The History of Live Attenuated Centrin Gene-Deleted Leishmania Vaccine Candidates
Abstract
1. Introduction
2. Vaccination Strategies against Leishmaniasis
2.1. First-Generation Vaccines
2.2. Second-Generation Vaccines
2.3. Third-Generation Vaccines
2.4. Live Attenuated Vaccines
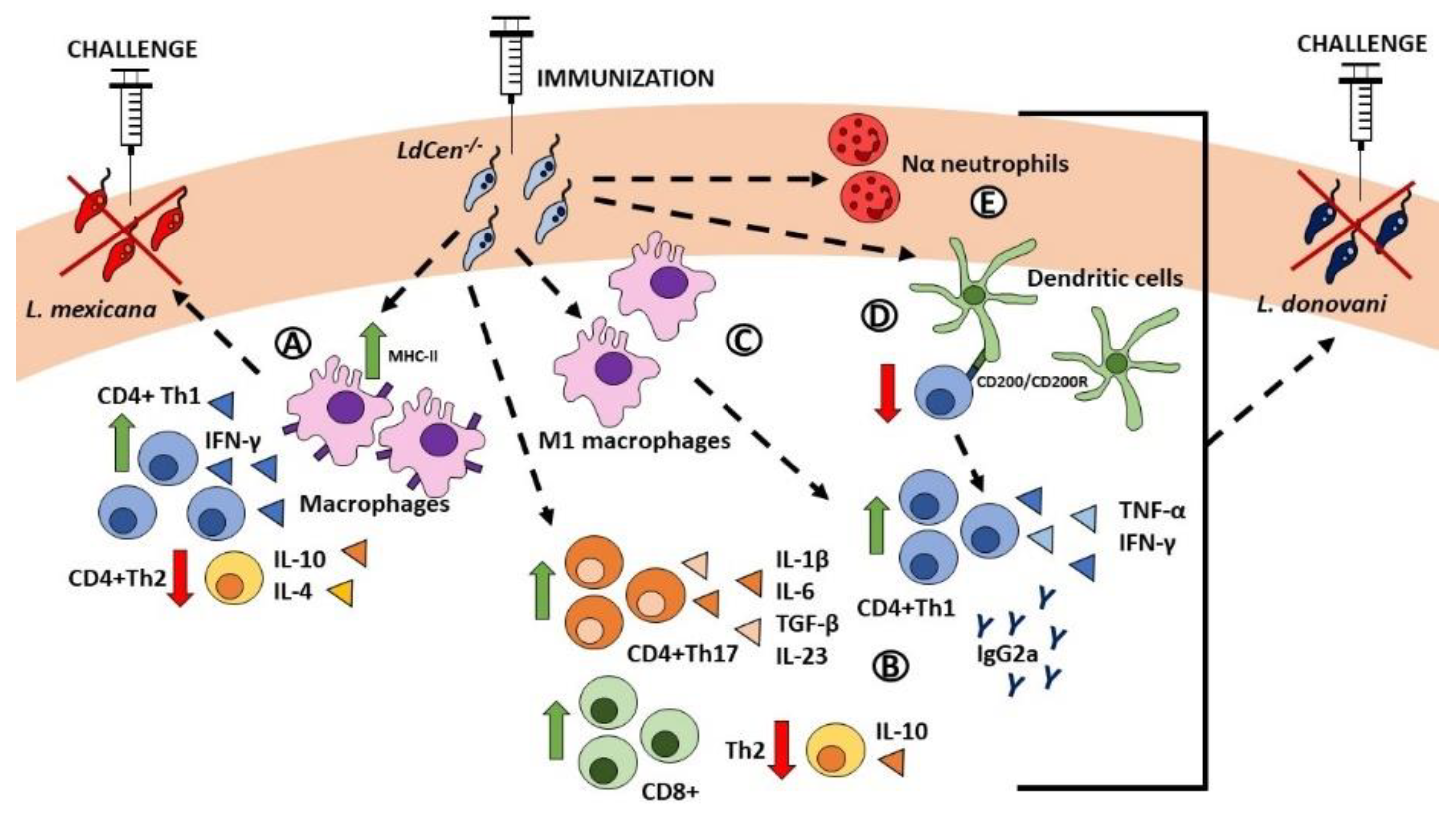
3. Leishmania centrin Knockout Parasites as a Vaccine Strategy against Leishmaniasis
3.1. Centrin-1-Deficient Leishmania donovani
3.2. L. donovani Cen-/- for the Vaccination of Reservoirs of the Infection
3.3. L. major Cen-/- as a Safer Alternative to L. donovani Cen-/- and Leishmanization
3.4. L. major Cen-/- Mutants Provide Safe and Long-Term Immunity
3.5. Towards Developing a Pan-Leishmania Vaccine
4. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martinez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Reithinger, R.; Dujardin, J.C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Basu, M.K.; Ray, M. Macrophage and Leishmania: An unacceptable coexistence. Crit. Rev. Microbiol. 2005, 31, 145–154. [Google Scholar] [CrossRef]
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- WHO. Leishmaniasis in high-burden countries: An epidemiological update based on data reported in 2014. Wkly. Epidemiol. Rec. 2016, 91, 10. [Google Scholar]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef]
- Zhang, W.W.; Karmakar, S.; Gannavaram, S.; Dey, R.; Lypaczewski, P.; Ismail, N.; Siddiqui, A.; Simonyan, V.; Oliveira, F.; Coutinho-Abreu, I.V.; et al. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat. Commun. 2020, 11, 3461. [Google Scholar] [CrossRef]
- Bumb, R.A.; Satoskar, A.R. Radiofrequency-induced heat therapy as first-line treatment for cutaneous leishmaniasis. Expert. Rev. Anti-Infect. Ther. 2011, 9, 623–625. [Google Scholar] [CrossRef]
- Row, R. The Curative Value of Leishmania Culture “Vaccine” in Oriental Sore. Br. Med. J. 1912, 1, 540–541. [Google Scholar] [CrossRef]
- Khamesipour, A.; Dowlati, Y.; Asilian, A.; Hashemi-Fesharki, R.; Javadi, A.; Noazin, S.; Modabber, F. Leishmanization: Use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine 2005, 23, 3642–3648. [Google Scholar] [CrossRef] [PubMed]
- Seyed, N.; Peters, N.C.; Rafati, S. Translating Observations from Leishmanization into Non-Living Vaccines: The Potential of Dendritic Cell-Based Vaccination Strategies against Leishmania. Front. Immunol. 2018, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, L. Leishmaniasis Vaccine: Where are We Today? J. Glob. Infect. Dis. 2010, 2, 177–185. [Google Scholar] [CrossRef]
- Moafi, M.; Rezvan, H.; Sherkat, R.; Taleban, R. Leishmania Vaccines Entered in Clinical Trials: A Review of Literature. Int. J. Prev. Med. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ali, N. Vaccine Development against Leishmania donovani. Front. Immunol. 2012, 3, 99. [Google Scholar] [CrossRef]
- Armijos, R.X.; Weigel, M.M.; Aviles, H.; Maldonado, R.; Racines, J. Field trial of a vaccine against New World cutaneous leishmaniasis in an at-risk child population: Safety, immunogenicity, and efficacy during the first 12 months of follow-up. J. Infect. Dis. 1998, 177, 1352–1357. [Google Scholar] [CrossRef]
- Alexander, J. A radioattenuated Leishmania major vaccine markedly increases the resistance of CBA mice to subsequent infection with Leishmania mexicana mexicana. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 646–649. [Google Scholar] [CrossRef]
- Mayrink, W.; da Costa, C.A.; Magalhaes, P.A.; Melo, M.N.; Dias, M.; Lima, A.O.; Michalick, M.S.; Williams, P. A field trial of a vaccine against American dermal leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 385–387. [Google Scholar] [CrossRef]
- Marzochi, K.B.; Marzochi, M.A.; Silva, A.F.; Grativol, N.; Duarte, R.; Confort, E.M.; Modabber, F. Phase 1 study of an inactivated vaccine against American tegumentary leishmaniasis in normal volunteers in Brazil. Mem. Inst. Oswaldo Cruz. 1998, 93, 205–212. [Google Scholar] [CrossRef][Green Version]
- Mendonca, S.C.; De Luca, P.M.; Mayrink, W.; Restom, T.G.; Conceicao-Silva, F.; Da-Cruz, A.M.; Bertho, A.L.; Da Costa, C.A.; Genaro, O.; Toledo, V.P.; et al. Characterization of human T lymphocyte-mediated immune responses induced by a vaccine against American tegumentary leishmaniasis. Am. J. Trop. Med. Hyg. 1995, 53, 195–201. [Google Scholar] [CrossRef]
- Palatnik-de-Sousa, C.B. Vaccines for leishmaniasis in the fore coming 25 years. Vaccine 2008, 26, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Velez, I.D.; del Pilar Agudelo, S.; Arbelaez, M.P.; Gilchrist, K.; Robledo, S.M.; Puerta, J.A.; Zicker, F.; Berman, J.; Modabber, F. Safety and immunogenicity of a killed Leishmania (L.) amazonensis vaccine against cutaneous leishmaniasis in Colombia: A randomized controlled trial. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 698–703. [Google Scholar] [CrossRef]
- Srivastava, S.; Shankar, P.; Mishra, J.; Singh, S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit. Vectors 2016, 9, 277. [Google Scholar] [CrossRef]
- Jeronimo, S.M.; Higgs, E.; Vedvick, T.; Mann, B.J.; Jernigan, J.; Petri, W.A., Jr.; Pearson, R.D. Identification of Leishmania chagasi antigens recognized by human lymphocytes. J. Infect. Dis. 1995, 172, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Ali, N. Progress in vaccine research and possible effector mechanisms in visceral leishmaniasis. Curr. Mol. Med. 2004, 4, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Giunchetti, R.C.; Correa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Roatt, B.M.; de Oliveira Aguiar-Soares, R.D.; de Souza, J.V.; das Dores Moreira, N.; Malaquias, L.C.; Mota e Castro, L.L.; et al. Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs. Vaccine 2007, 25, 7674–7686. [Google Scholar] [CrossRef] [PubMed]
- De Brito, R.C.F.; Cardoso, J.M.O.; Reis, L.E.S.; Vieira, J.F.; Mathias, F.A.S.; Roatt, B.M.; Aguiar-Soares, R.; Ruiz, J.C.; Resende, D.M.; Reis, A.B. Peptide Vaccines for Leishmaniasis. Front. Immunol. 2018, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Blackwell, J.M.; Castes, M.; Trujillo, D.; Convit, J.; Shaw, M.A. Immunotherapy with live BCG plus heat killed Leishmania induces a T helper 1-like response in American cutaneous leishmaniasis patients. Parasite Immunol. 2000, 22, 73–79. [Google Scholar] [CrossRef]
- Momeni, A.Z.; Jalayer, T.; Emamjomeh, M.; Khamesipour, A.; Zicker, F.; Ghassemi, R.L.; Dowlati, Y.; Sharifi, I.; Aminjavaheri, M.; Shafiei, A.; et al. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine 1999, 17, 466–472. [Google Scholar] [CrossRef]
- Sharifi, I.; FeKri, A.R.; Aflatonian, M.R.; Khamesipour, A.; Nadim, A.; Mousavi, M.R.; Momeni, A.Z.; Dowlati, Y.; Godal, T.; Zicker, F.; et al. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 1998, 351, 1540–1543. [Google Scholar] [CrossRef]
- Sharples, C.E.; Shaw, M.A.; Castes, M.; Convit, J.; Blackwell, J.M. Immune response in healthy volunteers vaccinated with BCG plus killed leishmanial promastigotes: Antibody responses to mycobacterial and leishmanial antigens. Vaccine 1994, 12, 1402–1412. [Google Scholar] [CrossRef]
- Armijos, R.X.; Weigel, M.M.; Romero, L.; Garcia, V.; Salazar, J. Field trial of a vaccine against new world cutaneous leishmaniasis in an at-risk child population: How long does protection last? J. Infect. Dis. 2003, 187, 1959–1961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Armijos, R.X.; Weigel, M.M.; Calvopina, M.; Hidalgo, A.; Cevallos, W.; Correa, J. Safety, immunogenecity, and efficacy of an autoclaved Leishmania amazonensis vaccine plus BCG adjuvant against New World cutaneous leishmaniasis. Vaccine 2004, 22, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, M.; Khamesipour, A.; Mobedi, I.; Zarei, Z.; Hashemi-Fesharki, R. Double-blind randomized efficacy field trial of alum precipitated autoclaved Leishmania major vaccine mixed with BCG against canine visceral leishmaniasis in Meshkin-Shahr district, I.R. Iran. Vaccine 2004, 22, 4097–4100. [Google Scholar] [CrossRef]
- Lee, N.H.; Lee, J.A.; Park, S.Y.; Song, C.S.; Choi, I.S.; Lee, J.B. A review of vaccine development and research for industry animals in Korea. Clin. Exp. Vaccine Res. 2012, 1, 18–34. [Google Scholar] [CrossRef]
- Nagill, R.; Kaur, S. Vaccine candidates for leishmaniasis: A review. Int. Immunopharmacol. 2011, 11, 1464–1488. [Google Scholar] [CrossRef]
- De Oliveira, B.C.; Duthie, M.S.; Alves Pereira, V.R. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum. Vaccines Immunother. 2020, 16, 919–930. [Google Scholar] [CrossRef]
- Alvar, J.; Croft, S.L.; Kaye, P.; Khamesipour, A.; Sundar, S.; Reed, S.G. Case study for a vaccine against leishmaniasis. Vaccine 2013, 31 (Suppl. 2), B244–B249. [Google Scholar] [CrossRef]
- Singh, B.; Sundar, S. Leishmaniasis: Vaccine candidates and perspectives. Vaccine 2012, 30, 3834–3842. [Google Scholar] [CrossRef]
- Duthie, M.S.; Raman, V.S.; Piazza, F.M.; Reed, S.G. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Coler, R.N.; Duthie, M.S.; Hofmeyer, K.A.; Guderian, J.; Jayashankar, L.; Vergara, J.; Rolf, T.; Misquith, A.; Laurance, J.D.; Raman, V.S.; et al. From mouse to man: Safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin. Transl. Immunol. 2015, 4, e35. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, H.; Moafi, M. An overview on Leishmania vaccines: A narrative review article. Vet. Res. Forum. 2015, 6, 1–7. [Google Scholar] [PubMed]
- Gillespie, P.M.; Beaumier, C.M.; Strych, U.; Hayward, T.; Hotez, P.J.; Bottazzi, M.E. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 2016, 34, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, J.; Kumar, S.; Trivedi, S.; Rai, V.K.; Singh, A.; Ashman, J.A.; Laughlin, E.M.; Coler, R.N.; Kahn, S.J.; Beckmann, A.M.; et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine 2011, 29, 3531–3537. [Google Scholar] [CrossRef]
- Llanos-Cuentas, A.; Calderon, W.; Cruz, M.; Ashman, J.A.; Alves, F.P.; Coler, R.N.; Bogatzki, L.Y.; Bertholet, S.; Laughlin, E.M.; Kahn, S.J.; et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with sodium stibogluconate for the treatment of mucosal leishmaniasis. Vaccine 2010, 28, 7427–7435. [Google Scholar] [CrossRef]
- Volpedo, G.; Huston, R.H.; Holcomb, E.A.; Pacheco-Fernandez, T.; Gannavaram, S.; Bhattacharya, P.; Nakhasi, H.L.; Satoskar, A.R. From infection to vaccination: Reviewing the global burden, history of vaccine development, and recurring challenges in global leishmaniasis protection. Expert Rev. Vaccines 2021, 20, 1431–1446. [Google Scholar] [CrossRef]
- Peters, N.C.; Kimblin, N.; Secundino, N.; Kamhawi, S.; Lawyer, P.; Sacks, D.L. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS Pathog. 2009, 5, e1000484. [Google Scholar] [CrossRef]
- Peters, N.C.; Bertholet, S.; Lawyer, P.G.; Charmoy, M.; Romano, A.; Ribeiro-Gomes, F.L.; Stamper, L.W.; Sacks, D.L. Evaluation of recombinant Leishmania polyprotein plus glucopyranosyl lipid A stable emulsion vaccines against sand fly-transmitted Leishmania major in C57BL/6 mice. J. Immunol. 2012, 189, 4832–4841. [Google Scholar] [CrossRef]
- Gomes, R.; Teixeira, C.; Oliveira, F.; Lawyer, P.G.; Elnaiem, D.E.; Meneses, C.; Goto, Y.; Bhatia, A.; Howard, R.F.; Reed, S.G.; et al. KSAC, a defined Leishmania antigen, plus adjuvant protects against the virulence of L. major transmitted by its natural vector Phlebotomus duboscqi. PLoS Negl. Trop. Dis. 2012, 6, e1610. [Google Scholar] [CrossRef]
- Rogers, M.E.; Sizova, O.V.; Ferguson, M.A.; Nikolaev, A.V.; Bates, P.A. Synthetic glycovaccine protects against the bite of leishmania-infected sand flies. J. Infect. Dis. 2006, 194, 512–518. [Google Scholar] [CrossRef]
- Tavares, N.M.; Silva, R.A.; Costa, D.J.; Pitombo, M.A.; Fukutani, K.F.; Miranda, J.C.; Valenzuela, J.G.; Barral, A.; de Oliveira, C.I.; Barral-Netto, M.; et al. Lutzomyia longipalpis saliva or salivary protein LJM19 protects against Leishmania braziliensis and the saliva of its vector, Lutzomyia intermedia. PLoS Negl. Trop. Dis. 2011, 5, e1169. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Oliveira, F.; Teixeira, C.; Meneses, C.; Gilmore, D.C.; Elnaiem, D.E.; Kamhawi, S.; Valenzuela, J.G. Immunity to sand fly salivary protein LJM11 modulates host response to vector-transmitted leishmania conferring ulcer-free protection. J. Investig. Dermatol. 2012, 132, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Collin, N.; Gomes, R.; Teixeira, C.; Cheng, L.; Laughinghouse, A.; Ward, J.M.; Elnaiem, D.E.; Fischer, L.; Valenzuela, J.G.; Kamhawi, S. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog. 2009, 5, e1000441. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Giorgobiani, E.; Guimaraes-Costa, A.B.; Abdeladhim, M.; Oristian, J.; Tskhvaradze, L.; Tsertsvadze, N.; Zakalashvili, M.; Valenzuela, J.G.; Kamhawi, S. Immunity to vector saliva is compromised by short sand fly seasons in endemic regions with temperate climates. Sci. Rep. 2020, 10, 7990. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Bahloul, C.; Robbana, C.; Askri, S.; Dellagi, K. A comparative evaluation of different DNA vaccine candidates against experimental murine leishmaniasis due to L. major. Vaccine 2004, 22, 1631–1639. [Google Scholar] [CrossRef]
- Gurunathan, S.; Sacks, D.L.; Brown, D.R.; Reiner, S.L.; Charest, H.; Glaichenhaus, N.; Seder, R.A. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 1997, 186, 1137–1147. [Google Scholar] [CrossRef]
- Xu, D.; Liew, F.Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology 1995, 84, 173–176. [Google Scholar]
- Walker, P.S.; Scharton-Kersten, T.; Rowton, E.D.; Hengge, U.; Bouloc, A.; Udey, M.C.; Vogel, J.C. Genetic immunization with glycoprotein 63 cDNA results in a helper T cell type 1 immune response and protection in a murine model of leishmaniasis. Hum. Gene Ther. 1998, 9, 1899–1907. [Google Scholar] [CrossRef]
- Lopez-Fuertes, L.; Perez-Jimenez, E.; Vila-Coro, A.J.; Sack, F.; Moreno, S.; Konig, S.A.; Junghans, C.; Wittig, B.; Timon, M.; Esteban, M. DNA vaccination with linear minimalistic (MIDGE) vectors confers protection against Leishmania major infection in mice. Vaccine 2002, 21, 247–257. [Google Scholar] [CrossRef]
- Marques-da-Silva, E.A.; Coelho, E.A.; Gomes, D.C.; Vilela, M.C.; Masioli, C.Z.; Tavares, C.A.; Fernandes, A.P.; Afonso, L.C.; Rezende, S.A. Intramuscular immunization with p36(LACK) DNA vaccine induces IFN-gamma production but does not protect BALB/c mice against Leishmania chagasi intravenous challenge. Parasitol. Res. 2005, 98, 67–74. [Google Scholar] [CrossRef]
- Melby, P.C.; Yang, J.; Zhao, W.; Perez, L.E.; Cheng, J. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 2001, 69, 4719–4725. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Samant, M. DNA vaccine against visceral leishmaniasis: A promising approach for prevention and control. Parasite Immunol. 2016, 38, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, R.M.; del Real, G.; Rodriguez, J.R.; Rodriguez, D.; Heljasvaara, R.; Lucas, P.; Larraga, V.; Esteban, M. A heterologous prime-boost regime using DNA and recombinant vaccinia virus expressing the Leishmania infantum P36/LACK antigen protects BALB/c mice from cutaneous leishmaniasis. Vaccine 2002, 20, 1226–1231. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A.; Shahbazi, S. Prime-boost vaccine strategy against viral infections: Mechanisms and benefits. Vaccine 2016, 34, 413–423. [Google Scholar] [CrossRef]
- Ramiro, M.J.; Zarate, J.J.; Hanke, T.; Rodriguez, D.; Rodriguez, J.R.; Esteban, M.; Lucientes, J.; Castillo, J.A.; Larraga, V. Protection in dogs against visceral leishmaniasis caused by Leishmania infantum is achieved by immunization with a heterologous prime-boost regime using DNA and vaccinia recombinant vectors expressing LACK. Vaccine 2003, 21, 2474–2484. [Google Scholar] [CrossRef]
- Ramos, I.; Alonso, A.; Marcen, J.M.; Peris, A.; Castillo, J.A.; Colmenares, M.; Larraga, V. Heterologous prime-boost vaccination with a non-replicative vaccinia recombinant vector expressing LACK confers protection against canine visceral leishmaniasis with a predominant Th1-specific immune response. Vaccine 2008, 26, 333–344. [Google Scholar] [CrossRef]
- Osman, M.; Mistry, A.; Keding, A.; Gabe, R.; Cook, E.; Forrester, S.; Wiggins, R.; Di Marco, S.; Colloca, S.; Siani, L.; et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017, 11, e0005527. [Google Scholar] [CrossRef]
- Younis, B.M.; Osman, M.; Khalil, E.A.G.; Santoro, F.; Furini, S.; Wiggins, R.; Keding, A.; Carraro, M.; Musa, A.E.A.; Abdarahaman, M.A.A.; et al. Safety and immunogenicity of ChAd63-KH vaccine in post-kala-azar dermal leishmaniasis patients in Sudan. Mol. Ther. 2021, 29, 2366–2377. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines-How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Gannavaram, S.; Dey, R.; Avishek, K.; Selvapandiyan, A.; Salotra, P.; Nakhasi, H.L. Biomarkers of safety and immune protection for genetically modified live attenuated leishmania vaccines against visceral leishmaniasis—Discovery and implications. Front. Immunol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Silvestre, R.; Cordeiro-da-Silva, A.; Ouaissi, A. Live attenuated Leishmania vaccines: A potential strategic alternative. Arch. Immunol. Ther. Exp. 2008, 56, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Peacock, C.S.; Seeger, K.; Harris, D.; Murphy, L.; Ruiz, J.C.; Quail, M.A.; Peters, N.; Adlem, E.; Tivey, A.; Aslett, M.; et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat. Genet. 2007, 39, 839–847. [Google Scholar] [CrossRef]
- Daneshvar, H.; Coombs, G.H.; Hagan, P.; Phillips, R.S. Leishmania mexicana and Leishmania major: Attenuation of wild-type parasites and vaccination with the attenuated lines. J. Infect. Dis. 2003, 187, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F.; Handman, E.; Spithill, T.W. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Aust. J. Exp. Biol. Med. Sci. 1984, 62 Pt 2, 145–153. [Google Scholar] [CrossRef]
- Gorczynski, R.M. Immunization of susceptible BALB/c mice against Leishmania braziliensis. II. Use of temperature-sensitive avirulent clones of parasite for vaccination purposes. Cell. Immunol. 1985, 94, 11–20. [Google Scholar] [CrossRef]
- Rivier, D.; Shah, R.; Bovay, P.; Mauel, J. Vaccine development against cutaneous leishmaniasis. Subcutaneous administration of radioattenuated parasites protects CBA mice against virulent Leishmania major challenge. Parasite Immunol. 1993, 15, 75–84. [Google Scholar] [CrossRef]
- Kimsey, P.B.; Theodos, C.M.; Mitchen, T.K.; Turco, S.J.; Titus, R.G. An avirulent lipophosphoglycan-deficient Leishmania major clone induces CD4+ T cells which protect susceptible BALB/c mice against infection with virulent L. major. Infect. Immun. 1993, 61, 5205–5213. [Google Scholar] [CrossRef]
- Papadopoulou, B.; Roy, G.; Breton, M.; Kundig, C.; Dumas, C.; Fillion, I.; Singh, A.K.; Olivier, M.; Ouellette, M. Reduced infectivity of a Leishmania donovani biopterin transporter genetic mutant and its use as an attenuated strain for vaccination. Infect. Immun. 2002, 70, 62–68. [Google Scholar] [CrossRef]
- Zhang, W.W.; Matlashewski, G. Characterization of the A2-A2rel gene cluster in Leishmania donovani: Involvement of A2 in visceralization during infection. Mol. Microbiol. 2001, 39, 935–948. [Google Scholar] [CrossRef]
- Silvestre, R.; Cordeiro-Da-Silva, A.; Santarem, N.; Vergnes, B.; Sereno, D.; Ouaissi, A. SIR2-deficient Leishmania infantum induces a defined IFN-gamma/IL-10 pattern that correlates with protection. J. Immunol. 2007, 179, 3161–3170. [Google Scholar] [CrossRef]
- Carrion, J.; Folgueira, C.; Soto, M.; Fresno, M.; Requena, J.M. Leishmania infantum HSP70-II null mutant as candidate vaccine against leishmaniasis: A preliminary evaluation. Parasit. Vectors 2011, 4, 150. [Google Scholar] [CrossRef] [PubMed]
- Santi, A.M.M.; Lanza, J.S.; Tunes, L.G.; Fiuza, J.A.; Roy, G.; Orfano, A.D.S.; de Carvalho, A.T.; Frezard, F.; Barros, A.L.B.; Murta, S.M.F.; et al. Growth arrested live-attenuated Leishmania infantum KHARON1 null mutants display cytokinesis defect and protective immunity in mice. Sci. Rep. 2018, 8, 11627. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Natarajan, G.; Bhattacharya, P.; Cummings, H.; Dagur, P.K.; Terrazas, C.; Selvapandiyan, A.; McCoy, J.P.; Duncan, R.; Satoskar, A.R.; et al. Characterization of cross-protection by genetically modified live-attenuated Leishmania donovani parasites against Leishmania mexicana. J. Immunol. 2014, 193, 3513–3527. [Google Scholar] [CrossRef]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.C.; Kumar, A.; Samant, M. Genetically modified live attenuated vaccine: A potential strategy to combat visceral leishmaniasis. Parasite Immunol. 2020, 42, e12732. [Google Scholar] [CrossRef]
- Zabala-Penafiel, A.; Todd, D.; Daneshvar, H.; Burchmore, R. The potential of live attenuated vaccines against Cutaneous Leishmaniasis. Exp. Parasitol. 2020, 210, 107849. [Google Scholar] [CrossRef]
- Titus, R.G.; Gueiros-Filho, F.J.; de Freitas, L.A.; Beverley, S.M. Development of a safe live Leishmania vaccine line by gene replacement. Proc. Natl. Acad. Sci. USA 1995, 92, 10267–10271. [Google Scholar] [CrossRef]
- Amaral, V.F.; Teva, A.; Oliveira-Neto, M.P.; Silva, A.J.; Pereira, M.S.; Cupolillo, E.; Porrozzi, R.; Coutinho, S.G.; Pirmez, C.; Beverley, S.M.; et al. Study of the safety, immunogenicity and efficacy of attenuated and killed Leishmania (Leishmania) major vaccines in a rhesus monkey (Macaca mulatta) model of the human disease. Mem. Inst. Oswaldo Cruz. 2002, 97, 1041–1048. [Google Scholar] [CrossRef]
- Saravia, N.G.; Escorcia, B.; Osorio, Y.; Valderrama, L.; Brooks, D.; Arteaga, L.; Coombs, G.; Mottram, J.; Travi, B.L. Pathogenicity and protective immunogenicity of cysteine proteinase-deficient mutants of Leishmania mexicana in non-murine models. Vaccine 2006, 24, 4247–4259. [Google Scholar] [CrossRef]
- Alexander, J.; Coombs, G.H.; Mottram, J.C. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 1998, 161, 6794–6801. [Google Scholar]
- Uzonna, J.E.; Spath, G.F.; Beverley, S.M.; Scott, P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J. Immunol. 2004, 172, 3793–3797. [Google Scholar] [CrossRef] [PubMed]
- Rivier, D.; Bovay, P.; Shah, R.; Didisheim, S.; Mauel, J. Vaccination against Leishmania major in a CBA mouse model of infection: Role of adjuvants and mechanism of protection. Parasite Immunol. 1999, 21, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Handman, E. Leishmaniasis: Current status of vaccine development. Clin. Microbiol. Rev. 2001, 14, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Janse, C.J.; Kappe, S.H.; Mikolajczak, S.A. Genetic engineering of attenuated malaria parasites for vaccination. Curr. Opin. Biotechnol. 2012, 23, 908–916. [Google Scholar] [CrossRef]
- Keitany, G.J.; Sack, B.; Smithers, H.; Chen, L.; Jang, I.K.; Sebastian, L.; Gupta, M.; Sather, D.N.; Vignali, M.; Vaughan, A.M.; et al. Immunization of mice with live-attenuated late liver stage-arresting Plasmodium yoelii parasites generates protective antibody responses to preerythrocytic stages of malaria. Infect. Immun. 2014, 82, 5143–5153. [Google Scholar] [CrossRef]
- Mikolajczak, S.A.; Lakshmanan, V.; Fishbaugher, M.; Camargo, N.; Harupa, A.; Kaushansky, A.; Douglass, A.N.; Baldwin, M.; Healer, J.; O’Neill, M.; et al. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol. Ther. 2014, 22, 1707–1715. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Sack, B.K.; Dankwa, D.; Minkah, N.; Nguyen, T.; Cardamone, H.; Kappe, S.H.I. A Plasmodium Parasite with Complete Late Liver Stage Arrest Protects against Preerythrocytic and Erythrocytic Stage Infection in Mice. Infect. Immun. 2018, 86, e00088-18. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Duncan, R.; Debrabant, A.; Bertholet, S.; Sreenivas, G.; Negi, N.S.; Salotra, P.; Nakhasi, H.L. Expression of a mutant form of Leishmania donovani centrin reduces the growth of the parasite. J. Biol. Chem. 2001, 276, 43253–43261. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Debrabant, A.; Duncan, R.; Muller, J.; Salotra, P.; Sreenivas, G.; Salisbury, J.L.; Nakhasi, H.L. Centrin gene disruption impairs stage-specific basal body duplication and cell cycle progression in Leishmania. J. Biol. Chem. 2004, 279, 25703–25710. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Dey, R.; Gannavaram, S.; Solanki, S.; Salotra, P.; Nakhasi, H.L. Generation of growth arrested Leishmania amastigotes: A tool to develop live attenuated vaccine candidates against visceral leishmaniasis. Vaccine 2014, 32, 3895–3901. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Dey, R.; Nylen, S.; Duncan, R.; Sacks, D.; Nakhasi, H.L. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J. Immunol. 2009, 183, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bhattacharya, P.; Dagur, P.K.; Karmakar, S.; Ismail, N.; Joshi, A.B.; Akue, A.D.; KuKuruga, M.; McCoy, J.P.; Dey, R.; et al. Live Attenuated Leishmania donovani Centrin Gene-Deleted Parasites Induce IL-23-Dependent IL-17-Protective Immune Response against Visceral Leishmaniasis in a Murine Model. J. Immunol. 2018, 200, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Gannavaram, S.; Ismail, N.; Kaul, A.; Gedda, M.R.; Nakhasi, H.L. Centrin-Deleted Leishmania donovani Parasites Help CD4+ T Cells to Acquire Th1 Phenotype and Multi-Functionality Through Downregulation of CD200-CD200R Immune Inhibitory Axis. Front. Immunol. 2018, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Dey, R.; Dagur, P.K.; Joshi, A.B.; Ismail, N.; Gannavaram, S.; Debrabant, A.; Akue, A.D.; KuKuruga, M.A.; Selvapandiyan, A.; et al. Live Attenuated Leishmania donovani Centrin Knock Out Parasites Generate Non-inferior Protective Immune Response in Aged Mice against Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2016, 10, e0004963. [Google Scholar] [CrossRef]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell. Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.D.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef]
- Bogdan, C. Macrophages as host, effector and immunoregulatory cells in leishmaniasis: Impact of tissue micro-environment and metabolism. Cytokine X 2020, 2, 100041. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dey, R.; Dagur, P.K.; Kruhlak, M.; Ismail, N.; Debrabant, A.; Joshi, A.B.; Akue, A.; Kukuruga, M.; Takeda, K.; et al. Genetically Modified Live Attenuated Leishmania donovani Parasites Induce Innate Immunity through Classical Activation of Macrophages That Direct the Th1 Response in Mice. Infect. Immun. 2015, 83, 3800–3815. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dey, R.; Saxena, A.; Karmakar, S.; Ismail, N.; Gannavaram, S.; Dagur, P.K.; Satoskar, M.; Satoskar, S.; De Paoli, S.; et al. Essential Role of Neutrophils in the Protective Immune Response Induced by a Live Attenuated. J. Immunol. 2020, 205, 3333–3347. [Google Scholar] [CrossRef]
- Ribeiro-Gomes, F.L.; Sacks, D. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front. Cell. Infect. Microbiol. 2012, 2, 59. [Google Scholar] [CrossRef]
- Singh, O.P.; Hasker, E.; Sacks, D.; Boelaert, M.; Sundar, S. Asymptomatic Leishmania infection: A new challenge for Leishmania control. Clin. Infect. Dis. 2014, 58, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Kaul, A.; Bhattacharya, P.; Gannavaram, S.; Nakhasi, H.L. Immunization with Live Attenuated Leishmania donovani Centrin-/- Parasites Is Efficacious in Asymptomatic Infection. Front. Immunol. 2017, 8, 1788. [Google Scholar] [CrossRef] [PubMed]
- WHO. Post-Kala-Azar dermal leishmaniasis: A manual for case management and control. In Proceedings of the Report of a WHO Consultative Meeting, Kolkata, India, 2–3 July 2012; WHO/HTM/NTD/IDM/2013.1. p. 29. [Google Scholar]
- Gedda, M.R.; Singh, B.; Kumar, D.; Singh, A.K.; Madhukar, P.; Upadhyay, S.; Singh, O.P.; Sundar, S. Post kala-azar dermal leishmaniasis: A threat to elimination program. PLoS Negl. Trop. Dis. 2020, 14, e0008221. [Google Scholar] [CrossRef] [PubMed]
- Avishek, K.; Kaushal, H.; Gannavaram, S.; Dey, R.; Selvapandiyan, A.; Ramesh, V.; Negi, N.S.; Dubey, U.S.; Nakhasi, H.L.; Salotra, P. Gene deleted live attenuated Leishmania vaccine candidates against visceral leishmaniasis elicit pro-inflammatory cytokines response in human PBMCs. Sci. Rep. 2016, 6, 33059. [Google Scholar] [CrossRef]
- Fiuza, J.A.; Santiago, H.A.C.; Selvapandiyan, A.; Gannavaram, S.; Ricci, N.D.; Bueno, L.L.; Bartholomeu, D.C.; Correa-Oliveira, R.; Nakhasi, H.L.; Fujiwara, R.T. Induction of immunogenicity by live attenuated Leishmania donovani centrin deleted parasites in dogs. Vaccine 2013, 31, 1785–1792. [Google Scholar] [CrossRef][Green Version]
- Fiuza, J.A.; Gannavaram, S.; Santiago, H.A.C.; Selvapandiyan, A.; Souza, D.M.; Passos, L.S.; de Mendonça, L.Z.; Lemos-Giunchetti, D.A.S.; Ricci, N.D.; Bartholomeu, D.C.; et al. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine 2015, 33, 280–288. [Google Scholar] [CrossRef]
- Viana, K.F.; Fiuza, J.A.; Gannavaram, S.; Dey, R.; Selvapandiyan, A.; Bartholomeu, D.C.; da Silveira-Lemos, D.; Bueno, L.L.; Dutra, W.O.; Fujiwara, R.T.; et al. Application of rapid in vitro co-culture system of macrophages and T-cell subsets to assess the immunogenicity of dogs vaccinated with live attenuated Leishmania donovani centrin deleted parasites (LdCen-/-). Parasit. Vectors 2016, 9, 250. [Google Scholar] [CrossRef]
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef]
- Srivastava, P.; Dayama, A.; Mehrotra, S.; Sundar, S. Diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 1–6. [Google Scholar] [CrossRef]
- Volpedo, G.; Pacheco-Fernandez, T.; Holcomb, E.A.; Cipriano, N.; Cox, B.; Satoskar, A.R. Mechanisms of Immunopathogenesis in Cutaneous Leishmaniasis And Post Kala-azar Dermal Leishmaniasis (PKDL). Front. Cell. Infect. Microbiol. 2021, 11, 685296. [Google Scholar] [CrossRef]
- Karmakar, S.; Ismail, N.; Oliveira, F.; Oristian, J.; Zhang, W.W.; Kaviraj, S.; Singh, K.P.; Mondal, A.; Das, S.; Pandey, K.; et al. Preclinical validation of a live attenuated dermotropic Leishmania vaccine against vector transmitted fatal visceral leishmaniasis. Commun. Biol. 2021, 4, 929. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Pacheco-Fernandez, T.; Holcomb, E.A.; Zhang, W.W.; Lypaczewski, P.; Cox, B.; Fultz, R.; Mishan, C.; Verma, C.; Huston, R.H.; et al. Centrin-deficient Leishmania mexicana confers protection against New World cutaneous leishmaniasis. NPJ Vaccines 2022, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Avendaño Rangel, F.; Reis-Cunha, J.L.; Marques, L.P.; Figueira, C.P.; Borba, P.B.; Viana, S.M.; Beneke, T.; Bartholomeu, D.C.; de Oliveira, C.I. Targeted Deletion of Centrin in Leishmania braziliensis Using CRISPR-Cas9-Based Editing. Front. Cell. Infect. Microbiol. 2021, 11, 790418. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Van Hoeven, N.; MacMillen, Z.; Picone, A.; Mohamath, R.; Erasmus, J.; Hsu, F.C.; Stinchcomb, D.T.; Reed, S.G. Heterologous Immunization with Defined RNA and Subunit Vaccines Enhances T Cell Responses That Protect against Leishmania donovani. Front. Immunol. 2018, 9, 2420. [Google Scholar] [CrossRef]
- Ashwin, H.; Sadlova, J.; Vojtkova, B.; Becvar, T.; Lypaczewski, P.; Schwartz, E.; Greensted, E.; Van Bocxlaer, K.; Pasin, M.; Lipinski, K.S.; et al. Characterization of a new Leishmania major strain for use in a controlled human infection model. Nat. Commun. 2021, 12, 215. [Google Scholar] [CrossRef]
- Cooper, M.M.; Loiseau, C.; McCarthy, J.S.; Doolan, D.L. Human challenge models: Tools to accelerate the development of malaria vaccines. Expert Rev. Vaccines 2019, 18, 241–251. [Google Scholar] [CrossRef]
- Ismail, N.; Karmakar, S.; Bhattacharya, P.; Takeda, K.; Hamano, S.; Matlashewski, G.; Satoskar, A.R.; Gannavaram, S.; Dey, R.; Nakhasi, H.L. Leishmania major centrin gene deleted parasites generate skin resident memory T cell immune response analogous to leishmanization. Front. Immunol. 2022. [Google Scholar] [CrossRef]
- Valian, H.K.; Rostami, M.N.; Tasbihi, M.; Mohammadi, A.M.; Eskandari, S.E.; Sarrafnejad, A.; Khamesipour, A. CCR7+ central and CCR7− effector memory CD4+ T cells in human cutaneous leishmaniasis. J. Clin. Immunol. 2013, 33, 220–234. [Google Scholar] [CrossRef]
- Pacheco-Fernandez, T.; Volpedo, G.; Gannavaram, S.; Bhattacharya, P.; Dey, R.; Satoskar, A.; Matlashewski, G.; Nakhasi, H.L. Revival of Leishmanization and Leishmanin. Front. Cell. Infect. Microbiol. 2021, 11, 639801. [Google Scholar] [CrossRef]
- Malvolti, S.; Malhame, M.; Mantel, C.F.; Le Rutte, E.A.; Kaye, P.M. Human leishmaniasis vaccines: Use cases, target population and potential global demand. PLoS Negl. Trop. Dis. 2021, 15, e0009742. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpedo, G.; Bhattacharya, P.; Gannavaram, S.; Pacheco-Fernandez, T.; Oljuskin, T.; Dey, R.; Satoskar, A.R.; Nakhasi, H.L. The History of Live Attenuated Centrin Gene-Deleted Leishmania Vaccine Candidates. Pathogens 2022, 11, 431. https://doi.org/10.3390/pathogens11040431
Volpedo G, Bhattacharya P, Gannavaram S, Pacheco-Fernandez T, Oljuskin T, Dey R, Satoskar AR, Nakhasi HL. The History of Live Attenuated Centrin Gene-Deleted Leishmania Vaccine Candidates. Pathogens. 2022; 11(4):431. https://doi.org/10.3390/pathogens11040431
Chicago/Turabian StyleVolpedo, Greta, Parna Bhattacharya, Sreenivas Gannavaram, Thalia Pacheco-Fernandez, Timur Oljuskin, Ranadhir Dey, Abhay R. Satoskar, and Hira L. Nakhasi. 2022. "The History of Live Attenuated Centrin Gene-Deleted Leishmania Vaccine Candidates" Pathogens 11, no. 4: 431. https://doi.org/10.3390/pathogens11040431
APA StyleVolpedo, G., Bhattacharya, P., Gannavaram, S., Pacheco-Fernandez, T., Oljuskin, T., Dey, R., Satoskar, A. R., & Nakhasi, H. L. (2022). The History of Live Attenuated Centrin Gene-Deleted Leishmania Vaccine Candidates. Pathogens, 11(4), 431. https://doi.org/10.3390/pathogens11040431






