The European Badger as a New Host for Dirofilaria immitis and an Update on the Distribution of the Heartworm in Wild Carnivores from Romania
Abstract
:1. Introduction
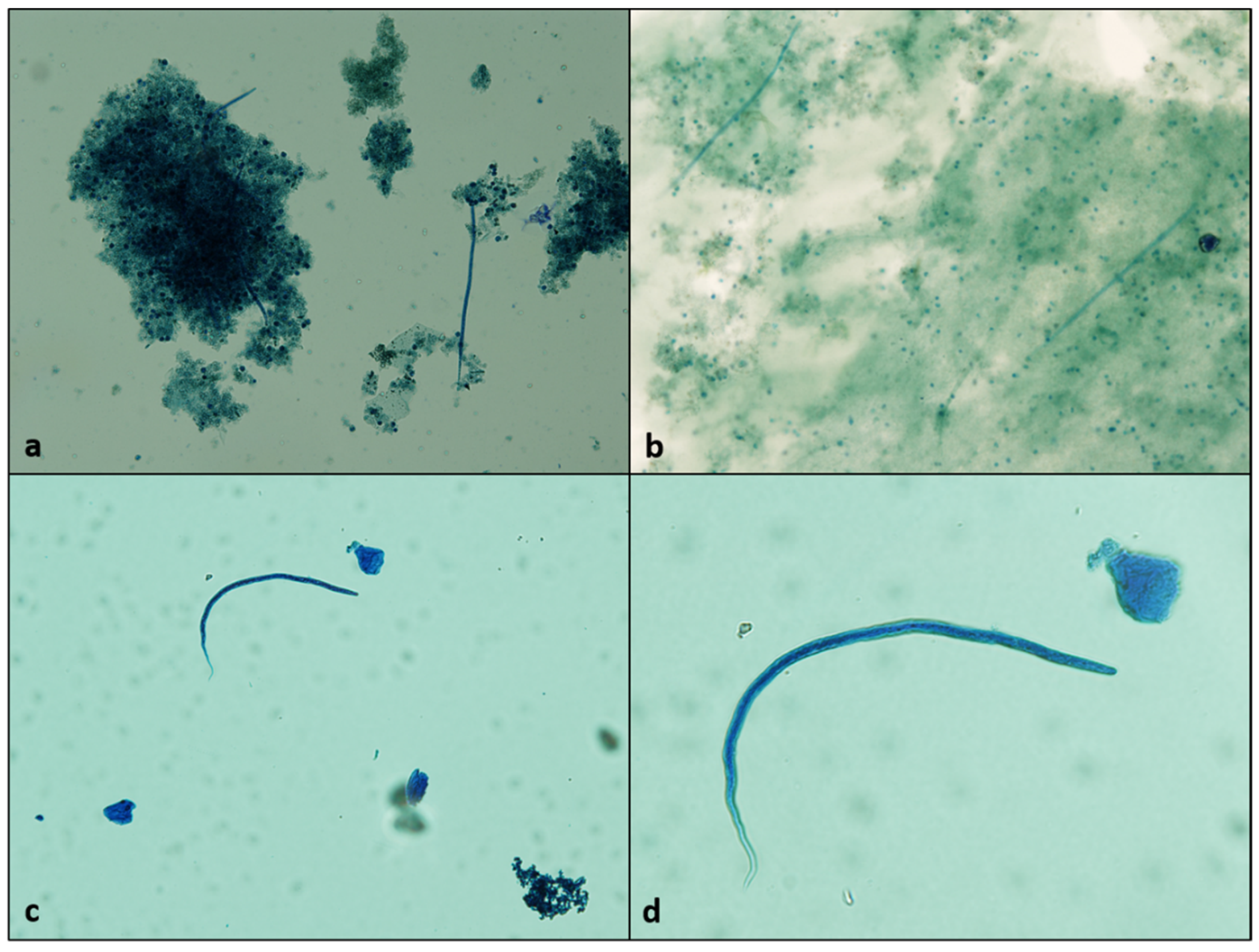
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otranto, D.; Deplazes, P. Zoonotic nematodes of wild carnivores. IJP Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Cancrini, G.; Scaramozzino, P.; Gabrielli, S.; Paolo, M.; Di Toma, L.; Romi, R. Aedes albopictus and Culex pipiens implicated as natural vectors of Dirofilaria repens in Central Italy. J. Med. Entomol. 2007, 44, 1064–1066. [Google Scholar] [PubMed]
- Capelli, G.; Frangipane di Regalbono, A.; Simonato, G.; Cassini, R.; Cazzin, S.; Cancrini, G.; Otranto, D.; Pietrobelli, M. Risk of canine and human exposure to Dirofilaria immitis infected mosquitoes in endemic areas of Italy. Parasit. Vectors 2013, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- McCall, J.W.; Genchi, C.; Kramer, L.H.; Guerrero, J.; Venco, L. Heartworm disease in animals and humans. Adv. Parasitol. 2008, 66, 193–285. [Google Scholar]
- Lan, J.; Fu, Y.; Yang, Z.; Zhang, Z.; Wang, C.; Luo, L.; Liu, L.; Gu, X.; Wang, S.; Peng, X.; et al. Treatment and prevention of natural heartworm (Dirofilaria immitis) infections in red pandas (Ailurus fulgens) with selamectin and ivermectin. Parasitol. Int. 2012, 61, 372–374. [Google Scholar] [CrossRef]
- Alho, A.M.; Marcelino, I.; Colella, V.; Flanagan, C.; Silva, N.; Correia, J.J.; Latrofa, M.S.; Otranto, D.; Madeira de Carvalho, L. Dirofilaria immitis in pinnipeds and a new host record. Parasit. Vectors 2017, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Otranto, D.; Cantacessi, C.; Dantas-Torres, F.; Brianti, E.; Pfeffer, M.; Genchi, C.; Guberti, V.; Capelli, G.; Deplazes, P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Vet. Parasitol. 2015, 213, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Mircean, V.; Dumitrache, M.O.; Györke, A.; Pantchev, N.; Jodies, R.; Mihalca, A.D.; Cozma, V. Seroprevalence and geographic distribution of Dirofilaria immitis and and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis) in dogs from Romania. Vector Borne Zoonotic Dis. 2012, 12, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Ionică, A.M.; Matei, I.A.; Mircean, V.; Dumitrache, M.O.; D’Amico, G.; Győrke, A.; Pantchev, N.; Annoscia, G.; Albrechtová, K.; Otranto, D.; et al. Current surveys on the prevalence and distribution of Dirofilaria spp. and Acanthocheilonema reconditum infections in dogs in Romania. Parasitol. Res. 2015, 114, 975–982. [Google Scholar] [CrossRef]
- Ciucă, L.; Genchi, M.; Kramer, L.; Mangia, C.; Miron, L.D.; Prete, L.D.; Maurelli, M.P.; Cringoli, G.; Rinaldi, L. Heat treatment of serum samples from stray dogs naturally exposed to Dirofilaria immitis and Dirofilaria repens in Romania. Vet. Parasitol. 2016, 225, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Ionică, A.M.; Matei, I.A.; D’Amico, G.; Daskalaki, A.A.; Juránková, J.; Ionescu, D.T.; Mihalca, A.D.; Modrý, D.; Gherman, C.M. Role of golden jackals (Canis aureus) as natural reservoirs of Dirofilaria spp. in Romania. Parasit. Vectors 2016, 9, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionică, A.M.; Matei, I.A.; D’Amico, G.; Ababii, J.; Daskalaki, A.A.; Sándor, A.D.; Enache, D.V.; Gherman, C.M.; Mihalca, A.D. Filarioid infections in wild carnivores: A multispecies survey in Romania. Parasit. Vectors 2017, 10, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penezić, A.; Selaković, S.; Pavlović, I.; Ćirović, D. First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia. Parasitol. Res. 2014, 113, 3281–3285. [Google Scholar] [CrossRef] [PubMed]
- Tolnai, Z.; Széll, Z.; Sproch, Á.; Szeredi, L.; Sréter, T. Dirofilaria immitis: An emerging parasite in dogs, red foxes and golden jackals in Hungary. Vet. Parasitol. 2014, 203, 339–342. [Google Scholar] [CrossRef]
- Georgieva, D.; Kirkova, Z.; Ivanov, A. A study on the incidence and diagnosis of dirofilariosis (heartworm disease) in carnivores. Bulg. J. Vet. Med. 2001, 4, 231–236. [Google Scholar]
- Kirkova, Z.; Ivanov, A.; Georgieva, D. Dirofilariosis in dogs and wild carnivores in Bulgaria. In Mappe Parassitologiche 8-Dirofilaria Immitis and D. repens in Dog and Cat and Human Infections; Genchi, C., Rinaldi, L., Cringoli, G., Eds.; Rolando Editore: Naples, Italy, 2007; p. 204. [Google Scholar]
- Panayotova-Pencheva, M.S.; Mirchev, R.L.; Trifinova, A.P. Dirofilaria immitis infection in carnivores from Bulgaria: 2012–2013 update. Bulg. J. Vet. Med. 2016, 19, 153–162. [Google Scholar] [CrossRef]
- Mañas, S.; Ferrer, D.; Castellà, J.; López-Martín, J.M. Cardiopulmonary helminth parasites of red foxes (Vulpes vulpes) in Catalonia, northeastern Spain. Vet. J. 2005, 169, 118–120. [Google Scholar] [CrossRef]
- Marconcini, A.; Magi, M.; Macchioni, G.; Sassetti, M. Filariosis in foxes in Italy. Vet. Res. Comm. 1996, 20, 316–319. [Google Scholar] [CrossRef]
- Magi, M.; Calderini, P.; Gabrielli, S.; Dell’Omodarme, M.; Macchioni, F.; Prati, M.C.; Cancrini, G. Vulpes vulpes: A possible wild reservoir for zoonotic filariae. Vector Borne Zoonotic Dis. 2008, 8, 249–252. [Google Scholar] [CrossRef]
- Kauhala, K.; Kowalczyk, R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: History of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011, 57, 584–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kido, N.; Wada, Y.; Takahashi, M.; Kamegaya, C.; Omiya, T.; Yamamoto, Y. Prevalence of Dirofilaria immitis infection in living raccoon dogs assessed by hematological examination. J. Vet. Med. Sci. 2011, 73, 845–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.Y.; Kim, J.T.; Yang, D.K.; Hyun, C. Prevalence of Dirofilaria immitis in Raccoon Dogs (Nyctereutes procyonoides) in Korea. J. Vet. Clin. 2013, 30, 453–455. [Google Scholar]
- Nakagaki, K.; Yoshida, M.; Nogami, S.; Nakagaki, K. Experimental infection of Dirofilaria immitis in raccoon dogs. J. Parasitol. 2007, 93, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Gherman, C.M.; Ionică, A.M.; Deak, G.; Chişamera, G.B.; Mihalca, A.D. Co-infection with Angiostrongylus chabaudi and Dirofilaria immitis in a wildcat, Felis silvestris from Romania—A case report. Acta Vet. Brno 2019, 88, 303–306. [Google Scholar] [CrossRef]
- Deak, G.; Ionică, A.M.; Pop, A.R.; Mihalca, A.D.; Gherman, C.M. New insights into the distribution of cardio-pulmonary nematodes in road killed wild felids from Romania. Parasit. Vectors, 2022; submitted. [Google Scholar]
- Campbell, W.C.; Blair, L.S. Dirofilaria immitis: Experimental infections in the ferret (Mustela putorius furo). J. Parasitol. 1978, 64, 119–122. [Google Scholar] [CrossRef]
- Torres, J.; Feliu, C.; Fernández-Morán, J.; Ruíz-Olmo, J.; Rosoux, R.; Santos Reis, M.; Miquel, J.; Fons, R. Helminth parasites of the Eurasian otter Lutra lutra in southwest Europe. J. Helminthol. 2004, 78, 353–359. [Google Scholar] [CrossRef]
- Saraiva, A.L.; Sousa, S.; Silva, J.; Andrade, S.; Botelho, N.; Canavarro, I.; Costa, M.; Ferreira, F.; Meier, K.; Silva, J.A.; et al. Dirofilaria immitis in an Eurasian Otter (Lutra lutra). J. Comp. Pathol. 2013, 148, 88. [Google Scholar] [CrossRef]
- Penezić, A.; Moriano, R.; Spasić, M.; Ćirović, D. First report of a naturally patent infection with Dirofilaria immitis in an otter (Lutra lutra). Parasitol. Res. 2018, 117, 929–931. [Google Scholar] [CrossRef]
- Anderson, R.C.; Bain, O. Keys to genera of the order Spirurida. In Keys to Nematode Parasites of Vertebrates; Anderson, R.C., Chabaud, A.G., Willmott, S., Eds.; Commonwealth Agricultural Bureau: Wallingford, CT, USA, 1976; pp. 59–116. [Google Scholar]
- Furtado, A.P.; Melo, F.T.V.; Giese, E.G.; dos Santos, J.N. Morphological redescription of Dirofilaria immitis. J. Parasitol. 2010, 96, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Magnis, J.; Lorentz, S.; Guardone, L.; Grimm, F.; Magi, M.; Naucke, T.J.; Deplazes, P. Morphometric analyses of canine blood microfilariae isolated by the Knott’s test enables Dirofilaria immitis and D. repens species-specific and Acanthocheilonema (syn. Dipetalonema) genus-specific diagnosis. Parasit. Vectors 2013, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rishniw, M.; Barr, S.C.; Simpson, K.W.; Frongillo, M.F.; Franz, M.; Dominguez Alpizar, J.L. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet. Parasitol. 2006, 135, 303–314. [Google Scholar] [CrossRef] [PubMed]

| Family | Species | Examined | Dirofilaria immitis | ||
|---|---|---|---|---|---|
| n | % | 95% CI | |||
| Canidae | Canis aureus | 63 | 12 | 19.05 | 10.25–30.91 |
| Canis lupus | 8 | 0 | 0 | 0–36.94 | |
| Nyctereutes procyonoides | 1 | 1 | 100 | 2.50–100 | |
| Vulpes vulpes | 60 | 4 | 6.67 | 1.85–16.20 | |
| Total | 132 | 17 | 12.88 | 7.68–19.82 | |
| Felidae | Felis silvestris | 43 | 2 | 4.65 | 0.57–15.81 |
| Lynx lynx | 5 | 0 | 0 | 0–52.18 | |
| Total | 48 | 2 | 4.17 | 0.51–14.25 | |
| Mustelidae | Meles meles | 115 | 1 | 0.87 | 0.02–4.75 |
| Mustela putorius | 80 | 0 | 0 | 0–4.51 | |
| Martes foina | 36 | 0 | 0 | 0–9.74 | |
| Lutra lutra | 21 | 0 | 0 | 0–16.11 | |
| Martes martes | 5 | 0 | 0 | 0–52.18 | |
| Mustela nivalis | 3 | 0 | 0 | 0–70.76 | |
| Mustela erminea | 1 | 0 | 0 | 0–97.50 | |
| Mustela lutreola | 1 | 0 | 0 | 0–97.50 | |
| Mustela eversmanii | 1 | 0 | 0 | 0–97.50 | |
| Vormela peregusna | 1 | 0 | 0 | 0–97.50 | |
| Total | 264 | 1 | 0.38 | 0.01–2.09 | |
| Ursidae | Ursus arctos | 15 | 0 | 0 | 0–21.80 |
| Total | 459 | 20 | 4.36 | 2.84–6.63 | |
| No. | Species | Region | Sex | Age | Dirofilaria immitis | |||
|---|---|---|---|---|---|---|---|---|
| F | M | Microfilariae | PCR | |||||
| 1 | Canis aureus | West | M | Juvenile | 1 | 1 | Negative | Negative |
| 2 | Canis aureus | South | F | Adult | 1 | 2 | Positive | Positive |
| 3 | Canis aureus | South | F | Adult | 1 | 2 | Negative | Positive |
| 4 | Canis aureus | Southeast | M | Adult | 2 | 1 | Negative | Negative |
| 5 | Canis aureus | South | F | Adult | 2 | 1 | Negative | Negative |
| 6 | Canis aureus | South | M | Adult | 4 | 1 | Negative | Negative |
| 7 | Canis aureus | South | F | Juvenile | 6 | 1 | Negative | Positive |
| 8 | Canis aureus | Southwest | F | Adult | 1 | 0 | Negative | Negative |
| 9 | Canis aureus | South | M | Adult | 1 | 0 | Negative | Negative |
| 10 | Canis aureus | Southeast | M | not recorded | 5 | 0 | Negative | Negative |
| 11 | Canis aureus | South | M | Adult | 0 | 1 | Negative | Negative |
| 12 | Canis aureus | Southeast | M | Juvenile | 0 | 1 | Negative | Negative |
| 13 | Vulpes vulpes | South | M | Adult | 1 | 1 | Positive | Positive |
| 14 | Vulpes vulpes | South | F | Juvenile | 2 | 0 | Negative | Negative |
| 15 | Vulpes vulpes | South | F | not recorded | 0 | 1 | Negative | Negative |
| 16 | Vulpes vulpes | South | M | Juvenile | 0 | 1 | Negative | Negative |
| 17 | Nyctereutes procyonoides | Southeast | F | Adult | 1 | 0 | Negative | Negative |
| 18 | Felis silvestris | Southeast | M | Adult | 0 | 1 | Negative | Negative |
| 19 | Felis silvestris | South | F | Adult | 0 | 1 | Negative | Negative |
| 20 | Meles meles | South | M | Adult | 1 | 2 | Positive | Positive |
| Species | Examined | Region | ||||||
|---|---|---|---|---|---|---|---|---|
| SE | S | SW | W | NW | C | NE | ||
| Canidae | ||||||||
| Canis aureus | 63 | 28 | 19 | 11 | 3 | 1 | 1 | - |
| Canis lupus | 8 | - | - | - | 2 | 1 | 5 | - |
| Nyctereutes procyonoides | 1 | 1 | - | - | - | - | - | - |
| Vulpes vulpes | 60 | 8 | 42 | - | 2 | 6 | 2 | - |
| Felidae | ||||||||
| Felis silvestris | 43 | 8 | 2 | - | 5 | 22 | 5 | 1 |
| Lynx lynx | 5 | - | - | 1 | - | 3 | 1 | - |
| Mustelidae | ||||||||
| Meles meles | 115 | 3 | 6 | 1 | 12 | 75 | 18 | - |
| Mustela putorius | 80 | 16 | 60 | 1 | - | 1 | 2 | - |
| Martes foina | 36 | 9 | 4 | 1 | - | 20 | 1 | 1 |
| Lutra lutra | 21 | 7 | 7 | - | 3 | 1 | 1 | 2 |
| Martes martes | 5 | - | - | - | - | 5 | - | - |
| Mustela nivalis | 3 | - | 1 | - | 1 | 1 | - | - |
| Mustela erminea | 1 | - | - | - | - | 1 | - | - |
| Mustela lutreola | 1 | 1 | - | - | - | - | - | - |
| Mustela eversmanii | 1 | - | 1 | - | - | - | - | - |
| Vormela peregusna | 1 | 1 | - | - | - | - | - | - |
| Ursidae | ||||||||
| Ursus arctos | 15 | - | - | - | - | 2 | 13 | - |
| Total | 459 | 82 | 142 | 15 | 28 | 139 | 49 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionică, A.M.; Deak, G.; Boncea, R.; Gherman, C.M.; Mihalca, A.D. The European Badger as a New Host for Dirofilaria immitis and an Update on the Distribution of the Heartworm in Wild Carnivores from Romania. Pathogens 2022, 11, 420. https://doi.org/10.3390/pathogens11040420
Ionică AM, Deak G, Boncea R, Gherman CM, Mihalca AD. The European Badger as a New Host for Dirofilaria immitis and an Update on the Distribution of the Heartworm in Wild Carnivores from Romania. Pathogens. 2022; 11(4):420. https://doi.org/10.3390/pathogens11040420
Chicago/Turabian StyleIonică, Angela Monica, Georgiana Deak, Radu Boncea, Călin Mircea Gherman, and Andrei Daniel Mihalca. 2022. "The European Badger as a New Host for Dirofilaria immitis and an Update on the Distribution of the Heartworm in Wild Carnivores from Romania" Pathogens 11, no. 4: 420. https://doi.org/10.3390/pathogens11040420
APA StyleIonică, A. M., Deak, G., Boncea, R., Gherman, C. M., & Mihalca, A. D. (2022). The European Badger as a New Host for Dirofilaria immitis and an Update on the Distribution of the Heartworm in Wild Carnivores from Romania. Pathogens, 11(4), 420. https://doi.org/10.3390/pathogens11040420









