Exploring Rapid and Effective Screening Methods for Anti-SARS-CoV-2 Neutralizing Antibodies in COVID-19 Convalescent Patients and Longitudinal Vaccinated Populations
Abstract
:1. Introduction
2. Results
2.1. General Characteristics of Convalescent Patients up to 6 Months (CP-6M) from COVID-19
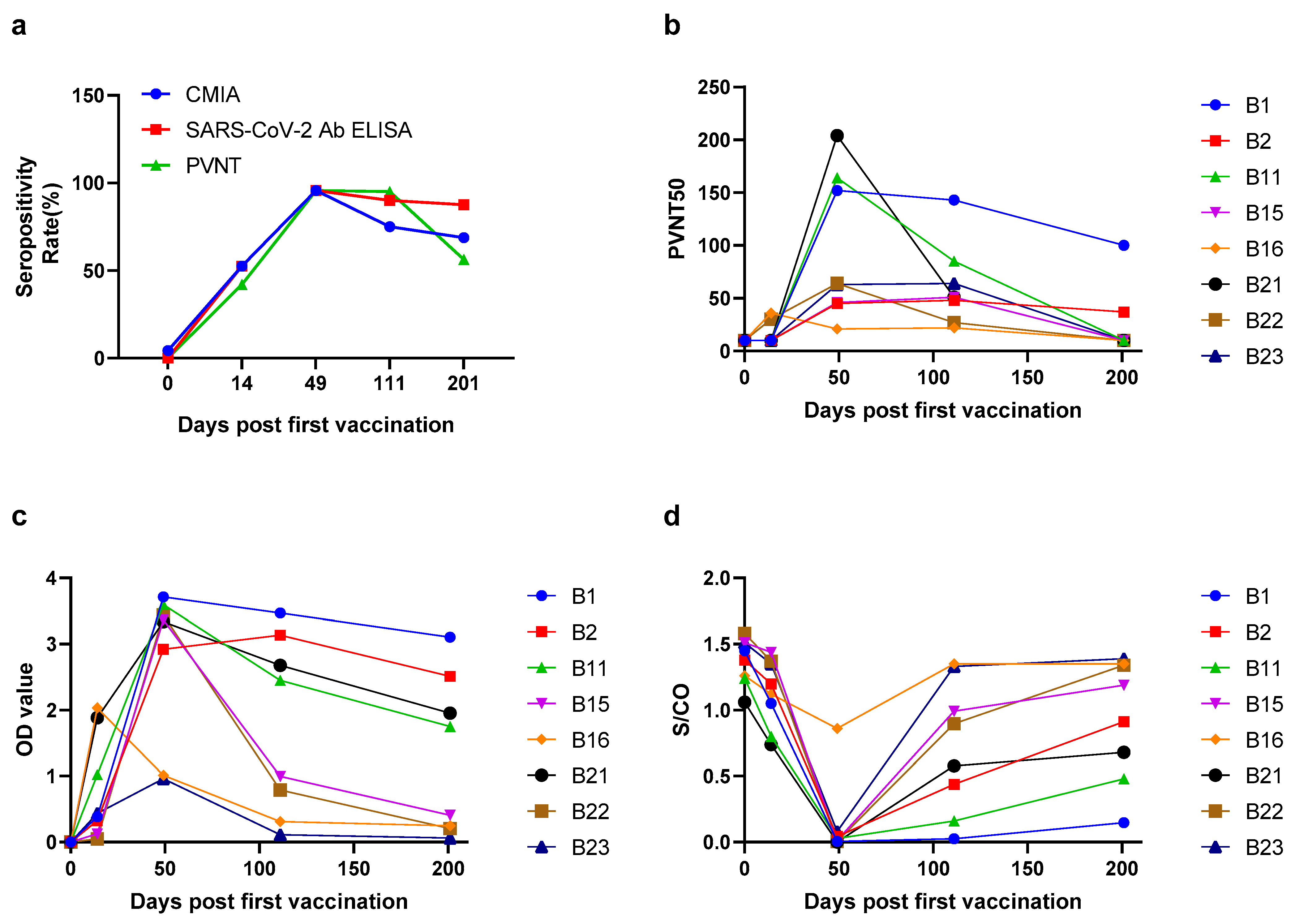
2.2. The Seropositivity and Antibody Levels over Times in Vaccinated Population
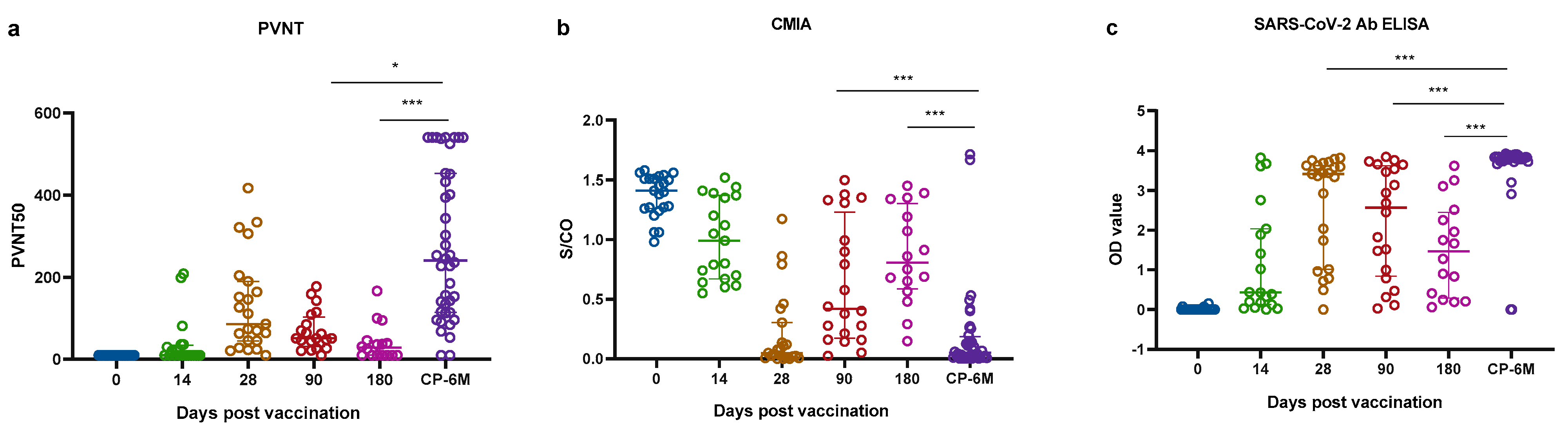
2.3. Comparisons of Antibody Responses in Two Cohorts
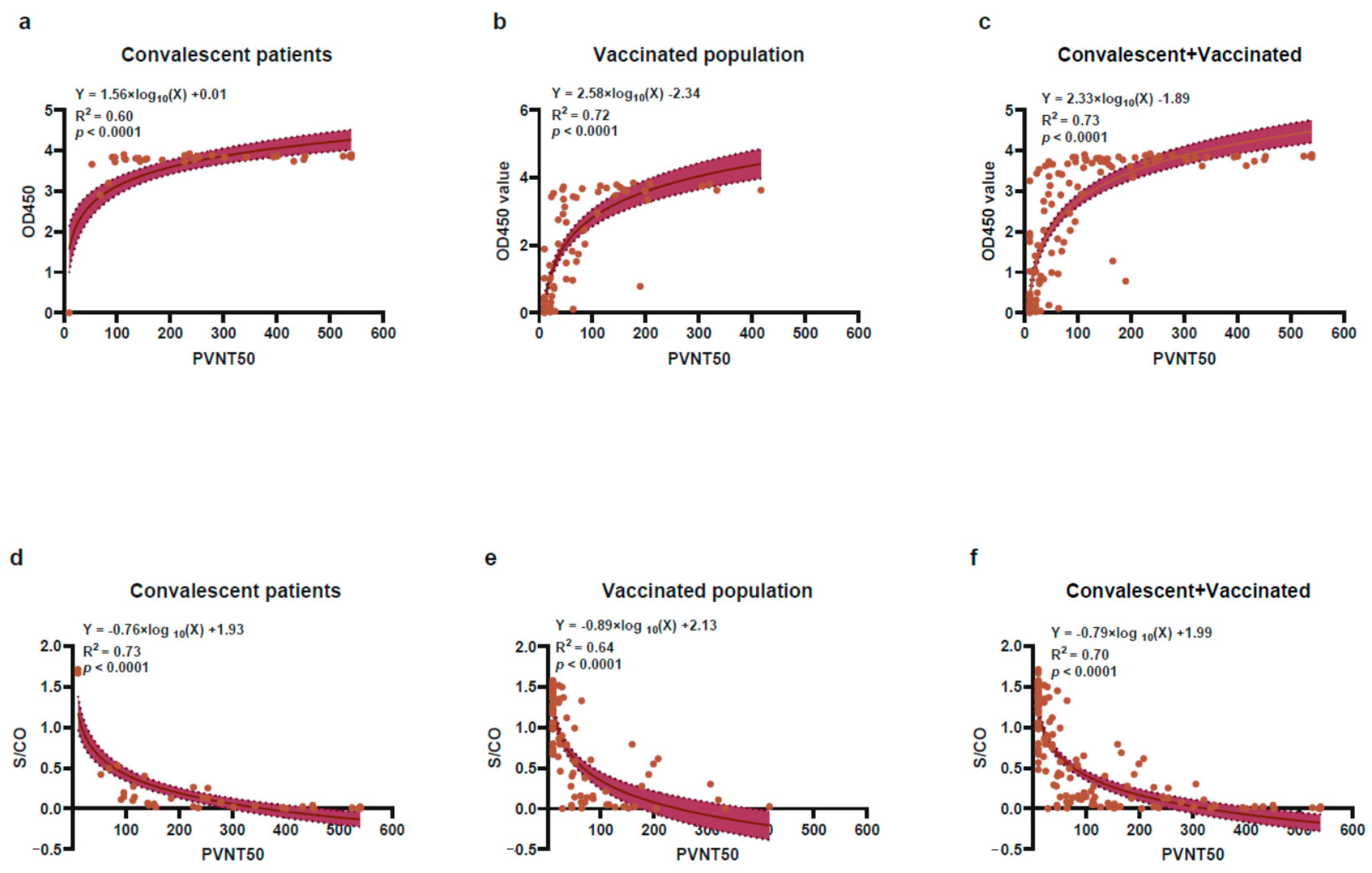
2.4. The Correlation of Antibody Response between CMIA or SARS-CoV-2 Ab ELISA with PVNT Method
3. Discussion
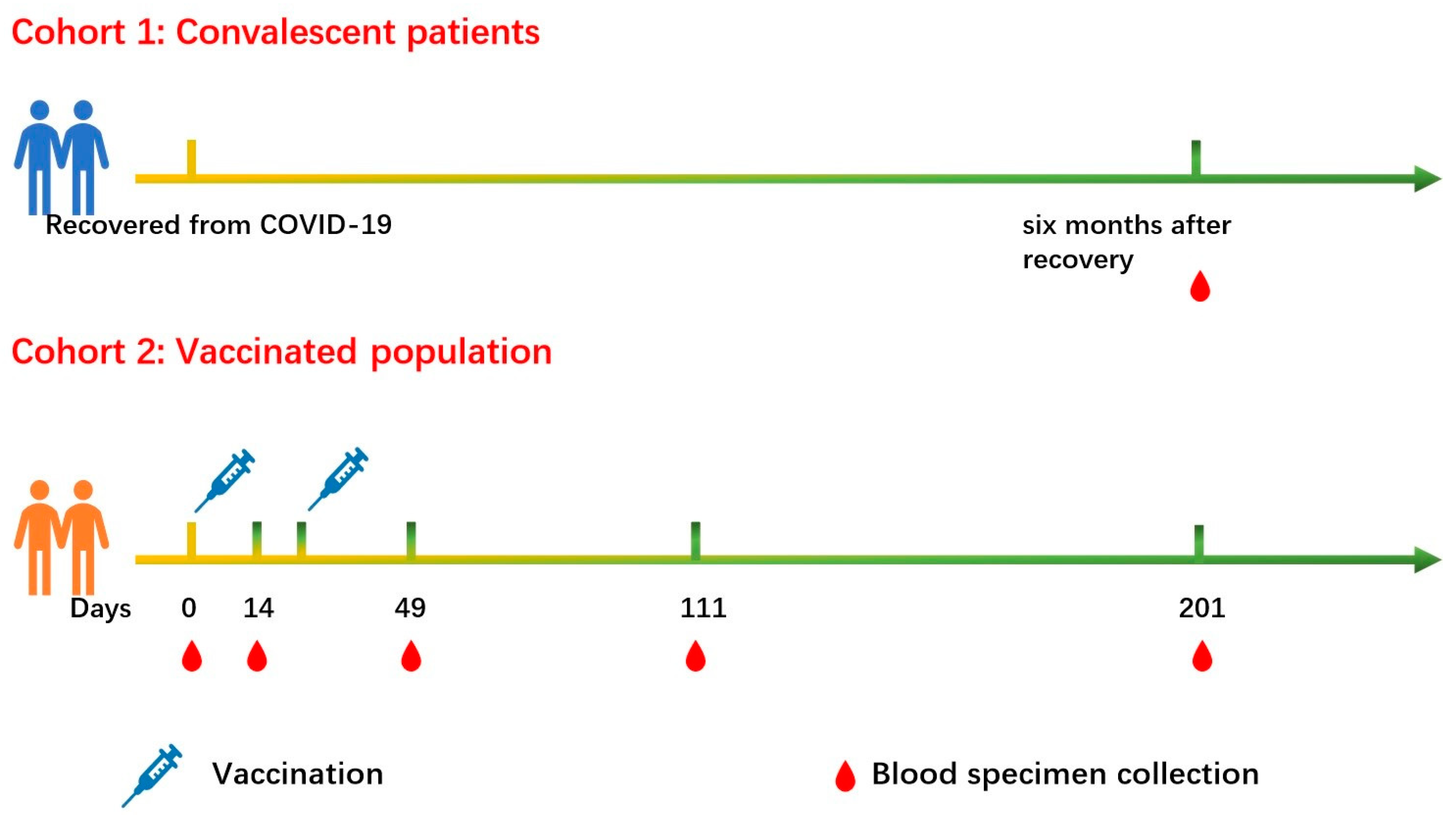
4. Materials and Methods
4.1. Study Participants
4.2. WANTAI SARS-CoV-2 Ab Enzyme-Linked Immunosorbent Assay (ELISA)
4.3. Chemiluminescent Microparticle Immunoassay (CMIA)
4.4. Pseudovirus Neutralization Test (PVNT)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef] [PubMed]
- McAndrews, K.M.; Dowlatshahi, D.P.; Dai, J.; Becker, L.M.; Hensel, J.; Snowden, L.M.; Leveille, J.M.; Brunner, M.R.; Holden, K.W.; Hopkins, N.S.; et al. Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity. JCI Insight 2020, 5, e142386. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Yuan, M.; Wan, Y.; Liu, C.; Li, Y.; Liu, Z.; Lin, C.; Chen, J. Identification and characterization of a monoclonal antibody blocking the SARS-CoV-2 spike protein-ACE2 interaction. Cell. Mol. Immunol. 2021, 18, 1562–1564. [Google Scholar] [CrossRef]
- Yao, H.; Song, Y.; Chen, Y.; Wu, N.; Xu, J.; Sun, C.; Zhang, J.; Weng, T.; Zhang, Z.; Wu, Z.; et al. Molecular Architecture of the SARS-CoV-2 Virus. Cell 2020, 183, 730–738.e13. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Xin, Q.; Pan, Y.; Hu, Y.; Li, J.; Chu, Y.; Feng, Y.; Wang, Q. Neutralizing Antibody Responses to Severe Acute Respiratory Syndrome Coronavirus 2 in Coronavirus Disease 2019 Inpatients and Convalescent Patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 2688–2694. [Google Scholar] [CrossRef]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Rapid isolation of potent SARS-CoV-2 neutralizing antibodies and protection in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Gal Levin, E.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- L’Huillier, A.G.; Meyer, B.; Andrey, D.O.; Arm-Vernez, I.; Baggio, S.; Didierlaurent, A.; Eberhardt, C.S.; Eckerle, I.; Grasset-Salomon, C.; Huttner, A.; et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: A prospective longitudinal study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 784.e1–784.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, Y.; Xiao, M.; Chen, L.; Zhao, Y.; Haiwei, Z.; Long, P.; Zhou, Y.; Xu, X.; Lei, Y.; et al. Dynamics of the SARS-CoV-2 antibody response up to 10 months after infection. Cell. Mol. Immunol. 2021, 18, 1832–1834. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ren, L.; Yang, J.; Guo, L.; Feng, L.; Ma, C.; Wang, X.; Leng, Z.; Tong, X.; Zhou, W.; et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: A longitudinal, population-level, cross-sectional study. Lancet 2021, 397, 1075–1084. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, F.; Xu, X.; Ling, Y.; Huang, W.; Zhu, Z.; Guo, M.; Lin, Y.; Fu, Z.; Liang, D.; et al. Durability of neutralizing antibodies and T-cell response post SARS-CoV-2 infection. Front. Med. 2020, 14, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liang, B.; Chen, C.; Wang, H.; Fang, Y.; Shen, S.; Yang, X.; Wang, B.; Chen, L.; Chen, Q.; et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat. Commun. 2021, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Choe, P.G.; Kim, K.H.; Kang, C.K.; Suh, H.J.; Kang, E.; Lee, S.Y.; Kim, N.J.; Yi, J.; Park, W.B.; Oh, M.D. Antibody Responses 8 Months after Asymptomatic or Mild SARS-CoV-2 Infection. Emerg. Infect. Dis. 2021, 27, 928–931. [Google Scholar] [CrossRef]
- Pirofski, L.A. Disease severity and durability of the SARS-CoV-2 antibody response: A view through the lens of the second year of the pandemic. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e1345–e1347. [Google Scholar] [CrossRef]
- Jeewandara, C.; Aberathna, I.S.; Pushpakumara, P.D.; Kamaladasa, A.; Guruge, D.; Jayathilaka, D.; Gunasekara, B.; Tanussiya, S.; Kuruppu, H.; Ranasinghe, T.; et al. Antibody and T cell responses to Sinopharm/BBIBP-CorV in naïve and previously infected individuals in Sri Lank. medRxiv 2021. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef]
- Pan, H.; Wu, Q.; Zeng, G.; Yang, J.; Jiang, D.; Deng, X.; Chu, K.; Zheng, W.; Zhu, F.; Yu, H.; et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18–59 years: Interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Lim, W.W.; Mak, L.; Leung, G.M.; Cowling, B.J.; Peiris, M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe 2021, 2, e423. [Google Scholar] [CrossRef]
- Riepler, L.; Rossler, A.; Falch, A.; Volland, A.; Borena, W.; von Laer, D.; Kimpel, J. Comparison of Four SARS-CoV-2 Neutralization Assays. Vaccines 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- von Rhein, C.; Scholz, T.; Henss, L.; Kronstein-Wiedemann, R.; Schwarz, T.; Rodionov, R.N.; Corman, V.M.; Tonn, T.; Schnierle, B.S. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. J. Virol. Methods 2021, 288, 114031. [Google Scholar] [CrossRef] [PubMed]
- Mendrone-Junior, A.; Dinardo, C.L.; Ferreira, S.C.; Nishya, A.; Salles, N.A.; de Almeida Neto, C.; Hamasaki, D.T.; Facincani, T.; de Oliveira Alves, L.B.; Machado, R.R.G.; et al. Correlation between SARS-CoV-2 antibody screening by immunoassay and neutralizing antibody testing. Transfusion 2021, 61, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Kinsley, R.; Pronost, S.; De Bock, M.; Temperton, N.; Daly, J.M.; Paillot, R.; Scott, S. Evaluation of a Pseudotyped Virus Neutralisation Test for the Measurement of Equine Influenza Virus-Neutralising Antibody Responses Induced by Vaccination and Infection. Vaccines 2020, 8, 466. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef]
- Valdivia, A.; Torres, I.; Latorre, V.; Frances-Gomez, C.; Albert, E.; Gozalbo-Rovira, R.; Alcaraz, M.J.; Buesa, J.; Rodriguez-Diaz, J.; Geller, R.; et al. Inference of SARS-CoV-2 spike-binding neutralizing antibody titers in sera from hospitalized COVID-19 patients by using commercial enzyme and chemiluminescent immunoassays. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 485–494. [Google Scholar] [CrossRef]
- Tang, M.S.; Case, J.B.; Franks, C.E.; Chen, R.E.; Anderson, N.W.; Henderson, J.P.; Diamond, M.S.; Gronowski, A.M.; Farnsworth, C.W. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin. Chem. 2020, 66, 1538–1547. [Google Scholar] [CrossRef]
- Rowntree, L.C.; Chua, B.Y.; Nicholson, S.; Koutsakos, M.; Hensen, L.; Douros, C.; Selva, K.; Mordant, F.L.; Wong, C.Y.; Habel, J.R.; et al. Robust correlations across six SARS-CoV-2 serology assays detecting distinct antibody features. Clin. Transl. Immunol. 2021, 10, e1258. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59, e02438–20. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, G.; Vos, E.R.A.; van den Hoogen, L.L.; van Boven, M.; Schepp, R.M.; Smits, G.; van Vliet, J.; Woudstra, L.; Wijmenga-Monsuur, A.J.; van Hagen, C.C.E.; et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Gurevich, M.; Falb, R.; Dreyer-Alster, S.; Sonis, P.; Mandel, M. SARS-CoV-2 antibody dynamics and B-cell memory response over time in COVID-19 convalescent subjects. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1349.e1–1349.e6. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Fischinger, S.; Siddiqui, S.M.; Chen, Z.; Yu, J.; Gebre, M.; Atyeo, C.; Gorman, M.J.; Zhu, A.L.; Kang, J.; et al. Discrete SARS-CoV-2 antibody titers track with functional humoral stability. Nat. Commun. 2021, 12, 1018. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Li, Y.; Lai, D.Y.; Zhang, H.N.; Jiang, H.W.; Tian, X.; Ma, M.L.; Qi, H.; Meng, Q.F.; Guo, S.J.; Wu, Y.; et al. Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 1095–1097. [Google Scholar] [CrossRef]
- Dupont, L.; Snell, L.B.; Graham, C.; Seow, J.; Merrick, B.; Lechmere, T.; Maguire, T.J.A.; Hallett, S.R.; Pickering, S.; Charalampous, T.; et al. Neutralizing antibody activity in convalescent sera from infection in humans with SARS-CoV-2 and variants of concern. Nat. Microbiol. 2021, 6, 1433–1442. [Google Scholar] [CrossRef]
- Huang, B.; Dai, L.; Wang, H.; Hu, Z.; Yang, X.; Tan, W.; Gao, G.F. Serum sample neutralisation of BBIBP-CorV and ZF2001 vaccines to SARS-CoV-2 501Y.V2. Lancet Microbe 2021, 2, e285. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef] [PubMed]
- China NHCo New Coronavirus Pneumonia Prevention and Control Program (8th). Available online: http://www.nhc.gov.cn/xcs/zhengcwj/202104/7de0b3837c8b4606a0594aeb0105232b/files/f192ac6e5567469db4f0a8691ca18907.pdf (accessed on 1 December 2021).

| Characteristics | All Patients (n = 40) |
|---|---|
| Age, y | |
| Median (IQR) | 52.0 (38.3–59.8) |
| ≤60 | 30 |
| >60 | 10 |
| Gender | |
| Male | 24 |
| Female | 16 |
| Disease severity | |
| Non-severe | |
| Mild | 3 |
| Moderate | 12 |
| Severe | |
| Severe | 17 |
| Critical | 8 |
| Comorbidities | |
| Liver diseases | 19 |
| Hypertension | 11 |
| Diabetes | 5 |
| Cardiovascular diseases | 5 |
| Pulmonary diseases | 5 |
| Rheumatic immune diseases | 1 |
| Without Comorbidities | 9 |
| PVNT, Median (IQR) | CMIA, Median (IQR) | SARS-CoV-2 Ab ELISA, Median (IQR) | |
|---|---|---|---|
| Before vaccination | <20 (/) | 1.41 (1.26–1.51) | 0.002 (0.001–0.003) |
| 14 days after 1st vaccination | <20 (<20–36) | 0.99 (0.67–1.37) | 0.43 (0.124–2.04) |
| 28 days after 2nd vaccination | 86 (45–190) | 0.05 (0.01–0.30) | 3.41(1.01–3.63) |
| 90 days after 2nd vaccination | 51(31–103) | 0.42 (0.173–1.229) | 2.56 (0.84–3.62) |
| 180 days after 2nd vaccination | 31 (<20–42.5) | 0.72 (0.59–1.10) | 1.47 (0.37–2.31) |
| Convalescent, 6 months recovered from COVID-19 | 240.5 (114–452) | 0.052 (0.01–0.18) | 3.83 (3.77–3.86) |
| SARS-CoV-2 Ab ELISA | CMIA | |||||
|---|---|---|---|---|---|---|
| Convalescents | Vaccinated Population | Convalescents and Vaccinated Population | Convalescents | Vaccinated Population | Convalescents and Vaccinated Population | |
| Sensitivity | 100% | 91.8% | 95.1% | 100% | 85.7% | 91.3% |
| Specificity | 100% | 80.6% | 71.1% | 100% | 83.3% | 80.0% |
| NPV | 100% | 87.9% | 86.5% | 100% | 81.1% | 80.0% |
| PPV | 100% | 86.5% | 88.3% | 100% | 87.5% | 91.3% |
| Consistency | 1.00 | 0.73 | 0.70 | 1.00 | 0.69 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Li, D.; Liu, Z.; Ren, L.; Su, J.; Zhu, M.; Feng, Y.; Wang, Z.; Liu, Q.; Zhu, B.; et al. Exploring Rapid and Effective Screening Methods for Anti-SARS-CoV-2 Neutralizing Antibodies in COVID-19 Convalescent Patients and Longitudinal Vaccinated Populations. Pathogens 2022, 11, 171. https://doi.org/10.3390/pathogens11020171
Hu C, Li D, Liu Z, Ren L, Su J, Zhu M, Feng Y, Wang Z, Liu Q, Zhu B, et al. Exploring Rapid and Effective Screening Methods for Anti-SARS-CoV-2 Neutralizing Antibodies in COVID-19 Convalescent Patients and Longitudinal Vaccinated Populations. Pathogens. 2022; 11(2):171. https://doi.org/10.3390/pathogens11020171
Chicago/Turabian StyleHu, Caiqin, Dan Li, Zhanmou Liu, Li Ren, Junwei Su, Meiling Zhu, Yi Feng, Zheng Wang, Qiang Liu, Biao Zhu, and et al. 2022. "Exploring Rapid and Effective Screening Methods for Anti-SARS-CoV-2 Neutralizing Antibodies in COVID-19 Convalescent Patients and Longitudinal Vaccinated Populations" Pathogens 11, no. 2: 171. https://doi.org/10.3390/pathogens11020171
APA StyleHu, C., Li, D., Liu, Z., Ren, L., Su, J., Zhu, M., Feng, Y., Wang, Z., Liu, Q., Zhu, B., & Shao, Y. (2022). Exploring Rapid and Effective Screening Methods for Anti-SARS-CoV-2 Neutralizing Antibodies in COVID-19 Convalescent Patients and Longitudinal Vaccinated Populations. Pathogens, 11(2), 171. https://doi.org/10.3390/pathogens11020171





