticks biting humans in the urban area of Istanbul. Parasitol. Res. 2008, 102, 551–553. [Google Scholar] [CrossRef]
- Bursali, A.; Keskin, A.; Tekin, S. A review of the ticks (Acari: Ixodida) of Turkey: Species diversity, hosts and geographical distribution. Exp. Appl. Acarol. 2012, 57, 91–104. [Google Scholar] [CrossRef]
- Ceylan, O.; Xuan, X.; Sevinc, F. Primary Tick-Borne Protozoan and Rickettsial Infections of Animals in Turkey. Pathogens 2021, 10, 231. [Google Scholar] [CrossRef]
- Ozubek, S.; Bastos, R.G.; Alzan, H.F.; Inci, A.; Aktas, M.; Suarez, C.E. Bovine babesiosis in Turkey: Impact, current gaps, and opportunities for intervention. Pathogens 2020, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Altay, K.; Dumanli, N.; Holman, P.J.; Aktas, M. Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet. Parasitol. 2005, 127, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Altay, K.; Dumanli, N.; Aktas, M. Molecular identification, genetic diversity and distribution of Theileria and Babesia species infecting small ruminants. Vet. Parasitol. 2007, 147, 161–165. [Google Scholar] [CrossRef]
- Altay, K.; Aydin, M.F.; Dumanli, N.; Aktas, M. Molecular detection of Theileria and Babesia infections in cattle. Vet. Parasitol. 2008, 158, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Altay, K.; Dumanli, N. Molecular detection and identification of Anaplasma and Ehrlichia species in cattle from Turkey. Ticks Tick Borne Dis. 2011, 2, 62–65. [Google Scholar] [CrossRef]
- Kocan, K.M.; de la Fuente, J.; Cabezas-Cruz, A. The genus Anaplasma: New challenges after reclassification. Rev. Sci. Tech. 2015, 34, 577–586. [Google Scholar] [CrossRef]
- Aydin, M.F.; Özübek, S.; Aktaş, M. Molecular survey of Anaplasma and Ehrlichia species in cattle from Karaman of Turkey, including a novel tandem report of Anaplasma marginale msp1a gene. Ankara Üniv. Vet. Fak. Derg. 2019, 66, 255–260. [Google Scholar] [CrossRef]
- De la Fuente, J.; Van Den Bussche, R.A.; Kocan, K.M. Molecular phylogeny and biogeography of North American isolates of Anaplasma marginale (Rickettsiaceae: Ehrlichieae). Vet. Parasitol. 2001, 97, 65–76. [Google Scholar] [CrossRef]
- De la Fuente, J.; Passos, L.M.; Van Den Bussche, R.A.; Ribeiro, M.F.; Facury-Filho, E.J.; Kocan, K.M. Genetic diversity and molecular phylogeny of Anaplasma marginale isolates from Minas Gerais, Brazil. Vet. Parasitol. 2004, 121, 307–316. [Google Scholar] [CrossRef]
- Birdane, F.M.; Sevinc, F.; Derinbay, O. Anaplasma marginale infections in dairy cattle: Clinical disease with high seroprevalence. Bull. Vet. Inst. Pulawy. 2006, 50, 467–470. [Google Scholar]
- Aktas, M.; Altay, K.; Ozubek, S.; Dumanli, N. A survey of ixodid ticks feeding on cattle and prevalence of tick-borne pathogens in the Black Sea region of Turkey. Vet. Parasitol. 2012, 187, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M. A survey of ixodid tick species and molecular identification of tick-borne pathogens. Vet. Parasitol. 2014, 200, 276–283. [Google Scholar] [CrossRef]
- Aktas, M.; Vatansever, Z.; Ozubek, S. Molecular evidence for trans-stadial and transovarial transmission of Babesia occultans in Hyalomma marginatum and Rhipicephalus turanicus in Turkey. Vet. Parasitol. 2014, 204, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Özübek, S. Bovine anaplasmosis in Turkey: First laboratory confirmed clinical cases caused by Anaplasma phagocytophilum. Vet. Microbiol. 2015, 178, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; de Vos, A.J. Studies on a bovine Babesia transmitted by Hyalomma marginatum rufipes Koch, 1844. Onderstepoort J. Vet. Res. 1981, 48, 215–223. [Google Scholar] [PubMed]
- Dipeolu, O.O.; Amoo, A. The presence of kinetes of a Babesia species in the haemolymph smears of engorged Hyalomma ticks in Nigeria. Vet. Parasitol. 1984, 17, 41–46. [Google Scholar] [CrossRef]
- Orkun, O. Molecular investigation of the natural transovarial transmission of tick-borne pathogens in Turkey. Vet. Parasitol. 2019, 273, 97–104. [Google Scholar] [CrossRef]
- Kilicoglu, Y.; Cagirgan, A.A.; Serdar; Kaya, S.; Durmaz, Y.; Gur, Y. Molecular investigation, isolation and phylogenetic analsysis of Coxiella burnetii from aborted fetus and ticks. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101571. [Google Scholar] [CrossRef]
- Altay, Ç.G.; Emre, Z.; Canpolat, S.; Vatansever, Y.; Düzgün, A. Detection of Coxiella burnetii from ticks by polymerase chain reaction and restriction fragment length polymorphism. Ankara. Univ. Vet. Fak. Derg. 2013, 60, 263–268. [Google Scholar] [CrossRef]
- Esemu, S.N.; Besong, W.O.; Ndip, R.N.; Ndip, L.M. Prevalence of Ehrlichia ruminantium in adult Amblyomma variegatum collected from cattle in Cameroon. Exp. Appl. Acarol. 2013, 59, 377–387. [Google Scholar] [CrossRef]
- Mtshali, K.; Khumalo, Z.; Nakao, R.; Grab, D.; Sugimoto, C.; Thekisoe, O. Molecular detection of zoonotic tick-borne pathogens from ticks collected from ruminants in four South African provinces. J. Vet. Med. Sci. 2015, 77, 1573–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.F.; Niu, Q.L.; Liu, Z.J.; Yang, J.F.; Chen, Z.; Guan, G.Q.; Liu, G.Y.; Luo, J.X.; Yin, H. Molecular epidemiological surveillance to assess emergence and re-emergence of tick-borne infections in tick samples from China evaluated by nested PCRs. Acta Trop. 2016, 158, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Orkun, O.; Çakmak, A.; Nalbantoğlu, S.; Karaer, Z. Turkey tick news: A molecular investigation into the presence of tick-borne pathogens in host-seeking ticks in Anatolia; Initial evidence of putative vectors and pathogens, and footsteps of a secretly rising vector tick, Haemaphysalis parva. Ticks Tick Borne Dis. 2020, 11, 101373. [Google Scholar] [CrossRef] [PubMed]
- Orkun, O.; Emir, H. Identification of tick-borne pathogens in ticks collected from wild animals in Turkey. Parasitol. Res. 2020, 119, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Xeridat, B.; Raoult, D. Isolation of a Rickettsia related to Astrakhan fever rickettsia from a patient in Chad. Ann. N. Y. Acad. Sci. 2003, 990, 152–157. [Google Scholar] [CrossRef]
- Ferrolho, J.; Antunes, S.; Santos, A.S.; Velez, R.; Padre, L.; Cabezas-Cruz, A.; Santos-Silva, M.M.; Domingos, A. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick Borne Dis. 2016, 7, 443–448. [Google Scholar] [CrossRef]
- Duron, O.; Binetruy, F.; Noël, V.; Cremaschi, J.; McCoy, K.D.; Arnathau, C.; Plantard, O.; Goolsby, J.; Pérez de León, A.A.; Heylen, D.J.A.; et al. Evolutionary changes in symbiont community structure in ticks. Mol. Ecol. 2017, 26, 2905–2921. [Google Scholar] [CrossRef] [Green Version]
- Karim, S.; Budachetri, K.; Mukherjee, N.; Williams, J.; Kausar, A.; Hassan, M.J.; Adamson, S.; Dowd, S.E.; Apanskevich, D.; Arijo, A.; et al. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017, 11, e0005681. [Google Scholar] [CrossRef]
- Ghafar, A.; Cabezas-Cruz, A.; Galon, C.; Obregon, D.; Gasser, R.B.; Moutailler, S.; Jabbar, A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasit. Vectors 2020, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, Y.; Lalzar, I.; Klasson, L. Distinctive Genome Reduction Rates Revealed by Genomic Analyses of Two Coxiella-Like Endosymbionts in Ticks. Genome Biol. Evol. 2015, 7, 1779–1796. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of Domestic Animals in the Mediterranean Region: A Guide to Identification of Species; University of Zaragoza: Zaragoza, Spain, 2004. [Google Scholar]
- Walker, A.R.; Bouattour, A.; Camicas, J.J.; Estrada-pena, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003; pp. 1–1221. [Google Scholar]
- Adjou Moumouni, P.F.; Terkawi, M.A.; Jirapattharasate, C.; Cao, S.; Liu, M.; Nakao, R.; Umemiya-Shirafuji, R.; Yokoyama, N.; Sugimoto, C.; Fujisaki, K.; et al. Molecular detection of spotted fever group rickettsiae in Amblyomma variegatum ticks from Benin. Ticks Tick Borne Dis. 2016, 7, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Black, W.C.; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georges, K.; Loria, G.R.; Riili, S.; Greco, A.; Caracappa, S.; Jongejan, F. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 2001, 99, 273–286. [Google Scholar] [CrossRef]
- Tay, S.T.; Koh, F.X.; Kho, K.L.; Ong, B.L. Molecular survey and sequence analysis of Anaplasma spp. in cattle and ticks in a Malaysian farm. Trop. Biomed. 2014, 31, 769–776. [Google Scholar] [PubMed]
- Pesquera, C.; Portillo, A.; Palomar, A.M.; Oteo, J.A. Investigation of tick-borne bacteria (Rickettsia spp., Anaplasma spp., Ehrlichia spp. and Borrelia spp.) in ticks collected from Andean tapirs, cattle and vegetation from a protected area in Ecuador. Parasit. Vectors 2015, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Terkawi, M.A.; Huyen, N.X.; Shinuo, C.; Inpankaew, T.; Maklon, K.; Aboulaila, M. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet. Parasitol. 2011, 178, 201–207. [Google Scholar] [CrossRef]
- Ota, N.; Mizuno, D.; Kuboki, N.; Igarashi, I.; Nakamura, Y.; Yamashina, H. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan. J. Vet. Med. Sci. 2009, 71, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Martín-Sánchez, J.; Viseras, J.; Adroher, F.J.; García-Fernández, P. Nested polymerase chain reaction for detection of Theileria annulata and comparison with conventional diagnostic techniques: Its use in epidemiology studies. Parasitol. Res. 1999, 85, 243–245. [Google Scholar] [CrossRef]
- Ybañez, A.P.; Sivakumar, T.; Battsetseg, B.; Battur, B.; Altangerel, K.; Matsumoto, K.; Yokoyama, N.; Inokuma, H. Specific molecular detection and characterization of Anaplasma marginale in Mongolian cattle. J. Vet. Med. Sci. 2013, 75, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Tumwebaze, M.A.; Byamukama, B.; Tayebwa, D.S.; Byaruhanga, J.; Angwe, M.K.; Galon, E.M.; Liu, M.; Lee, S.H.; Ringo, A.E.; Adjou Moumouni, P.F.; et al. First molecular detection of Babesia ovis, Theileria spp., Anaplasma spp., and Ehrlichia ruminantium in goats from western Uganda. Pathogens 2020, 9, 895. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, M.; Moumouni, P.F.; Liu, X.; Efstratiou, A.; Liu, Z.; Liu, Y.; Tao, H.; Guo, H.; Wang, G.; et al. First molecular detection of tick-borne pathogens in dogs from Jiangxi, China. J. Vet. Med. Sci. 2017, 79, 248–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; McBride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anstead, C.A.; Chilton, N.B. A novel Rickettsia species detected in Vole Ticks (Ixodes angustus) from Western Canada. Appl. Environ. Microbiol. 2013, 79, 7583–7589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringo, A.E.; Adjou Moumouni, P.F.; Lee, S.H.; Liu, M.; Khamis, Y.H.; Gao, Y.; Guo, H.; Zheng, W.; Efstratiou, A.; Galon, E.M.; et al. Molecular detection and characterization of tick-borne protozoan and rickettsial pathogens isolated from cattle on Pemba Island, Tanzania. Ticks Tick Borne Dis. 2018, 9, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rannala, B. Molecular phylogenetics: Principles and practice. Nat. Rev. Genet. 2012, 13, 303–314. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).