Proteomic Profiling and In Silico Characterization of the Secretome of Anisakis simplex Sensu Stricto L3 Larvae
Abstract
1. Introduction
2. Results
2.1. Mass Spectrometry-Based Identification
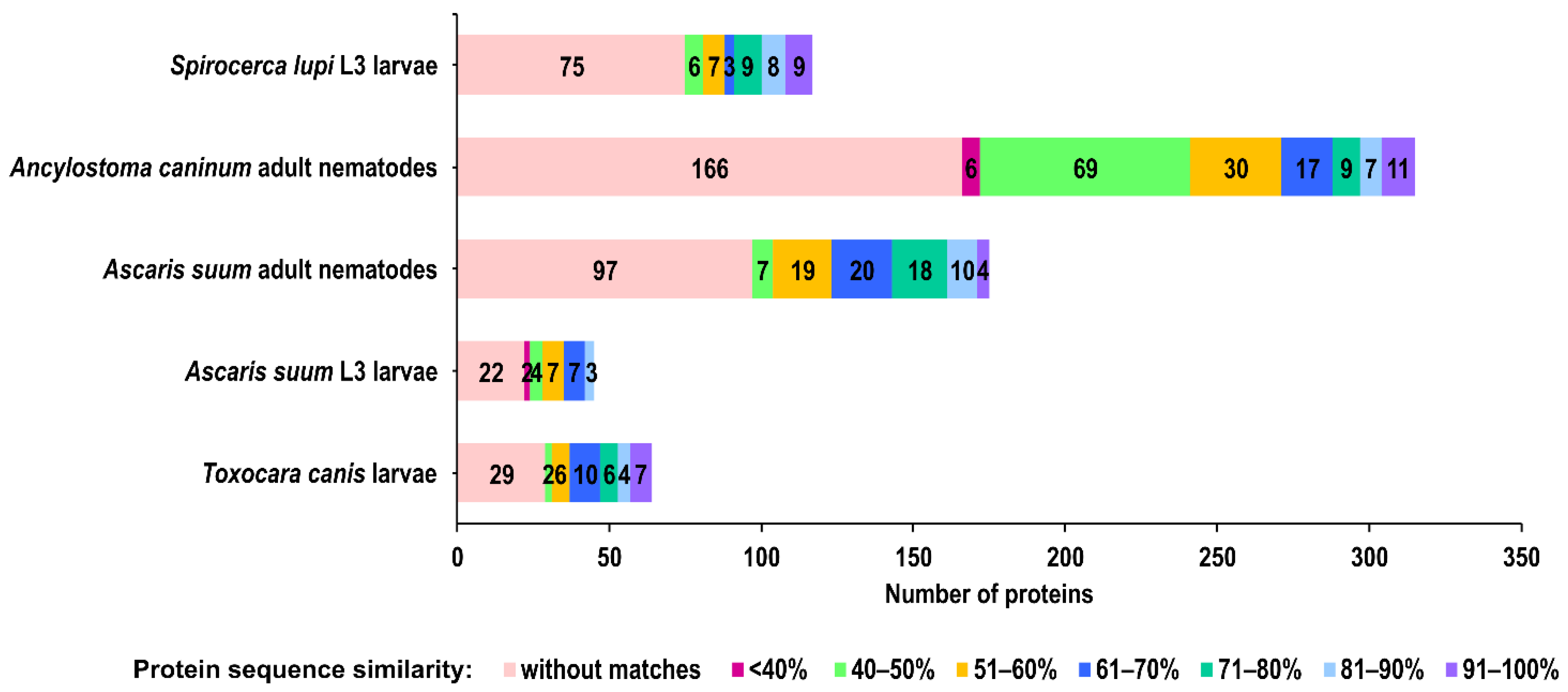
2.2. Comparison of A. simplex Secretome Proteins with Secretome Proteins of Other Nematodes
2.3. Protein Family Classification
2.4. Secretory Pathway Prediction
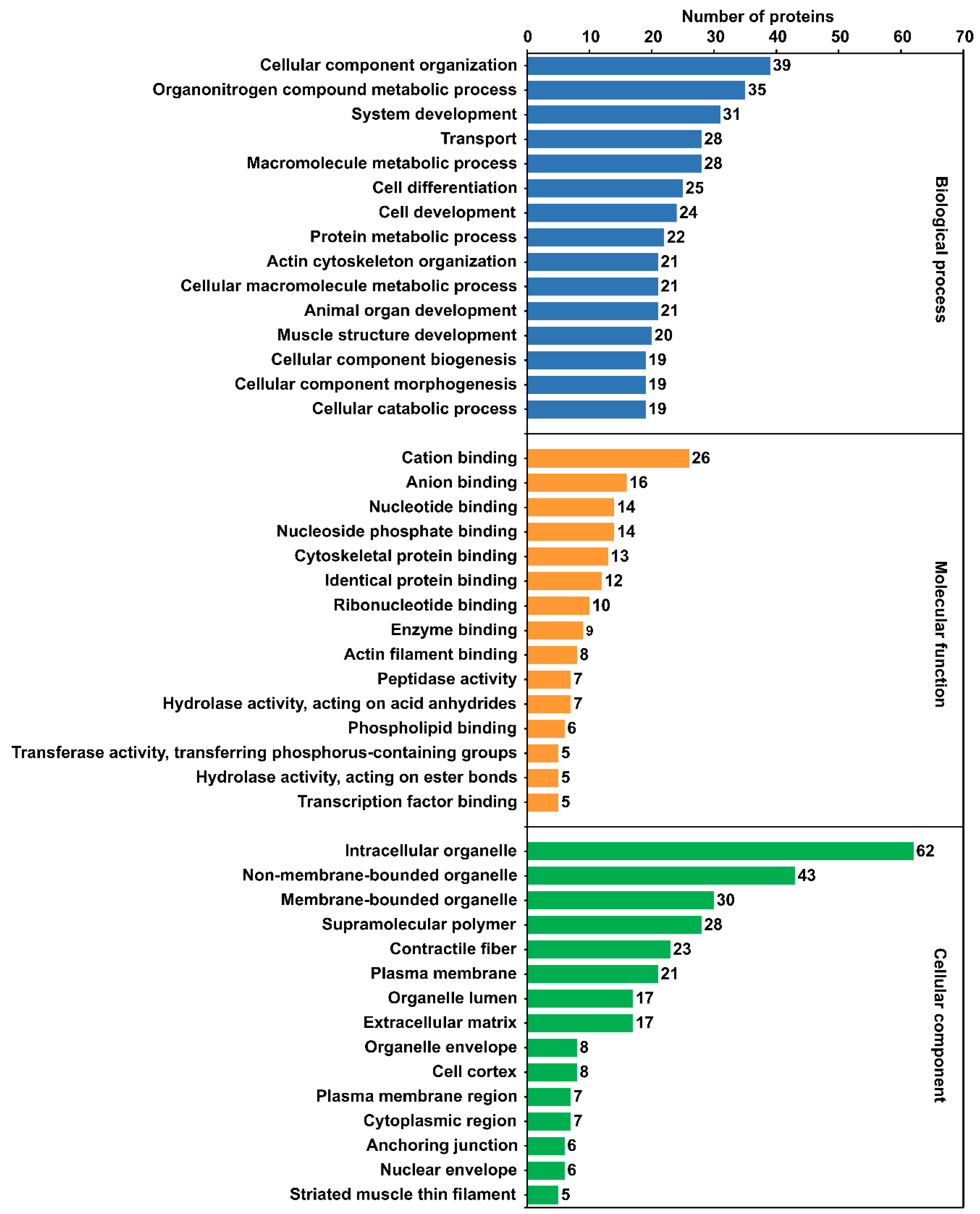
2.5. Gene Ontology (GO) Annotation and Enrichment Analysis
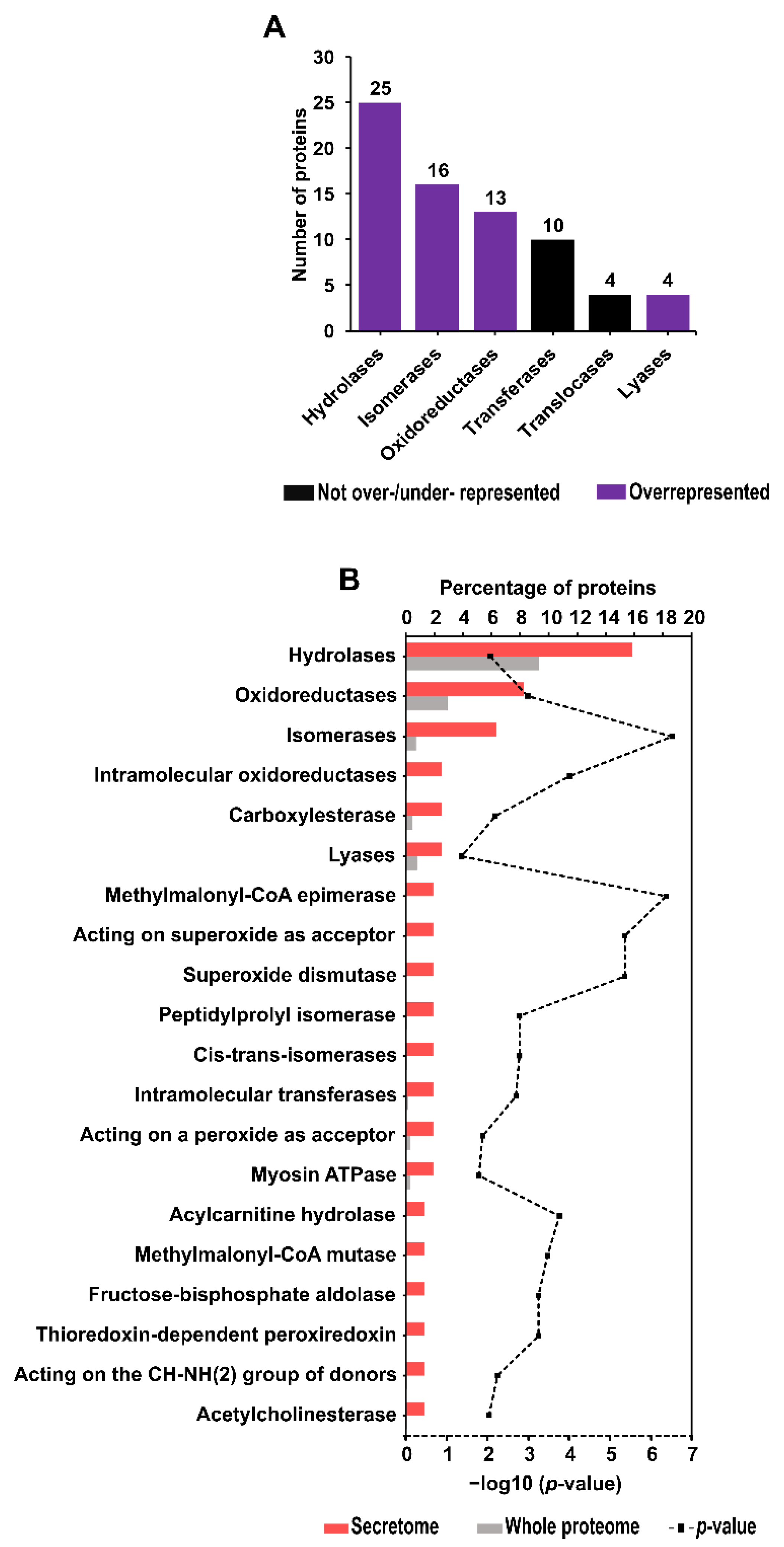
2.6. Enzyme Identification and Enrichment Analysis
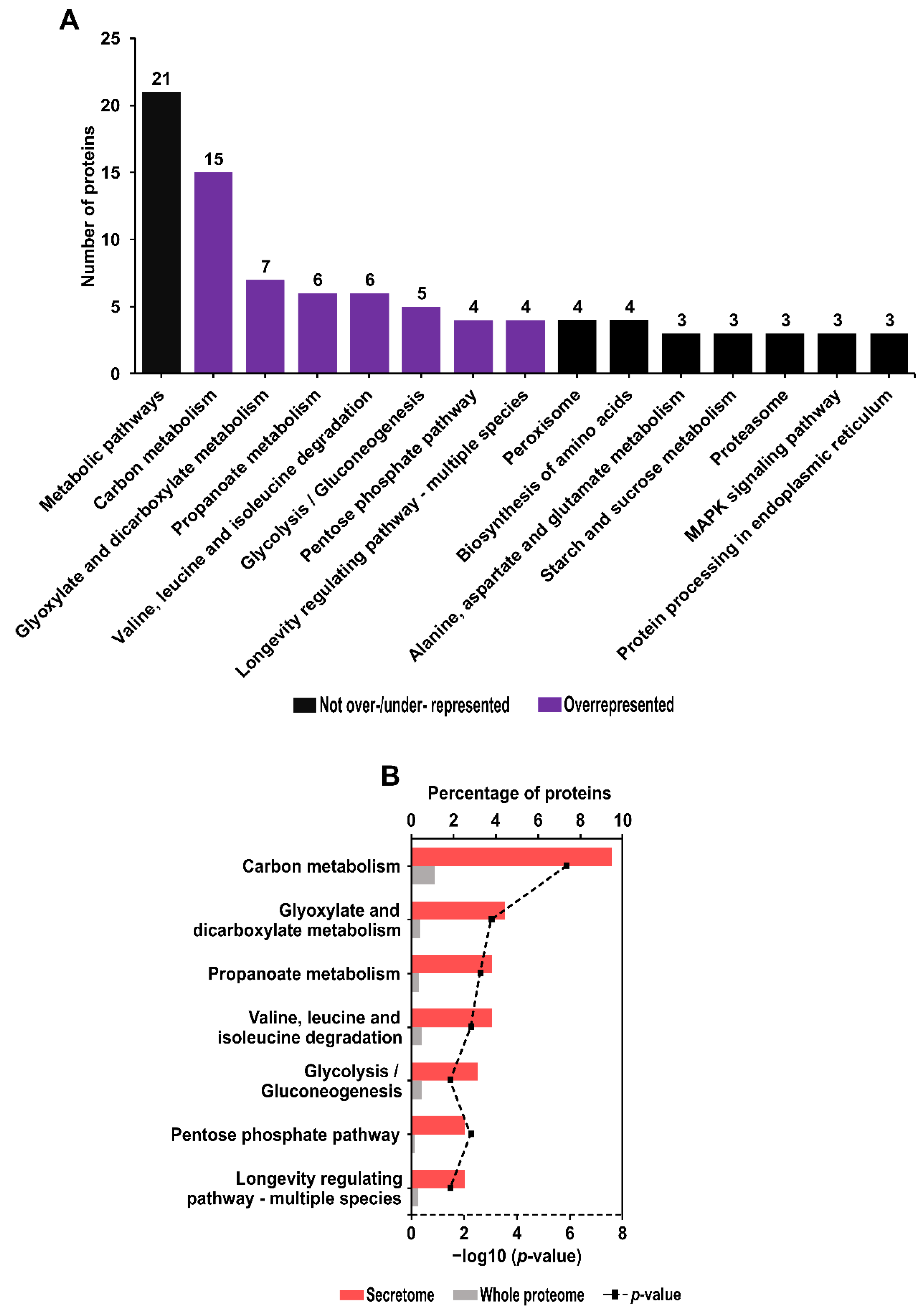
2.7. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Identification and Enrichment Analysis
2.8. Identification of Essential Proteins
2.9. Identification of Potential Pathogenicity-Related Proteins
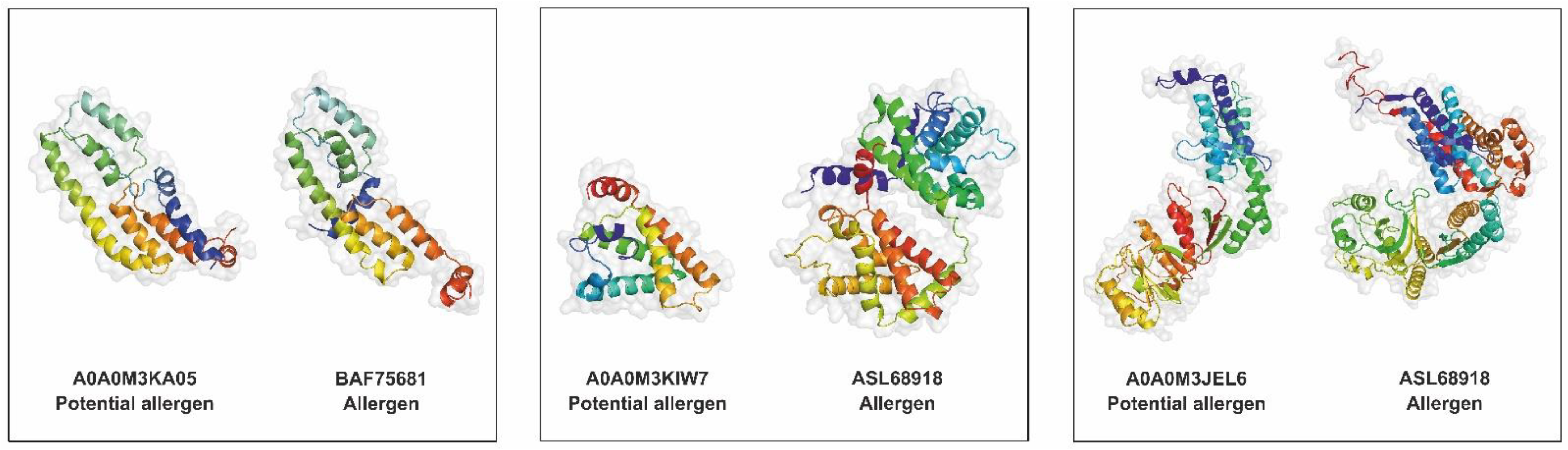
2.10. Allergen and Potential Allergen Identification
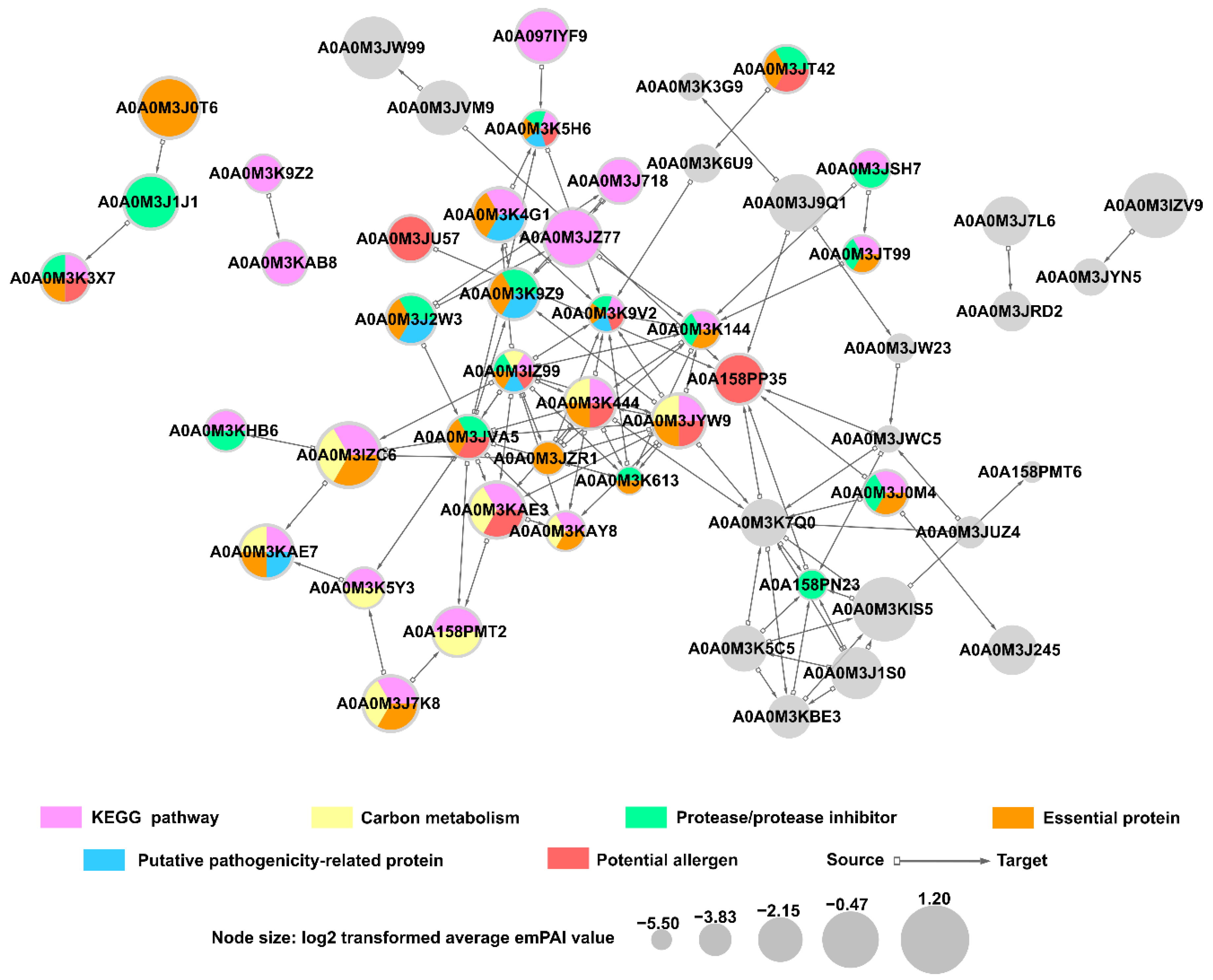
2.11. Predicted Protein-Protein Interactions in A. simplex (s.s.) Secretome
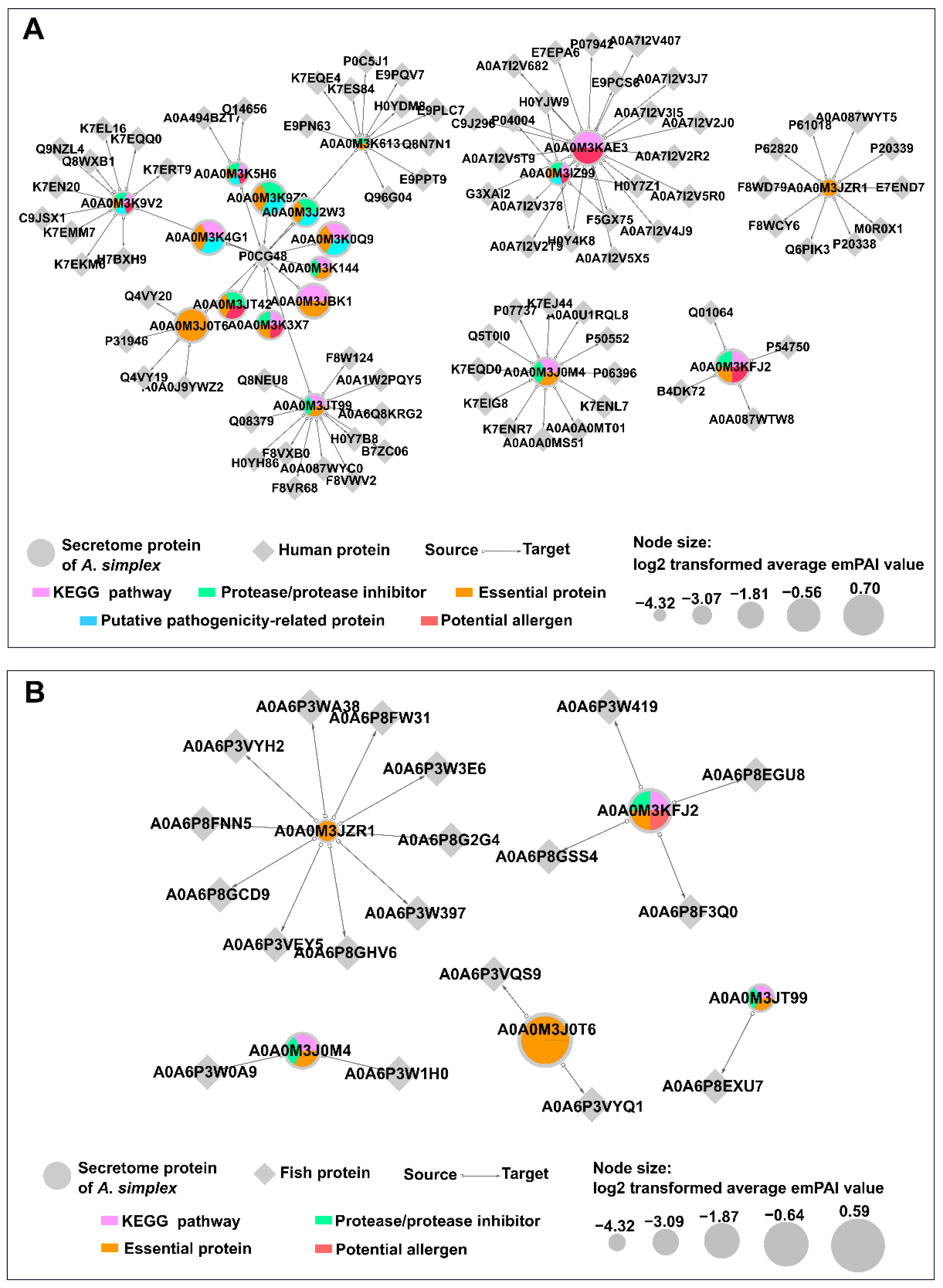
2.12. Predicted Host-Parasite Protein Interactions
3. Discussion
4. Materials and Methods
4.1. A. simplex (s.s.) L3 Larvae Collection and Identification
4.2. ES Proteins Preparation
4.3. SDS-PAGE
4.4. Sample Processing and LC-MS/MS Analysis
4.5. Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattiucci, S.; Cipriani, P.; Levsen, A.; Paoletti, M.; Nascetti, G. Molecular epidemiology of Anisakis and anisakiasis: An ecological and evolutionary road map. Adv. Parasitol. 2018, 99, 93–263. [Google Scholar] [PubMed]
- Palomba, M.; Mattiucci, S.; Crocetta, F.; Osca, D.; Santoro, M. Insights into the role of deep-sea squids of the genus Histioteuthis (Histioteuthidae) in the life cycle of ascaridoid parasites in the Central Mediterranean Sea waters. Sci. Rep. 2021, 11, 7135. [Google Scholar] [CrossRef]
- Kent, A.J.; Pert, C.C.; Briers, R.A.; Diele, K.; Rueckert, S. Increasing intensities of Anisakis simplex third-stage larvae (L3) in Atlantic salmon of coastal waters of Scotland. Parasites Vectors 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Levsen, A.; Cipriani, P.; Mattiucci, S.; Gay, M.; Hastie, L.C.; MacKenzie, K.; Pierce, G.J.; Svanevik, C.S.; Højgaard, D.P.; Nascetti, G. Anisakis species composition and infection characteristics in Atlantic mackerel, Scomber scombrus, from major European fishing grounds—reflecting changing fish host distribution and migration pattern. Fish. Res. 2018, 202, 112–121. [Google Scholar] [CrossRef]
- Santoro, M.; Palomba, M.; Mattiucci, S.; Osca, D.; Crocetta, F. New parasite records for the sunfish Mola mola in the Mediterranean Sea and their potential use as biological tags for long-distance host migration. Front. Vet. Sci. 2020, 7, 579728. [Google Scholar] [CrossRef] [PubMed]
- Orphanet. Prevalence and Incidence of Rare Diseases. Prevalence and Incidence of Rare Diseases: Bibliographic Data. 2021. Available online: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf (accessed on 2 November 2021).
- Bao, M.; Pierce, G.J.; Strachan, N.J.; Pascual, S.; González-Muñoz, M.; Levsen, A. Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: Challenges in risk assessment. Trends Food Sci. Technol. 2019, 86, 298–310. [Google Scholar] [CrossRef]
- Rahmati, A.R.; Kiani, B.; Afshari, A.; Moghaddas, E.; Williams, M.; Shamsi, S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: A systematic review. Parasitol. Res. 2020, 119, 3585–3594. [Google Scholar] [CrossRef]
- Mladineo, I.; Trumbić, Ž.; Radonić, I.; Vrbatović, A.; Hrabar, J.; Bušelić, I. Anisakis simplex complex: Ecological significance of recombinant genotypes in an allopatric area of the Adriatic Sea inferred by genome-derived simple sequence repeats. Int. J. Parasitol. 2017, 47, 215–223. [Google Scholar] [CrossRef]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef]
- Daschner, A.; Alonso-Gómez, A.; Cabañas, R.; Suarez-de-Parga, J.-M.; López-Serrano, M.-C. Gastroallergic anisakiasis: Borderline between food allergy and parasitic disease—Clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J. Allergy Clin. Immunol. 2000, 105, 176–181. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.E. Anisakis—Immunology of a foodborne parasitosis. Parasite Immunol. 2016, 38, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Kochanowski, M.; Różycki, M.; Dąbrowska, J.; Bełcik, A.; Karamon, J.; Sroka, J.; Cencek, T. Proteomic and bioinformatic investigations of heat-treated Anisakis simplex third-stage larvae. Biomolecules 2020, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Audicana, M.A.T.; Ansotegui, I.J.; de Corres, L.F.; Kennedy, M.W. Anisakis simplex: Dangerous—Dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef]
- Pravettoni, V.; Primavesi, L.; Piantanida, M. Anisakis simplex: Current knowledge. Eur Ann. Allergy Clin. Immunol. 2012, 44, 150. [Google Scholar]
- Sánchez-Alonso, I.; Carballeda-Sangiao, N.; Gonzalez-Munoz, M.; Navas, A.; Arcos, S.C.; Mendizábal, A.; Tejada, M.; Careche, M. Pathogenic potential of Anisakis L3 after freezing in domestic freezers. Food Control 2018, 84, 61–69. [Google Scholar] [CrossRef]
- Romero, M.C.; Valero, A.; Navarro, M.C.; Hierro, I.; Barón, S.D.; Martín-Sánchez, J. Experimental demonstration of pathogenic potential of Anisakis physeteris and Anisakis paggiae in Wistar rats. Parasitol. Res. 2014, 113, 4377–4386. [Google Scholar] [CrossRef]
- Jeon, C.-H.; Kim, J.-H. Pathogenic potential of two sibling species, Anisakis simplex (s.s.) and Anisakis pegreffii (Nematoda: Anisakidae): In vitro and in vivo studies. BioMed Res. Int. 2015, 2015, 983656. [Google Scholar] [CrossRef]
- Napoletano, C.; Mattiucci, S.; Colantoni, A.; Battisti, F.; Zizzari, I.G.; Rahimi, H.; Nuti, M.; Rughetti, A. Anisakis pegreffii impacts differentiation and function of human dendritic cells. Parasite Immunol. 2018, 40, e12527. [Google Scholar] [CrossRef]
- Palomba, M.; Cipriani, P.; Giulietti, L.; Levsen, A.; Nascetti, G.; Mattiucci, S. Differences in gene expression profiles of seven target proteins in third-stage larvae of Anisakis simplex (sensu stricto) by sites of infection in blue whiting (Micromesistius poutassou). Genes 2020, 11, 559. [Google Scholar] [CrossRef]
- Zamora, V.; Andreu-Ballester, J.C.; Rodero, M.; Cuéllar, C. Anisakis simplex: Immunomodulatory effects of larval antigens on the activation of Toll like Receptors. Int. Immunopharmacol. 2021, 100, 108120. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Perneel, J.; Vieri, M.K.; Colebunders, R.; Kumar-Singh, S. The Secretome of Filarial Nematodes and Its Role in Host-Parasite Interactions and Pathogenicity in Onchocerciasis-Associated Epilepsy. Front. Cell. Infect. Microbiol. 2021, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.B.; Oviedo, Y.; Cooper, P.J.; Pacheco, L.G.; Pinheiro, C.S.; Ferreira, F.; Briza, P.; Alcantara-Neves, N.M. The somatic proteins of Toxocara canis larvae and excretory-secretory products revealed by proteomics. Vet. Parasitol. 2018, 259, 25–34. [Google Scholar] [CrossRef]
- Wang, T.; Van Steendam, K.; Dhaenens, M.; Vlaminck, J.; Deforce, D.; Jex, A.R.; Gasser, R.B.; Geldhof, P. Proteomic analysis of the excretory-secretory products from larval stages of Ascaris suum reveals high abundance of glycosyl hydrolases. PLoS Negl. Trop. Dis. 2013, 7, e2467. [Google Scholar] [CrossRef] [PubMed]
- Morante, T.; Shepherd, C.; Constantinoiu, C.; Loukas, A.; Sotillo, J. Revisiting the Ancylostoma caninum secretome provides new information on hookworm–host interactions. Proteomics 2017, 17, 1700186. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Baneth, G. Secretome of the carcinogenic helminth Spirocerca lupi reveals specific parasite proteins associated with its different life stages. Vet. Parasitol. 2019, 275, 108935. [Google Scholar] [CrossRef]
- Boysen, A.T.; Whitehead, B.; Stensballe, A.; Carnerup, A.; Nylander, T.; Nejsum, P. Fluorescent labeling of helminth extracellular vesicles using an in vivo whole organism approach. Biomedicines 2020, 8, 213. [Google Scholar] [CrossRef]
- Hansen, E.P.; Fromm, B.; Andersen, S.D.; Marcilla, A.; Andersen, K.L.; Borup, A.; Williams, A.R.; Jex, A.R.; Gasser, R.B.; Young, N.D. Exploration of extracellular vesicles from Ascaris suum provides evidence of parasite–host cross talk. J. Extracell. Vesicles 2019, 8, 1578116. [Google Scholar] [CrossRef]
- Harischandra, H.; Yuan, W.; Loghry, H.J.; Zamanian, M.; Kimber, M.J. Profiling extracellular vesicle release by the filarial nematode Brugia malayi reveals sex-specific differences in cargo and a sensitivity to ivermectin. PLoS Negl. Trop. Dis. 2018, 12, e0006438. [Google Scholar] [CrossRef]
- Eichenberger, R.M.; Ryan, S.; Jones, L.; Buitrago, G.; Polster, R.; Montes de Oca, M.; Zuvelek, J.; Giacomin, P.R.; Dent, L.A.; Engwerda, C.R. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front. Immunol. 2018, 9, 850. [Google Scholar] [CrossRef]
- Stryiński, R.; Mateos, J.; Pascual, S.; González, Á.F.; Gallardo, J.M.; Łopieńska-Biernat, E.; Medina, I.; Carrera, M. Proteome profiling of L3 and L4 Anisakis simplex development stages by TMT-based quantitative proteomics. J. Proteom. 2019, 201, 1–11. [Google Scholar] [CrossRef]
- Arcos, S.C.; Ciordia, S.; Roberston, L.; Zapico, I.; Jiménez-Ruiz, Y.; Gonzalez-Muñoz, M.; Moneo, I.; Carballeda-Sangiao, N.; Rodriguez-Mahillo, A.; Albar, J.P. Proteomic profiling and characterization of differential allergens in the nematodes Anisakis simplex sensu stricto and A. pegreffii. Proteomics 2014, 14, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Kochanowski, M.; Dąbrowska, J.; Różycki, M.; Karamon, J.; Sroka, J.; Cencek, T. Proteomic profiling reveals new insights into the allergomes of Anisakis simplex, Pseudoterranova decipiens, and Contracaecum osculatum. J. Parasitol. 2020, 106, 572–588. [Google Scholar] [CrossRef] [PubMed]
- Marzano, V.; Pane, S.; Foglietta, G.; Levi Mortera, S.; Vernocchi, P.; Onetti Muda, A.; Putignani, L. Mass Spectrometry Based-Proteomic Analysis of Anisakis spp.: A Preliminary Study towards a New Diagnostic Tool. Genes 2020, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Arcos, S.C.; Robertson, L.; Ciordia, S.; Sánchez-Alonso, I.; Careche, M.; Carballeda-Sanguiao, N.; Gonzalez-Muñoz, M.; Navas, A. Quantitative Proteomics Comparison of Total Expressed Proteomes of Anisakis simplex Sensu Stricto, A. pegreffii, and Their Hybrid Genotype. Genes 2020, 11, 913. [Google Scholar] [CrossRef]
- Polak, I.; Łopieńska-Biernat, E.; Stryiński, R.; Mateos, J.; Carrera, M. Comparative proteomics analysis of Anisakis simplex s.s—Evaluation of the response of invasive larvae to ivermectin. Genes 2020, 11, 710. [Google Scholar] [CrossRef]
- Fæste, C.K.; Jonscher, K.R.; Dooper, M.M.; Egge-Jacobsen, W.; Moen, A.; Daschner, A.; Egaas, E.; Christians, U. Characterisation of potential novel allergens in the fish parasite Anisakis simplex. EuPA Open Proteom. 2014, 4, 140–155. [Google Scholar] [CrossRef]
- Robertson, L.; Arcos, S.C.; Ciordia, S.; Carballeda-Sanguiao, N.; Mena, M.d.C.; Sánchez-Alonso, I.; Gonzalez-Muñoz, M.; Careche, M.; Navas, A. Immunoreactive Proteins in the Esophageal Gland Cells of Anisakis Simplex Sensu Stricto Detected by MALDI-TOF/TOF Analysis. Genes 2020, 11, 683. [Google Scholar] [CrossRef]
- Geary, J.; Satti, M.; Moreno, Y.; Madrill, N.; Whitten, D.; Headley, S.A.; Agnew, D.; Geary, T.; Mackenzie, C. First analysis of the secretome of the canine heartworm, Dirofilaria immitis. Parasites Vectors 2012, 5, 140. [Google Scholar] [CrossRef]
- Maeda, Y.; Palomares-Rius, J.E.; Hino, A.; Afrin, T.; Mondal, S.I.; Nakatake, A.; Maruyama, H.; Kikuchi, T. Secretome analysis of Strongyloides venezuelensis parasitic stages reveals that soluble and insoluble proteins are involved in its parasitism. Parasites Vectors 2019, 12, 21. [Google Scholar] [CrossRef]
- Song, H.; He, X.; Du, X.; Hua, R.; Xu, J.; He, R.; Xie, Y.; Gu, X.; Peng, X.; Yang, G. Molecular characterization and expression analysis of annexin B3 and B38 as secretory proteins in Echinococcus granulosus. Parasites Vectors 2021, 14, 21. [Google Scholar] [CrossRef]
- Song, X.; Hu, D.; Zhong, X.; Wang, N.; Gu, X.; Wang, T.; Peng, X.; Yang, G. Characterization of a secretory annexin in Echinococcus granulosus. Am. J. Trop. Med. Hyg. 2016, 94, 626. [Google Scholar] [CrossRef]
- Hofmann, A.; Osman, A.; Leow, C.Y.; Driguez, P.; McManus, D.P.; Jones, M.K. Parasite annexins–new molecules with potential for drug and vaccine development. Bioessays 2010, 32, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Ditgen, D.; Anandarajah, E.M.; Hansmann, J.; Winter, D.; Schramm, G.; Erttmann, K.D.; Liebau, E.; Brattig, N.W. Multifunctional thioredoxin-like protein from the gastrointestinal parasitic nematodes Strongyloides ratti and Trichuris suis affects mucosal homeostasis. J. Parasitol. Res. 2016, 2016, 8421597. [Google Scholar] [CrossRef] [PubMed]
- Fierro-González, J.C.; González-Barrios, M.; Miranda-Vizuete, A.; Swoboda, P. The thioredoxin TRX-1 regulates adult lifespan extension induced by dietary restriction in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2011, 406, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Bertini, R.; Zack Howard, O.; Dong, H.-F.; Oppenheim, J.J.; Bizzarri, C.; Sergi, R.; Caselli, G.; Pagliei, S.; Romines, B.; Wilshire, J.A. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 1999, 189, 1783–1789. [Google Scholar] [CrossRef]
- Cuesta-Astroz, Y.; de Oliveira, F.S.; Nahum, L.A.; Oliveira, G. Helminth secretomes reflect different lifestyles and parasitized hosts. Int. J. Parasitol. 2017, 47, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Fuentes, S.; Morales-Ruiz, V.; López-Recinos, D.; Guevara-Salinas, A.; Arce-Sillas, A.; Rodríguez, J.; Parada-Colin, C.; Adalid-Peralta, L. Biological role of excretory–secretory proteins in endemic parasites of Latin America and the Caribbean. J. Helminthol. 2020, 94, e53. [Google Scholar] [CrossRef] [PubMed]
- Młocicki, D.; Sulima, A.; Bień, J.; Näreaho, A.; Zawistowska-Deniziak, A.; Basałaj, K.; Sałamatin, R.; Conn, D.B.; Savijoki, K. Immunoproteomics and surfaceomics of the adult tapeworm Hymenolepis diminuta. Front. Immunol. 2018, 9, 2487. [Google Scholar] [CrossRef]
- Jeffery, C.J. Protein moonlighting: What is it, and why is it important? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160523. [Google Scholar] [CrossRef]
- Vanhamme, L.; Souopgui, J.; Ghogomu, S.; Ngale Njume, F. The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products. Pathogens 2020, 9, 975. [Google Scholar] [CrossRef]
- Bahlool, Q.Z.; Skovgaard, A.; Kania, P.W.; Buchmann, K. Effects of excretory/secretory products from Anisakis simplex (Nematoda) on immune gene expression in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 35, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, J.-O.; Jeon, C.-H.; Nam, U.-H.; Subramaniyam, S.; Yoo, S.-I.; Park, J.-H. Comparative transcriptome analyses of the third and fourth stage larvae of Anisakis simplex (Nematoda: Anisakidae). Mol. Biochem. Parasitol. 2018, 226, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Molina-Fernández, D.; Benítez, R.; Adroher, F.J.; Malagón, D. Differential proteolytic activity in Anisakis simplex s.s. and Anisakis pegreffii, two sibling species from the complex Anisakis simplex s.l., major etiological agents of anisakiasis. Acta Trop. 2019, 195, 44–50. [Google Scholar] [CrossRef]
- Yang, Y.; jun Wen, Y.; Cai, Y.N.; Vallée, I.; Boireau, P.; Liu, M.Y.; Cheng, S.P. Serine proteases of parasitic helminths. Korean J. Parasitol. 2015, 53, 1. [Google Scholar] [CrossRef] [PubMed]
- Haffner, A.; Guilavogui, A.Z.; Tischendorf, F.W.; Brattig, N.W. Onchocerca volvulus: Microfilariae secrete elastinolytic and males nonelastinolytic matrix-degrading serine and metalloproteases. Exp. Parasitol. 1998, 90, 26–33. [Google Scholar] [CrossRef]
- McKerrow, J.H.; Caffrey, C.; Kelly, B.; Loke, P.n.; Sajid, M. Proteases in parasitic diseases. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 497–536. [Google Scholar] [CrossRef]
- Britton, C.; Murray, L. A cathepsin L protease essential for Caenorhabditis elegans embryogenesis is functionally conserved in parasitic nematodes. Mol. Biochem. Parasitol. 2002, 122, 21–33. [Google Scholar] [CrossRef]
- Jolodar, A.; Fischer, P.; Büttner, D.W.; Miller, D.J.; Schmetz, C.; Brattig, N.W. Onchocerca volvulus: Expression and immunolocalization of a nematode cathepsin D-like lysosomal aspartic protease. Exp. Parasitol. 2004, 107, 145–156. [Google Scholar] [CrossRef]
- Podolska, M.; Nadolna, K. Acetylcholinesterase secreted by Anisakis simplex larvae (Nematoda: Anisakidae) parasitizing herring, Clupea harengus: An inverse relationship of enzyme activity in the host-parasite system. Parasitol. Res. 2014, 113, 2231–2238. [Google Scholar] [CrossRef]
- You, H.; Liu, C.; Du, X.; McManus, D.P. Acetylcholinesterase and nicotinic acetylcholine receptors in schistosomes and other parasitic helminths. Molecules 2017, 22, 1550. [Google Scholar] [CrossRef]
- Trumbić, Ž.; Hrabar, J.; Palevich, N.; Carbone, V.; Mladineo, I. Molecular and evolutionary basis for survival, its failure, and virulence factors of the zoonotic nematode Anisakis pegreffii. Genomics 2021, 113, 2891–2905. [Google Scholar] [CrossRef] [PubMed]
- Łopieńska-Biernat, E.; Paukszto, Ł.; Jastrzębski, J.P.; Myszczyński, K.; Polak, I.; Stryiński, R. Genome-wide analysis of Anisakis simplex sensu lato: The role of carbohydrate metabolism genes in the parasite’s development. Int. J. Parasitol. 2019, 49, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Łopieńska-Biernat, E.; Żółtowska, K.; Rokicki, J. The content of carbohydrates in larval stages of Anisakis simplex (Nematoda, Anisakidae). Helminthologia 2006, 43, 125–129. [Google Scholar] [CrossRef]
- Łopieńska-Biernat, E.; Stryiński, R.; Dmitryjuk, M.; Wasilewska, B. Infective larvae of Anisakis simplex (Nematoda) accumulate trehalose and glycogen in response to starvation and temperature stress. Biol. Open 2019, 8, bio040014. [Google Scholar] [CrossRef]
- Zečić, A.; Dhondt, I.; Braeckman, B.P. The nutritional requirements of Caenorhabditis elegans. Genes Nutr. 2019, 14, 15. [Google Scholar] [CrossRef]
- Luo, H.; Lin, Y.; Liu, T.; Lai, F.-L.; Zhang, C.-T.; Gao, F.; Zhang, R. DEG 15, an update of the Database of Essential Genes that includes built-in analysis tools. Nucleic Acids Res. 2021, 49, D677–D686. [Google Scholar] [CrossRef]
- Pfam. Available online: http://pfam.xfam.org/family/actin (accessed on 16 January 2022).
- Xue, R.; Meng, H.; Yin, J.; Xia, J.; Hu, Z.; Liu, H. The Role of Calmodulin vs. Synaptotagmin in Exocytosis. Front. Mol. Neurosci. 2021, 162, 691363. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Afgar, A.; Mohammadi, M.A.; Mortezaei, S.; Sadeghi, B.; Harandi, M.F. Calmodulin-specific small interfering RNA induces consistent expression suppression and morphological changes in Echinococcus granulosus. Sci. Rep. 2019, 9, 3894. [Google Scholar] [CrossRef]
- Lanneau, D.; de Thonel, A.; Maurel, S.; Didelot, C.; Garrido, C. Apoptosis versus cell differentiation: Role of heat shock proteins HSP90, HSP70 and HSP27. Prion 2007, 1, 53–60. [Google Scholar] [CrossRef]
- Sun, H.; Zhuo, X.; Zhao, X.; Yang, Y.; Chen, X.; Yao, C.; Du, A. The heat shock protein 90 of Toxoplasma gondii is essential for invasion of host cells and tachyzoite growth. Parasite 2017, 24, 22. [Google Scholar] [CrossRef]
- Neckers, L.; Tatu, U. Molecular chaperones in pathogen virulence: Emerging new targets for therapy. Cell Host Microbe 2008, 4, 519–527. [Google Scholar] [CrossRef]
- Sharma, O.P.; Kumar, M.S. Essential proteins and possible therapeutic targets of Wolbachia endosymbiont and development of FiloBase-a comprehensive drug target database for lymphatic filariasis. Sci. Rep. 2016, 6, 19842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Foster, J.M.; Kumar, S.; Fougere, M.; Carlow, C.K. Cofactor-independent phosphoglycerate mutase has an essential role in Caenorhabditis elegans and is conserved in parasitic nematodes. J. Biol. Chem. 2004, 279, 37185–37190. [Google Scholar] [CrossRef] [PubMed]
- Dhamodharan, R.; Hoti, S.; Sankari, T. Characterization of cofactor-independent phosphoglycerate mutase isoform-1 (Wb-iPGM) gene: A drug and diagnostic target from human lymphatic filarial parasite, Wuchereria bancrofti. Infect. Genet. Evol. 2012, 12, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kushwaha, S.; Mohd, S.; Pathak, M.; Misra-Bhattacharya, S. In vitro gene silencing of independent phosphoglycerate mutase (iPGM) in the filarial parasite Brugia malayi. Infect. Dis. Poverty 2013, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Chandra, S.; Baharia, R.K.; Misra, P.; Das, S.; Rawat, K.; Siddiqi, M.I.; Sundar, S.; Dube, A. Molecular, biochemical characterization and assessment of immunogenic potential of cofactor-independent phosphoglycerate mutase against Leishmania donovani: A step towards exploring novel vaccine candidate. Parasitology 2018, 145, 508–526. [Google Scholar] [CrossRef]
- Goulhen, F.; Grenier, D.; Mayrand, D. Oral microbial heat-shock proteins and their potential contributions to infections. Crit. Rev. Oral Biol. Med. 2003, 14, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, S.; Yamaguchi, H.; Osaki, T.; Taguchi, H. A virulence factor of Helicobacter pylori: Role of heat shock protein in mucosal inflammation after H. pylori infection. J. Clin. Gastroenterol. 1998, 27, S35–S39. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Cheng, Y.-S.; Grabner, D.S.; Chang, S.-H.; Shih, H.-H. Effect of different temperatures on the expression of the newly characterized heat shock protein 90 (Hsp90) in L3 of Anisakis spp. isolated from Scomber australasicus. Vet. Parasitol. 2014, 205, 540–550. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Cheng, Y.-S.; Shih, H.-H. Expression patterns and structural modelling of Hsp70 and Hsp90 in a fish-borne zoonotic nematode Anisakis pegreffii. Vet. Parasitol. 2015, 212, 281–291. [Google Scholar] [CrossRef]
- Dobbin, C.A.; Smith, N.C.; Johnson, A.M. Heat shock protein 70 is a potential virulence factor in murine Toxoplasma infection via immunomodulation of host NF-κB and nitric oxide. J. Immunol. 2002, 169, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.; Dehio, C. Intruders below the radar: Molecular pathogenesis of Bartonella spp. Clin. Microbiol. Rev. 2012, 25, 42–78. [Google Scholar] [CrossRef] [PubMed]
- Missall, T.A.; Pusateri, M.E.; Lodge, J.K. Thiol peroxidase is critical for virulence and resistance to nitric oxide and peroxide in the fungal pathogen, Cryptococcus neoformans. Mol. Microbiol. 2004, 51, 1447–1458. [Google Scholar] [CrossRef]
- Palacios, Ò.; Espart, A.; Espín, J.; Ding, C.; Thiele, D.J.; Atrian, S.; Capdevila, M. Full characterization of the Cu-, Zn-, and Cd-binding properties of CnMT1 and CnMT2, two metallothioneins of the pathogenic fungus Cryptococcus neoformans acting as virulence factors. Metallomics 2014, 6, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Russo, B.C.; O’Dee, D.M.; Schmitt, D.M.; Nau, G.J. The contribution of the glycine cleavage system to the pathogenesis of Francisella tularensis. Microbes Infect. 2014, 16, 300–309. [Google Scholar] [CrossRef]
- Shahbaaz, M.; Ahmad, F.; Hassan, M.I. Structure-based function analysis of putative conserved proteins with isomerase activity from Haemophilus influenzae. 3 Biotech 2015, 5, 741–763. [Google Scholar] [CrossRef]
- D’Amelio, S.; Lombardo, F.; Pizzarelli, A.; Bellini, I.; Cavallero, S. Advances in omic studies drive discoveries in the biology of Anisakid nematodes. Genes 2020, 11, 801. [Google Scholar] [CrossRef]
- Rodriguez-Mahillo, A.I.; Gonzalez-Muñoz, M.; Moneo, I. Identification and allergenic characterisation of a new isoform of the A. simplex allergen Ani s 4. Mol. Biochem. Parasitol. 2008, 160, 152–156. [Google Scholar] [CrossRef]
- Carballeda-Sangiao, N.; Olivares, F.; Rodriguez-Mahillo, A.I.; Careche, M.; Tejada, M.; Moneo, I.; González-Muñoz, M. Identification of autoclave-resistant Anisakis simplex allergens. J. Food Prot. 2014, 77, 605–609. [Google Scholar] [CrossRef]
- Maurer-Stroh, S.; Krutz, N.L.; Kern, P.S.; Gunalan, V.; Nguyen, M.N.; Limviphuvadh, V.; Eisenhaber, F.; Gerberick, G.F. AllerCatPro—prediction of protein allergenicity potential from the protein sequence. Bioinformatics 2019, 35, 3020–3027. [Google Scholar] [CrossRef]
- Ballestero, S.C.; De Barrio, M.; Baeza, M.; Sotés, M.R. Allergy to chironomid larvae (red midge larvae) in non professional handlers of fish food. J. Investig. Allergol. Clin. Immunol. 2006, 16, 63. [Google Scholar]
- Maldonado, J.L.; Hita, L.M.; Sáez, V.D.; Almendros, I.M.; López, A.V.; Bueno, M.C. Cross-reactivity between antigens of Anisakis simplex s.l. and other ascarid nematodes. Parasite 2004, 11, 219–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernardini, R.; Mistrello, G.; Novembre, E.; Roncarolo, D.; Zanotta, S.; Lombardi, E.; Cianferoni, A.; Pucci, N.; De Martino, M.; Vierucci, A. Cross-reactivity between IgE-binding proteins from Anisakis simplex and Dermatophagoides pteronyssinus. Int J. Immunopathol. Pharm. 2005, 18, 671–675. [Google Scholar] [CrossRef]
- Baird, F.J.; Su, X.; Aibinu, I.; Nolan, M.J.; Sugiyama, H.; Otranto, D.; Lopata, A.L.; Cantacessi, C. The Anisakis transcriptome provides a resource for fundamental and applied studies on allergy-causing parasites. PLoS Negl. Trop. Dis. 2016, 10, e0004845. [Google Scholar] [CrossRef] [PubMed]
- Llorens, C.; Arcos, S.C.; Robertson, L.; Ramos, R.; Futami, R.; Soriano, B.; Ciordia, S.; Careche, M.; González-Muñoz, M.; Jiménez-Ruiz, Y. Functional insights into the infective larval stage of Anisakis simplex s.s., Anisakis pegreffii and their hybrids based on gene expression patterns. BMC Genom. 2018, 19, 592. [Google Scholar] [CrossRef]
- Virginio, V.G.; Monteiro, K.M.; Drumond, F.; de Carvalho, M.O.; Vargas, D.M.; Zaha, A.; Ferreira, H.B. Excretory/secretory products from in vitro-cultured Echinococcus granulosus protoscoleces. Mol. Biochem. Parasitol. 2012, 183, 15–22. [Google Scholar] [CrossRef]
- Moreno, Y.; Gros, P.-P.; Tam, M.; Segura, M.; Valanparambil, R.; Geary, T.G.; Stevenson, M.M. Proteomic analysis of excretory-secretory products of Heligmosomoides polygyrus assessed with next-generation sequencing transcriptomic information. PLoS Negl. Trop. Dis. 2011, 5, e1370. [Google Scholar] [CrossRef]
- Bušelić, I.; Trumbić, Ž.; Hrabar, J.; Vrbatović, A.; Bočina, I.; Mladineo, I. Molecular and cellular response to experimental Anisakis pegreffii (Nematoda, Anisakidae) third-stage larval infection in rats. Front. Immunol. 2018, 9, 2055. [Google Scholar] [CrossRef]
- Fang, W.; Xu, S.; Wang, Y.; Ni, F.; Zhang, S.; Liu, J.; Chen, X.; Luo, D. ES proteins analysis of Angiostrongylus cantonensis: Products of the potential parasitism genes? Parasitol. Res. 2010, 106, 1027–1032. [Google Scholar] [CrossRef][Green Version]
- Villares, M.; Berthelet, J.; Weitzman, J.B. The clever strategies used by intracellular parasites to hijack host gene expression. Semin. Immunopathol. 2020, 42, 215–226. [Google Scholar] [CrossRef]
- Li, J.; Chai, Q.-Y.; Liu, C.H. The ubiquitin system: A critical regulator of innate immunity and pathogen–host interactions. Cell. Mol. Immunol. 2016, 13, 560–576. [Google Scholar] [CrossRef] [PubMed]
- Selkirk, M.E.; Denham, D.; Partono, F.; Maizels, R.M. Heat shock cognate 70 is a prominent immunogen in Brugian filariasis. J. Immunol. 1989, 143, 299–308. [Google Scholar]
- Maresca, B.; Kobayashi, G. Hsp70 in parasites: As an inducible protective protein and as an antigen. Experientia 1994, 50, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.E.; Martel, N.; Lo, H.P.; Xiong, Z.; Parton, R.G. A plasmid library of full-length zebrafish rab proteins for in vivo cell biology. Cell. Logist. 2017, 7, e1301151. [Google Scholar] [CrossRef][Green Version]
- Grzelak, S.; Moskwa, B.; Bień, J. Trichinella britovi muscle larvae and adult worms: Stage-specific and common antigens detected by two-dimensional gel electrophoresis-based immunoblotting. Parasites Vectors 2018, 11, 584. [Google Scholar] [CrossRef]
- Becerro-Recio, D.; González-Miguel, J.; Ucero, A.; Sotillo, J.; Martínez-Moreno, Á.; Pérez-Arévalo, J.; Cwiklinski, K.; Dalton, J.P.; Siles-Lucas, M. Recognition Pattern of the Fasciola hepatica Excretome/Secretome during the Course of an Experimental Infection in Sheep by 2D Immunoproteomics. Pathogens 2021, 10, 725. [Google Scholar] [CrossRef]
- Kochanowski, M.; González-Muñoz, M.; Gómez-Morales, M.Á.; Gottstein, B.; Dąbrowska, J.; Różycki, M.; Cencek, T.; Müller, N.; Boubaker, G. Comparative analysis of excretory-secretory antigens of Anisakis simplex, Pseudoterranova decipiens and Contracaecum osculatum regarding their applicability for specific serodiagnosis of human anisakidosis based on IgG-ELISA. Exp. Parasitol. 2019, 197, 9–15. [Google Scholar] [CrossRef]
- Zhu, X.; Gasser, R.B.; Podolska, M.; Chilton, N.B. Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int. J. Parasitol. 1998, 28, 1911–1921. [Google Scholar] [CrossRef]
- D’Amelio, S.; Mathiopoulos, K.; Santos, C.P.; Pugachev, O.; Webb, S.; Picanço, M.; Paggi, L. Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase-chain-reaction-based restriction fragment length polymorphism. Int. J. Parasitol. 2000, 30, 223–226. [Google Scholar] [CrossRef]
- Abollo, E.; Paggi, L.; Pascual, S.; D’Amelio, S. Occurrence of recombinant genotypes of Anisakis simplex ss and Anisakis pegreffii (Nematoda: Anisakidae) in an area of sympatry. Infect. Genet. Evol. 2003, 3, 175–181. [Google Scholar] [CrossRef]
- Mattiucci, S.; Acerra, V.; Paoletti, M.; Cipriani, P.; Levsen, A.; Webb, S.; Canestrelli, D.; Nascetti, G. No more time to stay ‘single’ in the detection of Anisakis pegreffii, A. simplex (ss) and hybridization events between them: A multi-marker nuclear genotyping approach. Parasitology 2016, 143, 998–1011. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Consortium, U. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014, 42, D503–D509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Poschmann, G.; Waldera-Lupa, D.; Rafiee, N.; Kollmann, M.; Stühler, K. OutCyte: A novel tool for predicting unconventional protein secretion. Sci. Rep. 2019, 9, 19448. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 29, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Ramana, J.; Gupta, D. ProtVirDB: A database of protozoan virulent proteins. Bioinformatics 2009, 25, 1568–1569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sayers, S.; Li, L.; Ong, E.; Deng, S.; Fu, G.; Lin, Y.; Yang, B.; Zhang, S.; Fa, Z.; Zhao, B. Victors: A web-based knowledge base of virulence factors in human and animal pathogens. Nucleic Acids Res. 2019, 47, D693–D700. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Nowotny, J.; Cao, R.; Cheng, J. 3Drefine: An interactive web server for efficient protein structure refinement. Nucleic Acids Res. 2016, 44, W406–W409. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Ammari, M.G.; Gresham, C.R.; McCarthy, F.M.; Nanduri, B. HPIDB 2.0: A curated database for host–pathogen interactions. Database 2016, 2016, baw103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nanduri, B. HPIDB—A unified resource for host-pathogen interactions. BMC Bioinform. 2010, 11, S16. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]


| Secretome Proteins | Extracellular Vesicle-Associated Proteins | Blast Similarity (%) | |||
|---|---|---|---|---|---|
| UniProt Accession No. | Protein Name | UniProt Accession No. | Protein Name | Organism | |
| A0A0M3K144 | Proteasome subunit alpha type | F1L7Z7 | Proteasome subunit alpha type | Ascaris suum | 98.79 |
| A0A0M3JSH7 | Proteasome subunit alpha type-3 | F1LBE7 | Proteasome subunit alpha type-3 | Ascaris suum | 94.59 |
| A0A0M3JVA5 | Triosephosphate isomerase | A0A0J9YA50 | Triosephosphate isomerase | Brugia malayi | 93.50 |
| A0A0M3K0Q9 | Chaperonin homolog Hsp-60, mitochondrial | A0A0N4YLK1 | Chaperonin homolog Hsp-60, mitochondrial | Nippostrongylus brasiliensis | 92.76 |
| A0A0M3KAY8 | Phosphoglycerate mutase | Q4VWF8 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | Brugia malayi | 92.33 |
| A0A0M3JT99 | Proteasome subunit alpha type | A0A0N4XV21 | Proteasome subunit alpha type | Nippostrongylus brasiliensis | 92.02 |
| A0A0M3J0T6 | 14-3-3-like protein 2 | A0A0N4XVA6 | 14-3-3-like protein 2 | Nippostrongylus brasiliensis | 90.94 |
| A0A0M3K444 | Fructose-bisphosphate aldolase | A0A0H5S7G0 | Fructose-bisphosphate aldolase | Brugia malayi | 90.93 |
| A0A0M3K9Z9 | Peroxiredoxin 1 | A0A0N4XCN5 | Peroxiredoxin 1 | Nippostrongylus brasiliensis | 90.72 |
| A0A0M3K4G1 | Uncharacterized protein | A0A0N4YLK1 | Chaperonin homolog Hsp-60, mitochondrial | Nippostrongylus brasiliensis | 90.71 |
| Secretome Protein | Protease/Protease Inhibitor | BLAST Similarity (%) | ||||
|---|---|---|---|---|---|---|
| UniProt Accession No. | Protein Name | MEROPS Accession No. | MEROPS Classification | Activity | Organism | |
| A0A0M3K144 | Proteasome subunit alpha type | MER1107563 | Subfamily T1A non-peptidase homologues (T01.UNA) | Threonine protease | Anisakis simplex | 100.00 |
| A0A0M3JT99 | Proteasome subunit alpha type | MER1107399 | Subfamily T1A unassigned peptidases (T01.UPA) | Threonine protease | Anisakis simplex | 100.00 |
| A0A0M3JSH7 | Proteasome subunit alpha type-3 | MER1107379 | Subfamily T1A non-peptidase homologues (T01.UNA) | Threonine protease | Anisakis simplex | 100.00 |
| A0A0M3K9E5 | M20_dimer domain-containing protein | MER1107182 | Subfamily M20F non-peptidase homologues (M20.UNF) | Metalloprotease | Anisakis simplex | 100.00 |
| A0A0M3JV41 | M20_dimer domain-containing protein | MER1107451 | pes-9 g.p. (Caenorhabditis elegans) (M20.A14) | Metalloprotease | Anisakis simplex | 100.00 |
| A0A0M3K810 | Dipeptidase C | MER1107162 | Subfamily M24B non-peptidase homologues (M24.UNB) | Metalloprotease | Anisakis simplex | 100.00 |
| A0A0M3JXL8 | Uncharacterized protein | MER1107491 | F54F11.2 g.p. domain 2 (Caenorhabditis elegans) (M13.A20) | Metalloprotease | Anisakis simplex | 100.00 |
| A0A0M3KCB1 | Uncharacterized protein | MER1107243 | Family M1 non-peptidase homologues (M01.UNW) | Metalloprotease | Anisakis simplex | 100.00 |
| A0A0M3K9X8 | Carboxylic ester hydrolase | MER1107193 | Family S9 non-peptidase homologues (S09.UNW) | Serine protease | Anisakis simplex | 100.00 |
| A0A0M3JYK8 | COesterase domain-containing protein | MER1107514 | Family S9 non-peptidase homologues (S09.UNW) | Serine protease | Anisakis simplex | 100.00 |
| Secretome Protein | Essential Protein | Blast Similarity (%) | |||
|---|---|---|---|---|---|
| UniProt Accession No. | Protein Name | DEG Accession No. | Protein Name | Organism | |
| A0A0M3J0M4 | Putative actin | DEG20290735 | Actin gamma 1 | Homo sapiens | 99.10 |
| A0A0M3KFJ2 | Calmodulin | DEG20070098 | Calmodulin CG8472-PA, isoform A | Drosophila melanogaster | 98.08 |
| A0A0M3K916 | Uncharacterized protein | DEG20051541 | Alpha-actinin-4 (Non-muscle alpha-actinin 4) (F-actin cross-linking protein) | Mus musculus | 91.16 |
| A0A0M3K5H6 | 78 kDa glucose-regulated protein | DEG20040148 | Immunoglobulin binding protein mRNA | Danio rerio | 89.98 |
| A0A0M3K613 | Elongation factor 2 | DEG20280147 | Translation elongation factor 2 | Bombyx mori | 88.93 |
| A0A0M3K9V2 | Heat shock 70 kDa protein cognate 1 | DEG20330753 | Heat shock protein family A (Hsp70) member 8 | Homo sapiens | 86.16 |
| A0A0M3K9Z9 | Peroxiredoxin 1 | DEG20201416 | Peroxiredoxin 2 | Homo sapiens | 85.49 |
| A0A0M3KAY8 | Phosphoglycerate mutase (2,3-diphosphoglycerate-independent) | DEG20020003 | F57B10.3a | Caenorhabditis elegans | 84.52 |
| A0A0M3K4G1 | Uncharacterized protein | DEG20290492 | Heat shock protein family D (Hsp60) member 1 | Homo sapiens | 84.44 |
| A0A0M3K8S1 | Methylmalonyl-CoA mutase | DEG20051827 | Methylmalonyl-CoA mutase, mitochondrial precursor (MCM) (Methylmalonyl-CoA isomerase) | Mus musculus | 83.97 |
| Secretome Protein | Pathogenicity-Related Protein | Blast Similarity (%) | ||||
|---|---|---|---|---|---|---|
| UniProt Accession No. | Protein Name | Database | NCBI Accession No. | Protein Name | Organism | |
| A0A0M3K9V2 | Heat shock 70 kDa protein cognate 1 | ProtVirDB | AAC72001 | Hsp70 | Toxoplasma gondii | 88.24 |
| VICTORS | BAB20284 | Hsp70 | Toxoplasma gondii | 79.64 | ||
| A0A0M3K5H6 | 78 kDa glucose-regulated protein | ProtVirDB | AAC72001 | Hsp70 | Toxoplasma gondii | 86.06 |
| VICTORS | BAB20284 | Hsp70 | Toxoplasma gondii | 80.36 | ||
| VFDB | NP_219906.1 | Molecular chaperone DnaK | Chlamydia trachomatis D/UW-3/CX | 70.23 | ||
| A0A0M3K4G1 | Uncharacterized protein | VICTORS | YP_989430 | Chaperonin GroEL | Bartonella bacilliformis KC583 | 79.22 |
| VFDB | YP_001039283 | Chaperonin GroEL | Ruminiclostridium thermocellum ATCC 27405 | 76.17 | ||
| A0A0M3IZ99 | Glucose-6-phosphate isomerase | VFDB | NP_439722.1 | Glucose-6-phosphate isomerase | Haemophilus influenzae Rd KW20 | 76.46 |
| A0A0M3K0Q9 | Chaperonin homolog Hsp-60, mitochondrial | VICTORS | YP_989430 | Chaperonin GroEL | Bartonella bacilliformis KC583 | 74.07 |
| VFDB | YP_003454101 | Molecular chaperone GroEL | Legionella longbeachae NSW150 | 73.48 | ||
| A0A0M3K9Z9 | Peroxiredoxin 1 | VICTORS | AAP68994 | Thiol-specific antioxidant protein 1 | Cryptococcus neoformans var. grubii | 72.77 |
| A0A0M3K6L1 | Superoxide dismutase [Cu-Zn] | VICTORS | XP_012053609 | Hypothetical protein CNAG_05449 | Cryptococcus neoformans var. grubii H99 | 71.96 |
| A0A0M3KAE7 | Glycine cleavage system H protein | VICTORS | YP_169453 | Glycine cleavage system H protein | Francisella tularensis subsp. tularensis SCHU S4 | 71.30 |
| A0A0M3J2W3 | Probable peroxiredoxin prdx-3 | VICTORS | AAP68994 | Thiol-specific antioxidant protein 1 | Cryptococcus neoformans var. grubii | 70.27 |
| Secretome Protein | FARRP Database Match | AllerCatPro Prediction | ||||
|---|---|---|---|---|---|---|
| UniProt Accession No. | Protein Name | NCBI Accession No. | Protein Name | Organism | Blast Similarity (%) | |
| A0A0M3KA05 | SXP/RAL-2 family protein 2 isoform 1 | BAF75681 | SXP/RAL-2 family protein 2 isoform 1 | Anisakis simplex | 100.00 | Strong evidence |
| A0A0M3KIW7 | Globin-like protein | ASL68918 | Hemoglobin | Anisakis simplex | 100.00 | Strong evidence |
| A0A0M3JEL6 | Globin-like protein | ASL68918 | Hemoglobin | Anisakis simplex | 100.00 | Strong evidence |
| A0A0M3JU57 | Troponin-like protein | CAB58171 | Troponin-like protein | Anisakis simplex | 99.38 | Strong evidence |
| A0A158PP35 | Paramyosin | Q9NJA9 | Paramyosin (Ani s 2) | Anisakis simplex | 92.72 | Strong evidence |
| A0A0M3K5H6 | 78 kDa glucose-regulated protein | ABF18258 | Heat shock cognate 70 | Aedes aegypti | 89.69 | Strong evidence |
| A0A0M3K8L6 | Uncharacterized protein | Q06811 | Polyprotein ABA-1 | Ascaris suum | 85.67 | Weak evidence |
| A0A0M3J5J0 | Uncharacterized protein | P46436 | Glutathione S-transferase 1 | Ascaris suum | 82.63 | Strong evidence |
| A0A0M3IZ99 | Glucose-6-phosphate isomerase | XP_026782721 | LOW QUALITY PROTEIN: glucose-6-phosphate isomerase b | Pangasianodon hypophthalmus | 82.27 | Strong evidence |
| A0A0M3K9V2 | Heat shock 70 kDa protein cognate 1 | AOD75395 | Heat shock-like protein | Tyrophagus putrescentiae | 81.52 | Strong evidence |
| A0A0M3JVA5 | Triosephosphate isomerase | AEB54655 | Triosephosphate isomerase | Procambarus clarkii | 80.74 | Weak evidence |
| A0A0M3JT42 | Peptidyl-prolyl cis-trans isomerase (PPIase) | AAP35065 | Der f Mal f 6 allergen | Dermatophagoides farinae | 78.26 | Weak evidence |
| A0A0M3K444 | Fructose-bisphosphate aldolase | XP_026771637 | Aldolase a, fructose-bisphosphate, b | Pangasianodon hypophthalmus | 76.32 | Weak evidence |
| A0A0M3KFJ2 | Calmodulin | ACL36923 | Troponin C | Tyrophagus putrescentiae | 76.00 | Weak evidence |
| A0A0M3JYW9 | Fructose-bisphosphate aldolase | ACH70901 | Aldolase a, fructose-bisphosphate 1 | Salmo salar | 74.93 | Strong evidence |
| A0A0M3K3X7 | Inorganic diphosphatase | QAT18643 | Allergen Der p 32 | Dermatophagoides pteronyssinus | 74.51 | Strong evidence |
| A0A0M3KAE3 | Transaldolase | AHY02994 | Transaldolase | Fusarium proliferatum | 71.16 | Weak evidence |
| A0A0M3JTF7 | Peptidase A1 domain-containing protein | XP_001657556 | Lysosomal aspartic protease | Aedes aegypti | 70.82 | Weak evidence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochanowski, M.; Dąbrowska, J.; Różycki, M.; Sroka, J.; Karamon, J.; Bełcik, A.; Korpysa-Dzirba, W.; Cencek, T. Proteomic Profiling and In Silico Characterization of the Secretome of Anisakis simplex Sensu Stricto L3 Larvae. Pathogens 2022, 11, 246. https://doi.org/10.3390/pathogens11020246
Kochanowski M, Dąbrowska J, Różycki M, Sroka J, Karamon J, Bełcik A, Korpysa-Dzirba W, Cencek T. Proteomic Profiling and In Silico Characterization of the Secretome of Anisakis simplex Sensu Stricto L3 Larvae. Pathogens. 2022; 11(2):246. https://doi.org/10.3390/pathogens11020246
Chicago/Turabian StyleKochanowski, Maciej, Joanna Dąbrowska, Mirosław Różycki, Jacek Sroka, Jacek Karamon, Aneta Bełcik, Weronika Korpysa-Dzirba, and Tomasz Cencek. 2022. "Proteomic Profiling and In Silico Characterization of the Secretome of Anisakis simplex Sensu Stricto L3 Larvae" Pathogens 11, no. 2: 246. https://doi.org/10.3390/pathogens11020246
APA StyleKochanowski, M., Dąbrowska, J., Różycki, M., Sroka, J., Karamon, J., Bełcik, A., Korpysa-Dzirba, W., & Cencek, T. (2022). Proteomic Profiling and In Silico Characterization of the Secretome of Anisakis simplex Sensu Stricto L3 Larvae. Pathogens, 11(2), 246. https://doi.org/10.3390/pathogens11020246







