Characteristics and Clinical Course of Alveolar Echinococcosis in Patients with Immunosuppression-Associated Conditions: A Retrospective Cohort Study
Abstract
:1. Introduction
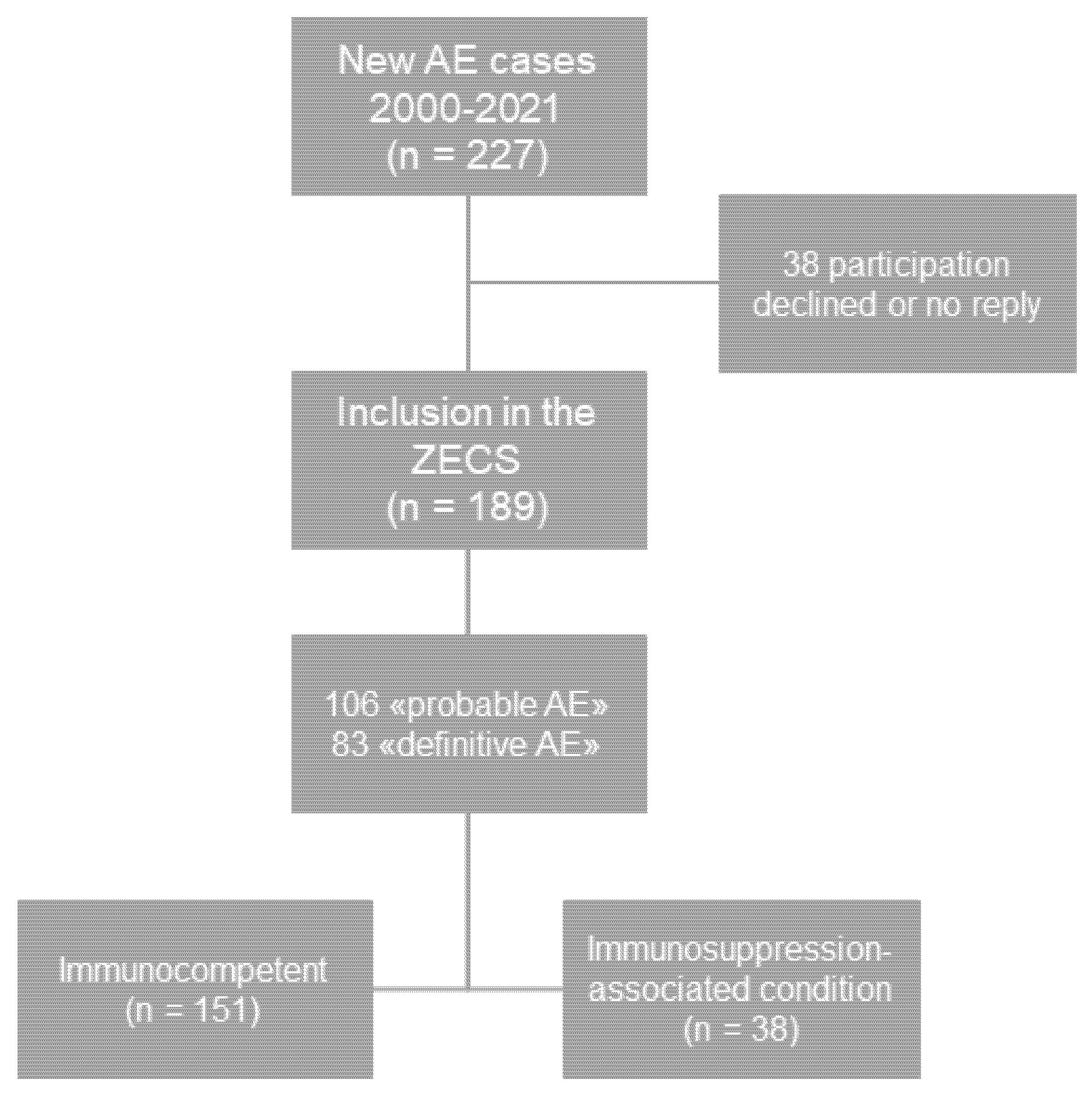
2. Methods
2.1. Study Population and Design
2.2. AE Serologic Testing
2.3. Analysis of Cross-Sectional Imaging
2.4. Delay of AE Diagnosis
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Total AE Cases and Immunosuppression-Associated Conditions
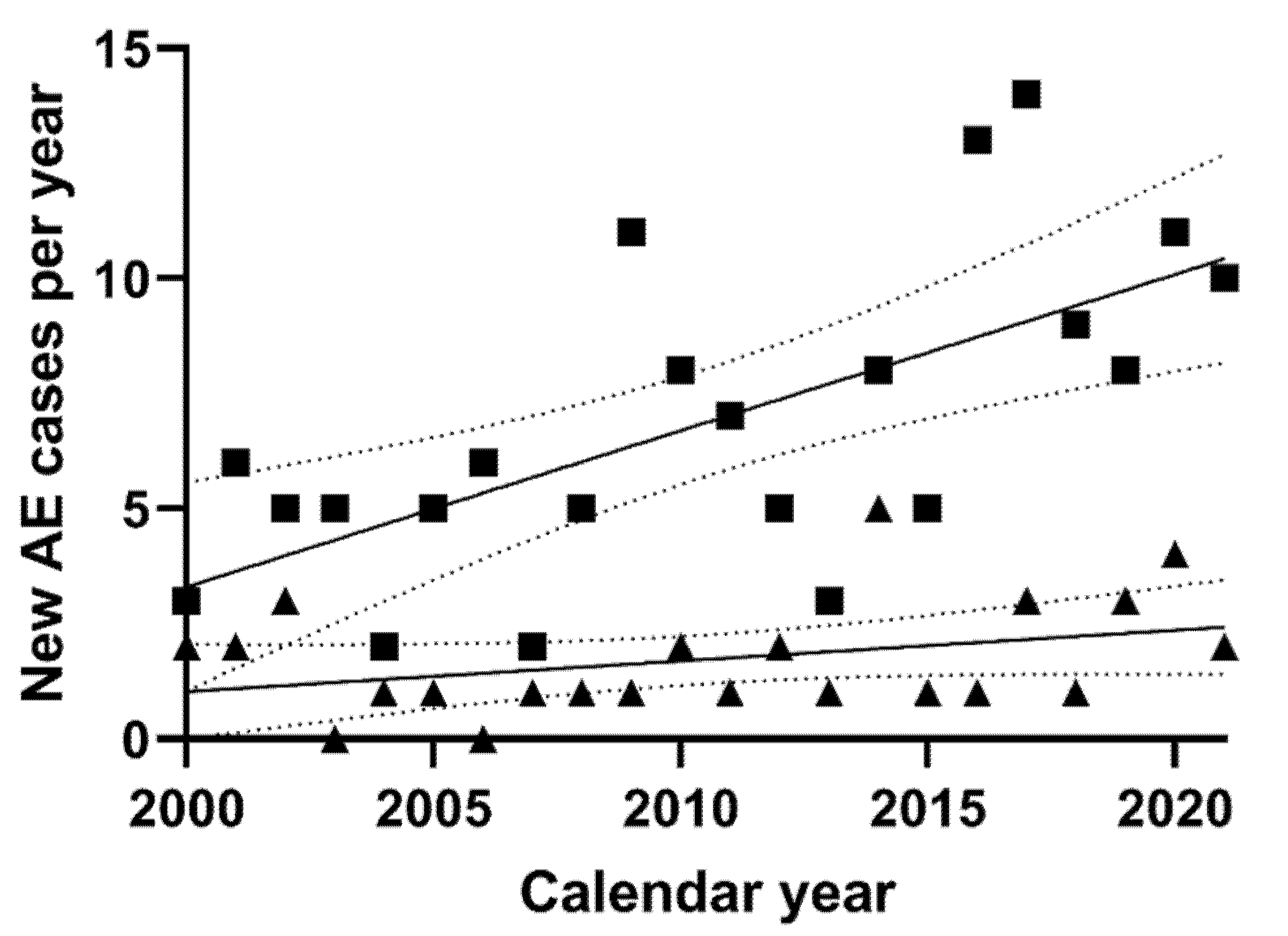
3.2. New AE Cases per Year
3.3. Clinical Characteristics at AE Diagnosis
3.4. Clinical Course of AE Patients with an IAC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Alveolar echinococcosis |
| CLL | Chronic lymphocytic leukemia |
| CT | Computed tomography |
| CTx | Chemotherapy |
| DNA | Deoxyribonucleic acid |
| EITB | Enzyme-linked immunoelectrotransfer blot |
| ELISA | Enzyme-linked immunosorbent assay |
| FDG | Fluorodeoxyglucose |
| IAC | Immunosuppression-associated condition |
| MRI | Magnetic resonance imaging |
| NLR | Neutrophil-to-lymphocyte ratio |
| PET | Positron emission tomography |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| US | Ultrasound |
| ZECS | Zurich Echinococcosis Cohort Study |
References
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [Green Version]
- Ammann, R.W.; Eckert, J. Cestodes. Echinococcus. Gastroenterol. Clin. N. Am. 1996, 25, 655–689. [Google Scholar] [CrossRef]
- Kern, P.; Menezes da Silva, A.; Akhan, O.; Müllhaupt, B.; Vizcaychipi, K.A.; Budke, C.; Vuitton, D.A. The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv. Parasitol. 2017, 96, 259–369. [Google Scholar] [CrossRef] [PubMed]
- Kadry, Z.; Renner, E.C.; Bachmann, L.M.; Attigah, N.; Renner, E.L.; Ammann, R.W.; Clavien, P.A. Evaluation of treatment and long-term follow-up in patients with hepatic alveolar echinococcosis. Br. J. Surg. 2005, 92, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Schweiger, A.; Deplazes, P.; Pohar, M.; Reichen, J.; Ammann, R.W.; Tarr, P.E.; Halkic, N.; Mullhaupt, B. Alveolar echinococcosis: From a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J. Hepatol. 2008, 49, 72–77. [Google Scholar] [CrossRef]
- Schweiger, A.; Ammann, R.W.; Candinas, D.; Clavien, P.A.; Eckert, J.; Gottstein, B.; Halkic, N.; Muellhaupt, B.; Prinz, B.M.; Reichen, J.; et al. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect. Dis. 2007, 13, 878–882. [Google Scholar] [CrossRef]
- Chauchet, A.; Grenouillet, F.; Knapp, J.; Richou, C.; Delabrousse, E.; Dentan, C.; Millon, L.; Di Martino, V.; Contreras, R.; Deconinck, E.; et al. Increased incidence and characteristics of alveolar echinococcosis in patients with immunosuppression-associated conditions. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.C.; Marx, M.; Peyerl-Hoffmann, G.; Kern, W.V. Spatial distribution and incidence trend of human alveolar echinococcosis in southwest Germany: Increased incidence and urbanization of the disease? Infection 2020, 48, 923–927. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Gebbers, D.; Gottstein, B.; Candinas, D.; Beldi, G. Elevated incidence of alveolar echinococcosis in immunocompromised patients. Food Waterborne Parasitol. 2019, 16, e00060. [Google Scholar] [CrossRef]
- Gottstein, B.; Deplazes, P. Alveolar echinococcosis: What triggers emergence in North America, Central Europe and Asia? Curr. Opin. Infect. Dis. 2021, 34, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bresson-Hadni, S.; Spahr, L.; Chappuis, F. Hepatic Alveolar Echinococcosis. Semin. Liver Dis. 2021, 41, 393–408. [Google Scholar] [CrossRef]
- Deplazes, P.; Hegglin, D.; Gloor, S.; Romig, T. Wilderness in the city: The urbanization of Echinococcus multilocularis. Trends Parasitol. 2004, 20, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Romig, T.; Thoma, D.; Weible, A.K. Echinococcus multilocularis--a zoonosis of anthropogenic environments? J. Helminthol. 2006, 80, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Soboslay, P.; Ortona, E.; Wang, J.; Siracusano, A.; Vuitton, D. Immunology of Alveolar and Cystic Echinococcosis (AE and CE). Adv. Parasitol. 2017, 96, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Vuitton, D.A.; Jones, M.K.; Craig, P.S.; McManus, D.P. Brain metastasis of alveolar echinococcosis in a hyperendemic focus of Echinococcus multilocularis infection. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 937–941. [Google Scholar] [CrossRef]
- Sailer, M.; Soelder, B.; Allerberger, F.; Zaknun, D.; Feichtinger, H.; Gottstein, B. Alveolar echinococcosis of the liver in a six-year-old girl with acquired immunodeficiency syndrome. J. Pediatri. 1997, 130, 320–323. [Google Scholar] [CrossRef]
- Bresson-Hadni, S.; Koch, S.; Beurton, I.; Vuitton, D.A.; Bartholomot, B.; Hrusovsky, S.; Heyd, B.; Lenys, D.; Minello, A.; Becker, M.C.; et al. Primary disease recurrence after liver transplantation for alveolar echinococcosis: Long-term evaluation in 15 patients. Hepatology 1999, 30, 857–864. [Google Scholar] [CrossRef]
- Armua-Fernandez, M.T.; Joekel, D.; Schweiger, A.; Eichenberger, R.M.; Matsumoto, J.; Deplazes, P. Successful intestinal Echinococcus multilocularis oncosphere invasion and subsequent hepatic metacestode establishment in resistant RccHan™: WIST rats after pharmacological immunosuppression. Parasitology 2016, 143, 1252–1260. [Google Scholar] [CrossRef]
- Joekel, D.E.; Nur, S.; Monné Rodriguez, J.; Kronenberg, P.A.; Kipar, A.; LeibundGut-Landmann, S.; Deplazes, P. Agranulocytosis leads to intestinal Echinococcus multilocularis oncosphere invasion and hepatic metacestode development in naturally resistant Wistar rats. Parasitology 2021, 148, 53–62. [Google Scholar] [CrossRef]
- Klastersky, J.; Aoun, M. Opportunistic infections in patients with cancer. Ann. Oncol. 2004, 15 (Suppl. S4), iv329–iv335. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Ko, C.H.; Wang, J.L.; Hsu, T.C.; Lin, C.Y. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: A nationally representative cohort study. Arthritis Res. Ther. 2019, 21, 211. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.A.; Cleveland, J.D. Epidemiology, Time Trends, and Outcomes of Serious Infections in Patients With Vasculitis: A Nineteen-Year National Study. Arthritis Care Res. 2021, 73, 1544–1551. [Google Scholar] [CrossRef]
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Bartoletti, M.; Giannella, M.; Lewis, R.; Caraceni, P.; Tedeschi, S.; Paul, M.; Schramm, C.; Bruns, T.; Merli, M.; Cobos-Trigueros, N.; et al. A prospective multicentre study of the epidemiology and outcomes of bloodstream infection in cirrhotic patients. Clin. Microbiol. Infect. 2018, 24, 546.e1–546.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, I.M.; Critchley, J.A.; DeWilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezgin, E.; Van Natta, M.L.; Thorne, J.E.; Puhan, M.A.; Jabs, D.A. Secular trends in opportunistic infections, cancers and mortality in patients with AIDS during the era of modern combination antiretroviral therapy. HIV Med. 2018, 19, 411–419. [Google Scholar] [CrossRef]
- Helfrich, M.; Dorschner, P.; Thomas, K.; Stosor, V.; Ison, M.G. A retrospective study to describe the epidemiology and outcomes of opportunistic infections after abdominal organ transplantation. Transpl. Infect. Dis. 2017, 19, e12691. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.; Wen, H.; Sato, N.; Vuitton, D.A.; Gruener, B.; Shao, Y.; Delabrousse, E.; Kratzer, W.; Bresson-Hadni, S. WHO classification of alveolar echinococcosis: Principles and application. Parasitol. Int. 2006, 55, S283–S287. [Google Scholar] [CrossRef]
- Schweiger, A.; Grimm, F.; Tanner, I.; Müllhaupt, B.; Bertogg, K.; Müller, N.; Deplazes, P. Serological diagnosis of echinococcosis: The diagnostic potential of native antigens. Infection 2012, 40, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Ammann, R.W.; Renner, E.C.; Gottstein, B.; Grimm, F.; Eckert, J.; Renner, E.L. Immunosurveillance of alveolar echinococcosis by specific humoral and cellular immune tests: Long-term analysis of the Swiss chemotherapy trial (1976–2001). J. Hepatol. 2004, 41, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ammann, R.W.; Stumpe, K.D.; Grimm, F.; Deplazes, P.; Huber, S.; Bertogg, K.; Fischer, D.R.; Mullhaupt, B. Outcome after Discontinuing Long-Term Benzimidazole Treatment in 11 Patients with Non-resectable Alveolar Echinococcosis with Negative FDG-PET/CT and Anti-EmII/3-10 Serology. PLoS Negl. Trop. Dis. 2015, 9, e0003964. [Google Scholar] [CrossRef] [PubMed]
- Deibel, A.; Stocker, D.; Meyer Zu Schwabedissen, C.; Husmann, L.; Kronenberg, P.A.; Grimm, F.; Deplazes, P.; Reiner, C.S.; Müllhaupt, B. Evaluation of a structured treatment discontinuation in patients with inoperable alveolar echinococcosis on long-term benzimidazole therapy: A retrospective cohort study. PLoS Negl. Trop. Dis. 2022, 16, e0010146. [Google Scholar] [CrossRef] [PubMed]
- Hotz, J.F.; Peters, L.; Kapp-Schwörer, S.; Theis, F.; Eberhardt, N.; Essig, A.; Grüner, B.; Hagemann, J.B. Evaluation of Serological Markers in Alveolar Echinococcosis Emphasizing the Correlation of PET-CTI Tracer Uptake with RecEm18 and Echinococcus-Specific IgG. Pathogens 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Bebezov, B.; Mamashev, N.; Umetaliev, T.; Ziadinov, I.; Craig, P.S.; Joekel, D.E.; Deplazes, P.; Grimm, F.; Torgerson, P.R. Intense Focus of Alveolar Echinococcosis, South Kyrgyzstan. Emerg. Infect. Dis. 2018, 24, 1119–1122. [Google Scholar] [CrossRef]
| Malignancy (15) | Breast cancer (4) |
| Prostate cancer (2) | |
| Colorectal cancer (2) | |
| Ovarian cancer (1) | |
| Non-small cell lung cancer (1) | |
| Parotid gland cancer (1) | |
| Carcinoid (1) | |
| Medullary thyroid cancer (1) | |
| Myelofibrosis (1) | |
| Morbus Waldenström (1) | |
| Chronic inflammatory disease (11) | Rheumatoid arthritis (3) |
| Chronic polyarthritis (3) | |
| Polymyalgia rheumatica (2) | |
| Psoriasis (1) | |
| Vasculitis (1) | |
| Collagenosis (1) | |
| Diabetes mellitus (8) | Type I (1) |
| Type II (7) | |
| Cirrhosis (4) | Child–Pugh A (1) |
| Child–Pugh B (2) | |
| Child–Pugh C (1) | |
| Organ transplantation (2) | Heart (1) |
| Liver (1) | |
| HIV/AIDS (1) | AIDS (1) |
| Medication (20) | Chemotherapy (8) |
| Immunosuppressive/anti-inflammatory therapy (12) |
| Immunocompetent (n = 151) | Immunosuppression-Associated Conditions (n = 38) | p-Value (Univariate) | |
|---|---|---|---|
| Age at diagnosis | 55 y; 12–80 y | 60.5 y; 39–77 y | 0.002 * |
| Sex | 82 f (54.3%) | 21 f (55.3%) | 0.916 |
| 69 m (45.7%) | 17 m (44.7%) | ||
| P | I: 61 (40.4%) | I: 22 (57.9%) | 0.126 |
| II: 28 (18.5%) | II: 7 (18.4%) | ||
| III: 36 (23.8%) | III: 3 (7.9%) | ||
| IV: 25 (16.6%) | IV: 5 (13.2%) | ||
| 0: 1 (0.7%) | 0: 1 (2.6%) | ||
| N | 0: 113 (74.8%) | 0: 34 (89.5%) | 0.052 |
| I: 37 (24.5%) | I: 3 (7.9%) | ||
| X: 1 (0.7%) | X: 1 (2.6%) | ||
| M | 0: 134 (88.7%) | 0: 35 (92.1%) | 0.547 |
| I: 17 # (11.3%) | I: 3 ## (7.9%) | ||
| Stage | I: 42 (27.8%) | I: 21 (55.3%) | 0.015 * |
| II: 20 (13.2%) | II: 6 (15.8%) | ||
| IIIa: 25 (16.6%) | IIIa: 3 (7.9%) | ||
| IIIb: 39 (25.8%) | IIIb: 4 (10.5%) | ||
| IV: 25 (16.6%) | IV: 4 (10.5%) | ||
| Pos. anti-Em18 ELISA | Yes: 70 (46.4%) | Yes: 10 (26.3%) | 0.034 * |
| No: 61 (40.4%) | No: 21 (55.3%) | ||
| Missing: 20 (13.2%) | Missing: 6 (15.8%) | ||
| Incidental finding | Yes: 60 (39.7%) | Yes: 30 (78.9%) No: 8 (21.1%) | 0.000 * |
| No: 84 (55.6%) | |||
| Missing: 7 (4.6%) | |||
| Delay of diagnosis | 16 d, 2–3954 d | 26.5 d, 2–650 d | 0.030 * |
| Missing: 10 (6.6%) | |||
| Lesion count | 1; 1–38 | 1; 1–17 | 0.555 |
| Missing: 7 (4.6%) | Missing: 4 (10.5%) | ||
| Lesion size, cum. | 103 mm; 15–792 mm | 86 mm; 13–390 mm | 0.052 |
| Missing: 7 (4.6%) | Missing: 4 (10.5%) | ||
| Lesion size, avg. | 64 mm; 9–213 mm | 44 mm; 8–151 mm | 0.036 * |
| Missing: 7 (4.6%) | Missing: 4 (10.5%) | ||
| Surgical resection | 56 (37.1%) | 14 (34.2%) | 0.742 |
| ID | Age | Sex | PNM | Stage | Em18 | IF | L.c. | L.s.c. | L.s.a. | Res. | Tfu | Prog. | IAC (Disease) | IAC (Medication) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | f | P1N0M0 | I | n.a. | yes | n.a. | n.a. | n.a. | yes # | 169 | no | CRC | 5FU, raltitrexed |
| 2 | 69 | m | P1N0M0 | I | - | yes | 1 | 28 | 28 | no | 42 | no | CPA | MTX, prednisone |
| 3 | 71 | f | P1N0M0 | I | n.a. | yes | n.a. | n.a. | n.a. | yes | 80 | no | RA | MTX |
| 4 | 59 | m | P3N0M0 | IIIa | + | yes | 1 | 91 | 91 | yes | 62 | no | DM II | --- |
| 5 | 61 | m | P4N1M0 | IV | n.a. | no | 1 | 141 | 141 | no | 230 | no | Psoriasis | --- |
| 6 | 39 | m | P3N0M0 | IIIa | n.a. | yes | 1 | 91 | 91 | no | 234 | no | AIDS | --- |
| 7 | 75 | f | P1N0M0 | I | n.a. | yes | n.a. | n.a. | n.a. | yes # | 54 | no | PMR | prednisone |
| 8 | 46 | m | P1N0M0 | I | n.a. | yes | 1 | 37 | 37 | yes # | 171 | no | Cirrhosis Child C | --- |
| 9 | 68 | f | P2N0M0 | II | + | yes | 1 | 85 | 85 | no | 43 | no | Vasculitis | prednisone |
| 10 | 51 | m | P2N0M0 | II | + | n.a. | 1 | 73 | 73 | no | 40 | no | BrCa | --- |
| 11 | 44 | f | P4N0M1 | IV | - | no | 3 | 196 | 65 | no | 161 | no | CPA | prednisone |
| 12 | 67 | f | P1N0M0 | I | - | yes | 1 | 13 | 13 | yes | 133 | no | BrCa | --- |
| 13 | 48 | m | P1N0M0 | I | + | yes | 3 | 58 | 19 | no | 128 | no | Cirrhosis Child B | --- |
| 14 | 71 | m | P2N1M1 | IV | - | no | 4 | 149 | 37 | no | 137 | no | RA, DM II | MTX, prednisone |
| 15 | 67 | f | P2N0M0 | II | - | yes | 1 | 84 | 84 | yes # | 113 | no | DM I | --- |
| 16 | 60 | m | P4N0M0 | IIIb | + | no | 1 | 125 | 125 | no | 107 | no | PGCa | --- |
| 17 | 54 | f | P3N0M0 | IIIa | + | yes | 1 | 151 | 151 | yes | 73 | no | Myelofibrosis | --- |
| 18 | 65 | f | P1N0M0 | I | - | yes | 2 | 74 | 37 | yes # | 92 | no | Cirrhosis Child A | --- |
| 19 | 63 | f | P1N0M0 | I | - | yes | 1 | 46 | 46 | no | 89 | yes * | PMR | prednisone |
| 20 | 66 | f | P1N0M0 | I | - | yes | 11 | 93 | 8 | no | 86 | no | DM II | --- |
| 21 | 55 | f | P2N0M0 | II | + | yes | 1 | 57 | 57 | no | 35 | no | mThyCa | --- |
| 22 | 76 | m | P1N1M0 | IIIb | - | no | 1 | 126 | 126 | no | 21 | no | CPA | leflunomid, MTX |
| 23 | 65 | m | P1N0M0 | I | - | yes | 4 | 125 | 31 | no | 74 | no | heart TPL | tacrolimus, MMF, prednisone |
| 24 | 61 | f | P2N0M0 | II | - | no | 1 | 55 | 55 | yes # | 70 | no | BrCa | unknown CTx-agent |
| 25 | 54 | f | P1N0M0 | I | - | yes | 7 | 91 | 13 | no | 54 | no | BrCa | unknown CTx-agent |
| 26 | 60 | f | P1N0M0 | I | + | yes | 5 | 117 | 23 | no | 54 | no | CRC | oxaliplatin, capecitabine |
| 27 | 55 | m | P1N1M0 | IIIb | + | no | 2 | 87 | 44 | no | 52 | no | Carcinoid | oxaliplatin |
| 28 | 55 | f | P1N0M0 | I | - | yes | 2 | 87 | 44 | yes | 31 | no | RA | anti-TNFa, tofacitinib |
| 29 | 58 | f | P1N0M0 | I | - | yes | 1 | 45 | 45 | no | 41 | no | DM II | --- |
| 30 | 67 | f | P1N0M0 | I | - | no | 2 | 115 | 58 | yes # | 32 | no | liver TPL ## | tacrolimus, MMF |
| 31 | 69 | m | P4N0M0 | IIIb | + | no | 17 | 390 | 23 | no | 28 | no | PrCa | --- |
| 32 | 77 | f | P4N0M0 | IIIb | - | yes | 2 | 114 | 57 | no | 1 | n.a. | Collagenosis | prednisone |
| 33 | 61 | f | P1N0M0 | I | - | yes | 2 | 84 | 42 | yes | 16 | no | OvCa | carboplatin, paclitaxel |
| 34 | 60 | m | P1N0M0 | I | - | yes | 6 | 84 | 14 | no | 20 | no | DM II | --- |
| 35 | 64 | m | P1N0M0 | I | - | yes | 3 | 40 | 13 | no | 9 | yes ** | DM II | --- |
| 36 | 60 | f | P1N0M0 | I | - | yes | 1 | 29 | 29 | no | 8 | no | Waldenström | rituximab, bendamustin |
| 37 | 67 | m | PxNxM1 | IV | - | yes | n.a. | n.a. | n.a. | yes # | 8 | no | NSCLC, Cirrhosis Child B, DM II | carboplatin, permetrexed |
| 38 | 58 | m | P2N0M0 | II | - | yes | 1 | 64 | 64 | no | 6 | no | PrCa | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deibel, A.; Meyer zu Schwabedissen, C.; Husmann, L.; Grimm, F.; Deplazes, P.; Reiner, C.S.; Müllhaupt, B. Characteristics and Clinical Course of Alveolar Echinococcosis in Patients with Immunosuppression-Associated Conditions: A Retrospective Cohort Study. Pathogens 2022, 11, 441. https://doi.org/10.3390/pathogens11040441
Deibel A, Meyer zu Schwabedissen C, Husmann L, Grimm F, Deplazes P, Reiner CS, Müllhaupt B. Characteristics and Clinical Course of Alveolar Echinococcosis in Patients with Immunosuppression-Associated Conditions: A Retrospective Cohort Study. Pathogens. 2022; 11(4):441. https://doi.org/10.3390/pathogens11040441
Chicago/Turabian StyleDeibel, Ansgar, Cordula Meyer zu Schwabedissen, Lars Husmann, Felix Grimm, Peter Deplazes, Cäcilia S. Reiner, and Beat Müllhaupt. 2022. "Characteristics and Clinical Course of Alveolar Echinococcosis in Patients with Immunosuppression-Associated Conditions: A Retrospective Cohort Study" Pathogens 11, no. 4: 441. https://doi.org/10.3390/pathogens11040441
APA StyleDeibel, A., Meyer zu Schwabedissen, C., Husmann, L., Grimm, F., Deplazes, P., Reiner, C. S., & Müllhaupt, B. (2022). Characteristics and Clinical Course of Alveolar Echinococcosis in Patients with Immunosuppression-Associated Conditions: A Retrospective Cohort Study. Pathogens, 11(4), 441. https://doi.org/10.3390/pathogens11040441






