Enterotoxigenic Escherichia coli Heat-Stable Toxin and Ebola Virus Delta Peptide: Similarities and Differences
Abstract
1. Introduction
2. Heat-Stable Toxin II (STb)
2.1. Epidemiology
2.2. Structure
2.3. Pore Formation
2.4. Enterotoxigenic Activity of STb
2.5. Histological Changes
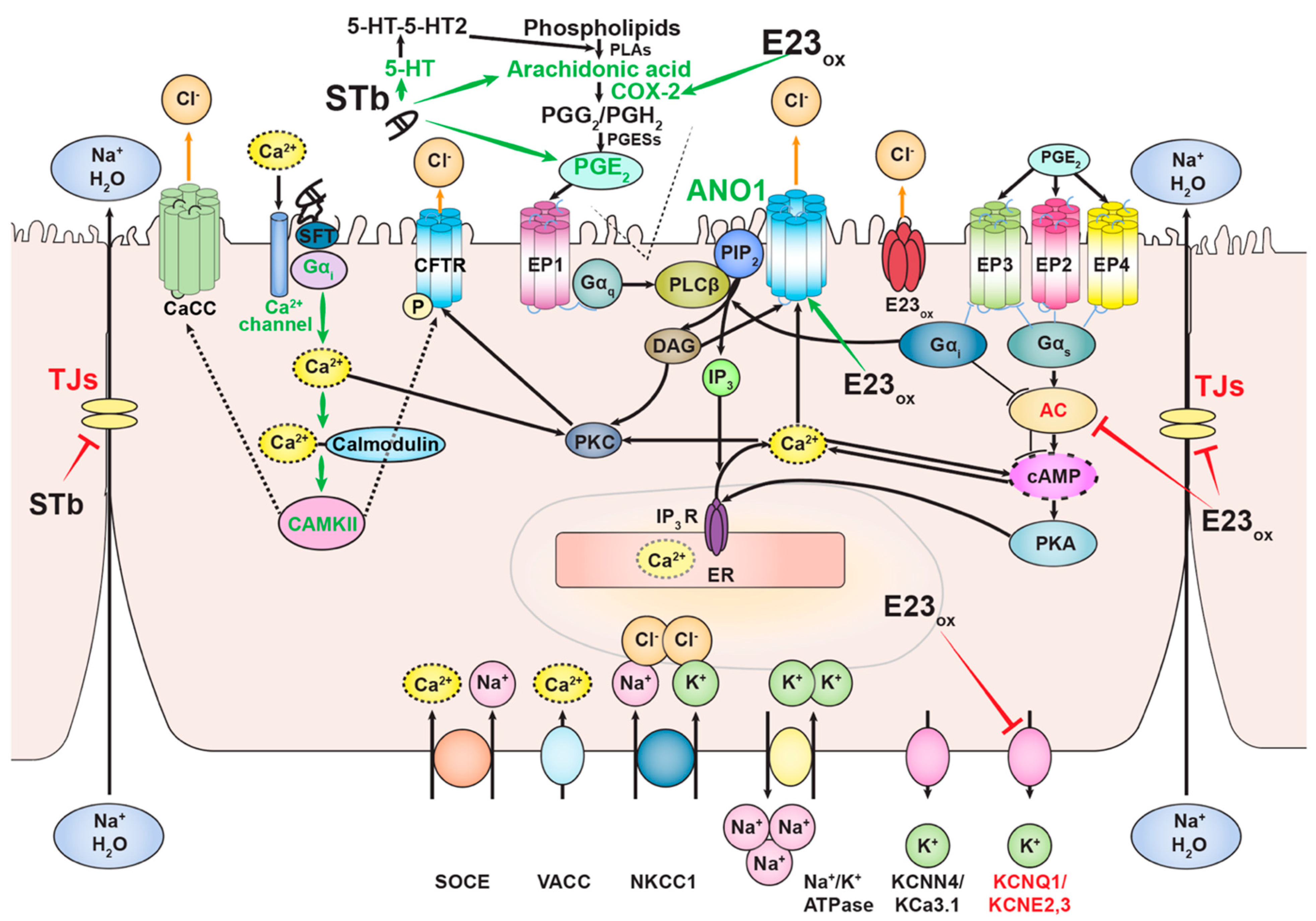
2.6. Mechanism of STb
3. Ebola Virus Delta Peptide
3.1. Epidemiology of Ebola Virus
3.2. Structure
3.3. Viroporin Activity
3.4. Enterotoxigenic Activity
3.5. Histological Changes
3.6. Mechanism of Ebola Virus Delta-Peptide-Induced Diarrhea
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Sack, D.A. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev. Vaccines 2012, 11, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Field, M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Investig. 2003, 111, 931–943. [Google Scholar] [CrossRef]
- Thapar, N.; Sanderson, I.R. Diarrhoea in children: An interface between developing and developed countries. Lancet 2004, 363, 641–653. [Google Scholar] [CrossRef]
- Steffen, R.; Hill, D.R.; DuPont, H.L. Traveler’s diarrhea: A clinical review. JAMA 2015, 313, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.W. Enterotoxins in acute infective diarrhoea. J. Infect. 1992, 24, 225–245. [Google Scholar] [CrossRef]
- Betley, M.J.; Miller, V.L.; Mekalanos, J.J. Genetics of bacterial enterotoxins. Annu. Rev. Microbiol. 1986, 40, 577–605. [Google Scholar] [CrossRef]
- Levine, M.M. Escherichia coli that cause diarrhea: Enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J. Infect. Dis. 1987, 155, 377–389. [Google Scholar] [CrossRef]
- Burgess, M.N.; Bywater, R.J.; Cowley, C.M.; Mullan, N.A.; Newsome, P.M. Biological evaluation of a methanol-soluble, heat-stable Escherichia coli enterotoxin in infant mice, pigs, rabbits, and calves. Infect. Immun. 1978, 21, 526–531. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Greenberg, R.N.; Dunn, J.A.; Abernathy, R.; Ryerse, J.S.; Guerrant, R.L. Effects of Escherichia coli heat-stable enterotoxin STb on intestines of mice, rats, rabbits, and piglets. Infect. Immun. 1984, 46, 639–643. [Google Scholar] [CrossRef]
- Lortie, L.A.; Dubreuil, J.D.; Harel, J. Characterization of Escherichia coli strains producing heat-stable enterotoxin b (STb) isolated from humans with diarrhea. J. Clin. Microbiol. 1991, 29, 656–659. [Google Scholar] [CrossRef]
- Okamoto, K.; Fujii, Y.; Akashi, N.; Hitotsubashi, S.; Kurazono, H.; Karasawa, T.; Takeda, Y. Identification and characterization of heat-stable enterotoxin II-producing Escherichia coli from patients with diarrhea. Microbiol. Immunol. 1993, 37, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.; Panchalingham, S.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob. Health 2019, 7, e568–e584. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 1999, 30, 259–284. [Google Scholar]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Escherichia coli STb enterotoxin. Microbiology (Reading) 1997, 143 Pt 6, 1783–1795. [Google Scholar] [CrossRef]
- Moon, H.W.; Schneider, R.A.; Moseley, S.L. Comparative prevalence of four enterotoxin genes among Escherichia coli isolated from swine. Am. J. Vet. Res. 1986, 47, 210–212. [Google Scholar]
- Chapman, T.A.; Wu, X.Y.; Barchia, I.; Bettelheim, K.A.; Driesen, S.; Trott, D.; Wilson, M.; Chin, J.J. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 2006, 72, 4782–4795. [Google Scholar] [CrossRef]
- Moredo, F.A.; Piñeyro, P.E.; Márquez, G.C.; Sanz, M.; Colello, R.; Etcheverría, A.; Padola, N.L.; Quiroga, M.A.; Perfumo, C.J.; Galli, L.; et al. Enterotoxigenic Escherichia coli Subclinical Infection in Pigs: Bacteriological and Genotypic Characterization and Antimicrobial Resistance Profiles. Foodborne Pathog. Dis. 2015, 12, 704–711. [Google Scholar] [CrossRef]
- Lee, C.H.; Moseley, S.L.; Moon, H.W.; Whipp, S.C.; Gyles, C.L.; So, M. Characterization of the gene encoding heat-stable toxin II and preliminary molecular epidemiological studies of enterotoxigenic Escherichia coli heat-stable toxin II producers. Infect. Immun. 1983, 42, 264–268. [Google Scholar] [CrossRef]
- Picken, R.N.; Mazaitis, A.J.; Maas, W.K.; Rey, M.; Heyneker, H. Nucleotide sequence of the gene for heat-stable enterotoxin II of Escherichia coli. Infect. Immun. 1983, 42, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Taillon, C.; Nadeau, E.; Mourez, M.; Dubreuil, J.D. Heterogeneity of Escherichia coli STb enterotoxin isolated from diseased pigs. J. Med. Microbiol. 2008, 57, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 2005, 295, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Kupersztoch, Y.M.; Tachias, K.; Moomaw, C.R.; Dreyfus, L.A.; Urban, R.; Slaughter, C.; Whipp, S. Secretion of methanol-insoluble heat-stable enterotoxin (STB): Energy- and secA-dependent conversion of pre-STB to an intermediate indistinguishable from the extracellular toxin. J. Bacteriol. 1990, 172, 2427–2432. [Google Scholar] [CrossRef]
- Dreyfus, L.A.; Urban, R.G.; Whipp, S.C.; Slaughter, C.; Tachias, K.; Kupersztoch, Y.M. Purification of the STB enterotoxin of Escherichia coli and the role of selected amino acids on its secretion, stability and toxicity. Mol. Microbiol. 1992, 6, 2397–2406. [Google Scholar] [CrossRef]
- Foreman, D.T.; Martinez, Y.; Coombs, G.; Torres, A.; Kupersztoch, Y.M. TolC and DsbA are needed for the secretion of STB, a heat-stable enterotoxin of Escherichia coli. Mol. Microbiol. 1995, 18, 237–245. [Google Scholar] [CrossRef]
- Fujii, Y.; Hayashi, M.; Hitotsubashi, S.; Fuke, Y.; Yamanaka, H.; Okamoto, K. Purification and characterization of Escherichia coli heat-stable enterotoxin II. J. Bacteriol. 1991, 173, 5516–5522. [Google Scholar] [CrossRef][Green Version]
- Sukumar, M.; Rizo, J.; Wall, M.; Dreyfus, L.A.; Kupersztoch, Y.M.; Gierasch, L.M. The structure of Escherichia coli heat-stable enterotoxin b by nuclear magnetic resonance and circular dichroism. Protein Sci. 1995, 4, 1718–1729. [Google Scholar] [CrossRef]
- Arriaga, Y.L.; Harville, B.A.; Dreyfus, L.A. Contribution of individual disulfide bonds to biological action of Escherichia coli heat-stable enterotoxin B. Infect. Immun. 1995, 63, 4715–4720. [Google Scholar] [CrossRef]
- Okamoto, K.; Baba, T.; Yamanaka, H.; Akashi, N.; Fujii, Y. Disulfide bond formation and secretion of Escherichia coli heat-stable enterotoxin II. J. Bacteriol. 1995, 177, 4579–4586. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Okamuro, Y.; Hitotsubashi, S.; Saito, A.; Akashi, N.; Okamoto, K. Effect of alterations of basic amino acid residues of Escherichia coli heat-stable enterotoxin II on enterotoxicity. Infect. Immun. 1994, 62, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Labrie, V.; Beausoleil, H.E.; Harel, J.; Dubreuil, J.D. Binding to sulfatide and enterotoxicity of various Escherichia coli STb mutants. Microbiology (Reading) 2001, 147, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Labrie, V.; Harel, J.; Dubreuil, J.D. Oligomerization of Escherichia coli enterotoxin b through its C-terminal hydrophobic alpha-helix. Biochim. Biophys. Acta 2001, 1535, 128–133. [Google Scholar] [CrossRef]
- Rousset, E.; Harel, J.; Dubreuil, J.D. Sulfatide from the pig jejunum brush border epithelial cell surface is involved in binding of Escherichia coli enterotoxin b. Infect. Immun. 1998, 66, 5650–5658. [Google Scholar] [CrossRef] [PubMed]
- Rousset, E.; Dubreuil, J.D. Evidence that Escherichia coli STb enterotoxin binds to lipidic components extracted from the pig jejunal mucosa. Toxicon 1999, 37, 1529–1537. [Google Scholar] [CrossRef]
- Sukumar, M.; Rizo, J.; Wall, M.; Dreyfus, L.A.; Kupersztoch, Y.M.; Gierasch, L.M. The Structure of Escherichia coli Heat-Stable Enterotoxin b. 1995. Available online: https://www.rcsb.org/structure/1ehs (accessed on 22 April 2021).
- Schrödinger, L. The PyMol Molecular Graphics System 2.4. Available online: www://www.pymol.org (accessed on 22 April 2021).
- Alouf, J.E. Pore-forming bacterial protein toxins: An overview. Curr. Top. Microbiol. Immunol. 2001, 257, 1–14. [Google Scholar]
- Geny, B.; Popoff, M.R. Bacterial protein toxins and lipids: Pore formation or toxin entry into cells. Biol. Cell 2006, 98, 667–678. [Google Scholar] [CrossRef]
- Gonçalves, C.; Vachon, V.; Schwartz, J.L.; Dubreuil, J.D. The Escherichia coli enterotoxin STb permeabilizes piglet jejunal brush border membrane vesicles. Infect. Immun. 2007, 75, 2208–2213. [Google Scholar] [CrossRef]
- Gonçalves, C.; Dubreuil, J.D. Effect of Escherichia coli STb toxin on NIH-3T3 cells. FEMS Immunol. Med. Microbiol. 2009, 55, 432–441. [Google Scholar] [CrossRef]
- Thress, K.; Kornbluth, S.; Smith, J.J. Mitochondria at the crossroad of apoptotic cell death. J. Bioenerg. Biomembr. 1999, 31, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Gogvadze, V.; Orrenius, S. Mitochondrial regulation of apoptotic cell death. Chem. Biol. Interact. 2006, 163, 4–14. [Google Scholar] [CrossRef]
- Gyles, C.L. Limitations of the infant mouse test for Escherichia coli heat stable enterotoxin. Can. J. Comp. Med. 1979, 43, 371–379. [Google Scholar] [PubMed]
- Kashiwazaki, M.; Nakamura, K.; Sugimoto, C.; Isayama, Y.; Akaike, Y. Diarrhea in piglets due to Escherichia coli that produce only porcine ileal loop-positive heat-stable enterotoxic component. Natl. Inst. Anim. Health Q (Tokyo) 1981, 21, 148–149. [Google Scholar]
- Rose, R.; Whipp, S.C.; Moon, H.W. Effects of Escherichia coli heat-stable enterotoxin b on small intestinal villi in pigs, rabbits, and lambs. Vet. Pathol. 1987, 24, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Whipp, S.C. Protease degradation of Escherichia coli heat-stable, mouse-negative, pig-positive enterotoxin. Infect. Immun. 1987, 55, 2057–2060. [Google Scholar] [CrossRef]
- Walsh, K.A. Trypsinogens and trypsins of various species. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1970; Volume 19, pp. 41–63. [Google Scholar]
- Whipp, S.C. Assay for enterotoxigenic Escherichia coli heat-stable toxin b in rats and mice. Infect. Immun. 1990, 58, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Whipp, S.C. Intestinal responses to enterotoxigenic Escherichia coli heat-stable toxin b in non-porcine species. Am. J. Vet. Res. 1991, 52, 734–737. [Google Scholar]
- Hitotsubashi, S.; Fujii, Y.; Yamanaka, H.; Okamoto, K. Some properties of purified Escherichia coli heat-stable enterotoxin II. Infect. Immun. 1992, 60, 4468–4474. [Google Scholar] [CrossRef]
- Whipp, S.C.; Moon, H.W.; Kemeny, L.J.; Argenzio, R.A. Effect of virus-induced destruction of villous epithelium on intestinal secretion induced by heat-stable Escherichia coli enterotoxins and prostaglandin E1 in swine. Am. J. Vet. Res. 1985, 46, 637–642. [Google Scholar]
- Whipp, S.C.; Moseley, S.L.; Moon, H.W. Microscopic alterations in jejunal epithelium of 3-week-old pigs induced by pig-specific, mouse-negative, heat-stable Escherichia coli enterotoxin. Am. J. Vet. Res. 1986, 47, 615–618. [Google Scholar] [PubMed]
- Sears, C.L.; Kaper, J.B. Enteric bacterial toxins: Mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 1996, 60, 167–215. [Google Scholar] [CrossRef]
- Rousset, E.; Harel, J.; Dubreuil, J.D. Binding characteristics of Escherichia coli enterotoxin b (STb) to the pig jejunum and partial characterization of the molecule involved. Microb. Pathog. 1998, 24, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Taillon, C.; Hancock, M.A.; Mourez, M.; Dubreuil, J.D. Biochemical and biological characterization of Escherichia coli STb His12 to Asn variant. Toxicon 2012, 59, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Labrie, V.; Harel, J.; Dubreuil, J.D. Escherichia coli heat-stable enterotoxin b (STb) in vivo internalization within rat intestinal epithelial cells. Vet. Res. 2002, 33, 223–228. [Google Scholar] [CrossRef]
- Dreyfus, L.A.; Harville, B.; Howard, D.E.; Shaban, R.; Beatty, D.M.; Morris, S.J. Calcium influx mediated by the Escherichia coli heat-stable enterotoxin B (STB). Proc. Natl. Acad. Sci. USA 1993, 90, 3202–3206. [Google Scholar] [CrossRef]
- Ransnäs, L.A.; Leiber, D.; Insel, P.A. Inhibition of subunit dissociation and release of the stimulatory G-protein, Gs, by beta gamma-subunits and somatostatin in S49 lymphoma cell membranes. Biochem. J. 1991, 280 Pt 2, 303–307. [Google Scholar] [CrossRef]
- Fujii, Y.; Nomura, T.; Yamanaka, H.; Okamoto, K. Involvement of Ca2+-calmodulin-dependent protein kinase II in the intestinal secretory action of Escherichia coli heat-stable enterotoxin II. Microbiol. Immunol. 1997, 41, 633–636. [Google Scholar] [CrossRef]
- Peterson, J.W.; Whipp, S.C. Comparison of the mechanisms of action of cholera toxin and the heat-stable enterotoxins of Escherichia coli. Infect. Immun. 1995, 63, 1452–1461. [Google Scholar] [CrossRef]
- Harville, B.A.; Dreyfus, L.A. Involvement of 5-hydroxytryptamine and prostaglandin E2 in the intestinal secretory action of Escherichia coli heat-stable enterotoxin B. Infect. Immun. 1995, 63, 745–750. [Google Scholar] [CrossRef]
- Felder, C.C.; Kanterman, R.Y.; Ma, A.L.; Axelrod, J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc. Natl. Acad. Sci. USA 1990, 87, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kondo, Y.; Okamoto, K. Involvement of prostaglandin E2 synthesis in the intestinal secretory action of Escherichia coli heat-stable enterotoxin II. FEMS Microbiol. Lett. 1995, 130, 259–265. [Google Scholar] [CrossRef]
- Ngendahayo Mukiza, C.; Dubreuil, J.D. Escherichia coli heat-stable toxin b impairs intestinal epithelial barrier function by altering tight junction proteins. Infect. Immun. 2013, 81, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Nassour, H.; Dubreuil, J.D. Escherichia coli STb enterotoxin dislodges claudin-1 from epithelial tight junctions. PLoS ONE 2014, 9, e113273. [Google Scholar] [CrossRef] [PubMed]
- Ebola haemorrhagic fever in Zaire, 1976. Bull. World Health Organ. 1978, 56, 271–293.
- Georges, A.J.; Leroy, E.M.; Renaut, A.A.; Benissan, C.T.; Nabias, R.J.; Ngoc, M.T.; Obiang, P.I.; Lepage, J.P.; Bertherat, E.J.; Bénoni, D.D.; et al. Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: Epidemiologic and health control issues. J. Infect. Dis. 1999, 179 (Suppl. S1), S65–S75. [Google Scholar] [CrossRef]
- Georges-Courbot, M.C.; Sanchez, A.; Lu, C.Y.; Baize, S.; Leroy, E.; Lansout-Soukate, J.; Tévi-Bénissan, C.; Georges, A.J.; Trappier, S.G.; Zaki, S.R.; et al. Isolation and phylogenetic characterization of Ebola viruses causing different outbreaks in Gabon. Emerg. Infect. Dis. 1997, 3, 59–62. [Google Scholar] [CrossRef]
- Lahm, S.A.; Kombila, M.; Swanepoel, R.; Barnes, R.F. Morbidity and mortality of wild animals in relation to outbreaks of Ebola haemorrhagic fever in Gabon, 1994-2003. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 64–78. [Google Scholar] [CrossRef]
- Outbreak(s) of Ebola haemorrhagic fever, Congo and Gabon, October 2001–July 2002. Wkly. Epidemiol. Rec. 2003, 78, 223–228.
- Leroy, E.M.; Rouquet, P.; Formenty, P.; Souquière, S.; Kilbourne, A.; Froment, J.M.; Bermejo, M.; Smit, S.; Karesh, W.; Swanepoel, R.; et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 2004, 303, 387–390. [Google Scholar] [CrossRef]
- Rouquet, P.; Froment, J.M.; Bermejo, M.; Kilbourn, A.; Karesh, W.; Reed, P.; Kumulungui, B.; Yaba, P.; Délicat, A.; Rollin, P.E.; et al. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg. Infect. Dis. 2005, 11, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Reisler, R.B.; Zeng, X.; Schellhase, C.W.; Bearss, J.J.; Warren, T.K.; Trefry, J.C.; Christopher, G.W.; Kortepeter, M.G.; Bavari, S.; Cardile, A.P. Ebola Virus Causes Intestinal Tract Architectural Disruption and Bacterial Invasion in Non-Human Primates. Viruses 2018, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Young, H.A.; Jahrling, P.B.; Davis, K.J.; Larsen, T.; Kagan, E.; Hensley, L.E. Pathogenesis of Ebola hemorrhagic fever in primate models: Evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am. J. Pathol. 2003, 163, 2371–2382. [Google Scholar] [CrossRef]
- Schieffelin, J.S.; Shaffer, J.G.; Goba, A.; Gbakie, M.; Gire, S.K.; Colubri, A.; Sealfon, R.S.; Kanneh, L.; Moigboi, A.; Momoh, M.; et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N. Engl. J. Med. 2014, 371, 2092–2100. [Google Scholar] [CrossRef]
- Dallatomasina, S.; Crestani, R.; Sylvester Squire, J.; Declerk, H.; Caleo, G.M.; Wolz, A.; Stinson, K.; Patten, G.; Brechard, R.; Gbabai, O.B.; et al. Ebola outbreak in rural West Africa: Epidemiology, clinical features and outcomes. Trop. Med. Int. Health 2015, 20, 448–454. [Google Scholar] [CrossRef]
- Mobula, L.M.; Nathalie, M.; Clive, H.; Brantly, K.; Plyler, W.; Brown, J.; Kauffeldt, B.; Eisenhut, D.; Cooper, L.A.; Fankhauser, J. Clinical Manifestations and Modes of Death among Patients with Ebola Virus Disease, Monrovia, Liberia, 2014. Am. J. Trop. Med. Hyg. 2018, 98, 1186–1193. [Google Scholar] [CrossRef]
- Weppelmann, T.A.; Donewell, B.; Haque, U.; Hu, W.; Magalhaes, R.J.S.; Lubogo, M.; Godbless, L.; Shabani, S.; Maeda, J.; Temba, H.; et al. Determinants of patient survival during the 2014 Ebola Virus Disease outbreak in Bong County, Liberia. Glob. Health Res. Policy 2016, 1, 5. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, B.; Teng, G.; Rong, Y.; Sun, L.; Zhang, J.; Du, N.; Liu, L.; Su, H.; Yuan, Y.; et al. Epidemiologic characteristics, clinical manifestations, and risk factors of 139 patients with Ebola virus disease in western Sierra Leone. Am. J. Infect. Control 2016, 44, 1285–1290. [Google Scholar] [CrossRef]
- Petti, S.; Messano, G.A.; Vingolo, E.M.; Marsella, L.T.; Scully, C. The face of Ebola: Changing frequency of haemorrhage in the West African compared with Eastern-Central African outbreaks. BMC Infect. Dis. 2015, 15, 564. [Google Scholar] [CrossRef][Green Version]
- Crowe, S.J.; Maenner, M.J.; Kuah, S.; Erickson, B.R.; Coffee, M.; Knust, B.; Klena, J.; Foday, J.; Hertz, D.; Hermans, V.; et al. Prognostic Indicators for Ebola Patient Survival. Emerg. Infect. Dis. 2016, 22, 217–223. [Google Scholar] [CrossRef]
- Hunt, L.; Gupta-Wright, A.; Simms, V.; Tamba, F.; Knott, V.; Tamba, K.; Heisenberg-Mansaray, S.; Tamba, E.; Sheriff, A.; Conteh, S.; et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: An observational cohort study. Lancet Infect. Dis. 2015, 15, 1292–1299. [Google Scholar] [CrossRef]
- Peters, J.L.; Cho, D.K.; Aluisio, A.R.; Kennedy, S.B.; Massaquoi, M.B.F.; Sahr, F.; Perera, S.M.; Levine, A.C. Environmental temperature and case fatality of patients with Ebola virus disease in Sierra Leone and Liberia, 2014–2015: A retrospective cohort study. Trop. Med. Int. Health 2019, 24, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Haaskjold, Y.L.; Bolkan, H.A.; Krogh, K.O.; Jongopi, J.; Lundeby, K.M.; Mellesmo, S.; Garces, P.S.; Josendal, O.; Opstad, A.; Svensen, E.; et al. Clinical Features of and Risk Factors for Fatal Ebola Virus Disease, Moyamba District, Sierra Leone, December 2014–February 2015. Emerg. Infect. Dis. 2016, 22, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Bah, E.I.; Lamah, M.C.; Fletcher, T.; Jacob, S.T.; Brett-Major, D.M.; Sall, A.A.; Shindo, N.; Fischer, W.A., 2nd; Lamontagne, F.; Saliou, S.M.; et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N. Engl. J. Med. 2015, 372, 40–47. [Google Scholar] [CrossRef]
- Hartley, M.A.; Young, A.; Tran, A.M.; Okoni-Williams, H.H.; Suma, M.; Mancuso, B.; Al-Dikhari, A.; Faouzi, M. Predicting Ebola Severity: A Clinical Prioritization Score for Ebola Virus Disease. PLoS Negl. Trop. Dis. 2017, 11, e0005265. [Google Scholar] [CrossRef] [PubMed]
- Moole, H.; Chitta, S.; Victor, D.; Kandula, M.; Moole, V.; Ghadiam, H.; Akepati, A.; Yerasi, C.; Uppu, A.; Dharmapuri, S.; et al. Association of clinical signs and symptoms of Ebola viral disease with case fatality: A systematic review and meta-analysis. J Community Hosp. Intern. Med. Perspect. 2015, 5, 28406. [Google Scholar] [CrossRef]
- Melnik, L.I.; Guha, S.; Ghimire, J.; Smither, A.R.; Beddingfield, B.J.; Hoffmann, A.R.; Sun, L.; Ungerleider, N.A.; Baddoo, M.C.; Flemington, E.K.; et al. Ebola virus delta peptide is an enterotoxin. Cell Rep. 2022, 38, 110172. [Google Scholar] [CrossRef]
- Volchkov, V.E.; Becker, S.; Volchkova, V.A.; Ternovoj, V.A.; Kotov, A.N.; Netesov, S.V.; Klenk, H.D. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 1995, 214, 421–430. [Google Scholar] [CrossRef]
- Sanchez, A.; Trappier, S.G.; Mahy, B.W.; Peters, C.J.; Nichol, S.T. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA 1996, 93, 3602–3607. [Google Scholar] [CrossRef]
- Volchkov, V.E.; Volchkova, V.A.; Slenczka, W.; Klenk, H.D.; Feldmann, H. Release of viral glycoproteins during Ebola virus infection. Virology 1998, 245, 110–119. [Google Scholar] [CrossRef]
- Volchkova, V.A.; Klenk, H.D.; Volchkov, V.E. Delta-peptide is the carboxy-terminal cleavage fragment of the nonstructural small glycoprotein sGP of Ebola virus. Virology 1999, 265, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Volchkova, V.A.; Dolnik, O.; Martinez, M.J.; Reynard, O.; Volchkov, V.E. Genomic RNA editing and its impact on Ebola virus adaptation during serial passages in cell culture and infection of guinea pigs. J. Infect. Dis. 2011, 204 (Suppl. S3), S941–S946. [Google Scholar] [CrossRef] [PubMed]
- Mehedi, M.; Hoenen, T.; Robertson, S.; Ricklefs, S.; Dolan, M.A.; Taylor, T.; Falzarano, D.; Ebihara, H.; Porcella, S.F.; Feldmann, H. Ebola virus RNA editing depends on the primary editing site sequence and an upstream secondary structure. PLoS Pathog. 2013, 9, e1003677. [Google Scholar] [CrossRef]
- Shabman, R.S.; Jabado, O.J.; Mire, C.E.; Stockwell, T.B.; Edwards, M.; Mahajan, M.; Geisbert, T.W.; Basler, C.F. Deep sequencing identifies noncanonical editing of Ebola and Marburg virus RNAs in infected cells. mBio 2014, 5, e02011. [Google Scholar] [CrossRef]
- Mehedi, M.; Falzarano, D.; Seebach, J.; Hu, X.; Carpenter, M.S.; Schnittler, H.J.; Feldmann, H. A new Ebola virus nonstructural glycoprotein expressed through RNA editing. J. Virol. 2011, 85, 5406–5414. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Yang, Z.Y.; Xu, L.; Nabel, G.J.; Crews, T.; Peters, C.J. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J. Virol. 1998, 72, 6442–6447. [Google Scholar] [CrossRef]
- Volchkova, V.A.; Feldmann, H.; Klenk, H.D.; Volchkov, V.E. The nonstructural small glycoprotein sGP of Ebola virus is secreted as an antiparallel-orientated homodimer. Virology 1998, 250, 408–414. [Google Scholar] [CrossRef]
- Wimley, W.C.; White, S.H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 1996, 3, 842–848. [Google Scholar] [CrossRef]
- Gallaher, W.R.; Garry, R.F. Modeling of the Ebola virus delta peptide reveals a potential lytic sequence motif. Viruses 2015, 7, 285–305. [Google Scholar] [CrossRef]
- Pokhrel, R.; Pavadai, E.; Gerstman, B.S.; Chapagain, P.P. Membrane pore formation and ion selectivity of the Ebola virus delta peptide. Phys. Chem. Chem. Phys. 2019, 21, 5578–5585. [Google Scholar] [CrossRef]
- He, J.; Melnik, L.I.; Komin, A.; Wiedman, G.; Fuselier, T.; Morris, C.F.; Starr, C.G.; Searson, P.C.; Gallaher, W.R.; Hristova, K.; et al. Ebola Virus Delta Peptide Is a Viroporin. J. Virol. 2017, 91, e00438-17. [Google Scholar] [CrossRef] [PubMed]
- Nieva, J.L.; Madan, V.; Carrasco, L. Viroporins: Structure and biological functions. Nat. Rev. Microbiol. 2012, 10, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.H.; Holsinger, L.J.; Lamb, R.A. Influenza virus M2 protein has ion channel activity. Cell 1992, 69, 517–528. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Leser, G.P.; Lamb, R.A. The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J. Cell Biol. 1996, 133, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.H.; Lamb, R.A. The M2 proton channels of influenza A and B viruses. J. Biol. Chem. 2006, 281, 8997–9000. [Google Scholar] [CrossRef]
- Pielak, R.M.; Chou, J.J. Influenza M2 proton channels. Biochim. Biophys. Acta 2011, 1808, 522–529. [Google Scholar] [CrossRef]
- Hout, D.R.; Mulcahy, E.R.; Pacyniak, E.; Gomez, L.M.; Gomez, M.L.; Stephens, E.B. Vpu: A multifunctional protein that enhances the pathogenesis of human immunodeficiency virus type 1. Curr. HIV Res. 2004, 2, 255–270. [Google Scholar] [CrossRef]
- Park, S.H.; Mrse, A.A.; Nevzorov, A.A.; Mesleh, M.F.; Oblatt-Montal, M.; Montal, M.; Opella, S.J. Three-dimensional structure of the channel-forming trans-membrane domain of virus protein “u” (Vpu) from HIV-1. J. Mol. Biol. 2003, 333, 409–424. [Google Scholar] [CrossRef]
- Dubé, M.; Bego, M.G.; Paquay, C.; Cohen, É.A. Modulation of HIV-1-host interaction: Role of the Vpu accessory protein. Retrovirology 2010, 7, 114. [Google Scholar] [CrossRef]
- Costin, J.M.; Rausch, J.M.; Garry, R.F.; Wimley, W.C. Viroporin potential of the lentivirus lytic peptide (LLP) domains of the HIV-1 gp41 protein. Virol. J. 2007, 4, 123. [Google Scholar] [CrossRef]
- Sung, J.H.; Shin, S.A.; Park, H.K.; Montelaro, R.C.; Chong, Y.H. Protective effect of glutathione in HIV-1 lytic peptide 1-induced cell death in human neuronal cells. J. Neurovirol. 2001, 7, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Meyer, J.C.; Bellamy, A.R.; Taylor, J.A. Rotavirus nonstructural glycoprotein NSP4 alters plasma membrane permeability in mammalian cells. J. Virol. 1997, 71, 9458–9465. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.R.; Arifullah, M.; Sastri, N.P.; Jeyakanthan, J.; Ueno, G.; Sekar, K.; Read, R.J.; Dodson, E.J.; Rao, D.C.; Suguna, K. Novel pentameric structure of the diarrhea-inducing region of the rotavirus enterotoxigenic protein NSP4. J. Virol. 2011, 85, 12721–12732. [Google Scholar] [CrossRef] [PubMed]
- Sastri, N.P.; Viskovska, M.; Hyser, J.M.; Tanner, M.R.; Horton, L.B.; Sankaran, B.; Prasad, B.V.; Estes, M.K. Structural plasticity of the coiled-coil domain of rotavirus NSP4. J. Virol. 2014, 88, 13602–13612. [Google Scholar] [CrossRef]
- Ball, J.M.; Tian, P.; Zeng, C.Q.; Morris, A.P.; Estes, M.K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 1996, 272, 101–104. [Google Scholar] [CrossRef]
- Morris, A.P.; Scott, J.K.; Ball, J.M.; Zeng, C.Q.; O’Neal, W.K.; Estes, M.K. NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am. J. Physiol. 1999, 277, G431–G444. [Google Scholar] [CrossRef]
- Ball, J.M.; Mitchell, D.M.; Gibbons, T.F.; Parr, R.D. Rotavirus NSP4: A multifunctional viral enterotoxin. Viral Immunol. 2005, 18, 27–40. [Google Scholar] [CrossRef]
- Ball, J.M.; Schroeder, M.E.; Williams, C.V.; Schroeder, F.; Parr, R.D. Mutational analysis of the rotavirus NSP4 enterotoxic domain that binds to caveolin-1. Virol. J. 2013, 10, 336. [Google Scholar] [CrossRef]
- Pérez, J.F.; Ruiz, M.C.; Chemello, M.E.; Michelangeli, F. Characterization of a membrane calcium pathway induced by rotavirus infection in cultured cells. J. Virol. 1999, 73, 2481–2490. [Google Scholar] [CrossRef]
- Tian, P.; Hu, Y.; Schilling, W.P.; Lindsay, D.A.; Eiden, J.; Estes, M.K. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J. Virol. 1994, 68, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Chang-Graham, A.L.; Perry, J.L.; Strtak, A.C.; Ramachandran, N.K.; Criglar, J.M.; Philip, A.A.; Patton, J.T.; Estes, M.K.; Hyser, J.M. Rotavirus Calcium Dysregulation Manifests as Dynamic Calcium Signaling in the Cytoplasm and Endoplasmic Reticulum. Sci. Rep. 2019, 9, 10822. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Estes, M.K.; Hu, Y.; Ball, J.M.; Zeng, C.Q.; Schilling, W.P. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J. Virol. 1995, 69, 5763–5772. [Google Scholar] [CrossRef] [PubMed]
- Hyser, J.M.; Collinson-Pautz, M.R.; Utama, B.; Estes, M.K. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity. mBio 2010, 1, e00265-10. [Google Scholar] [CrossRef]
- Pham, T.; Perry, J.L.; Dosey, T.L.; Delcour, A.H.; Hyser, J.M. The Rotavirus NSP4 Viroporin Domain is a Calcium-conducting Ion Channel. Sci. Rep. 2017, 7, 43487. [Google Scholar] [CrossRef]
- Crawford, S.E.; Hyser, J.M.; Utama, B.; Estes, M.K. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-β signaling is required for rotavirus replication. Proc. Natl. Acad. Sci. USA 2012, 109, E3405–E3413. [Google Scholar] [CrossRef]
- Tanwar, J.; Motiani, R.K. Role of SOCE architects STIM and Orai proteins in Cell Death. Cell Calcium 2018, 69, 19–27. [Google Scholar] [CrossRef]
- Bhowmick, R.; Halder, U.C.; Chattopadhyay, S.; Chanda, S.; Nandi, S.; Bagchi, P.; Nayak, M.K.; Chakrabarti, O.; Kobayashi, N.; Chawla-Sarkar, M. Rotaviral enterotoxin nonstructural protein 4 targets mitochondria for activation of apoptosis during infection. J. Biol. Chem. 2012, 287, 35004–35020. [Google Scholar] [CrossRef]
- Dickman, K.G.; Hempson, S.J.; Anderson, J.; Lippe, S.; Zhao, L.; Burakoff, R.; Shaw, R.D. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G757–G766. [Google Scholar] [CrossRef]
- Dong, Y.; Zeng, C.Q.; Ball, J.M.; Estes, M.K.; Morris, A.P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc. Natl. Acad. Sci. USA 1997, 94, 3960–3965. [Google Scholar] [CrossRef]
- Seo, N.S.; Zeng, C.Q.; Hyser, J.M.; Utama, B.; Crawford, S.E.; Kim, K.J.; Höök, M.; Estes, M.K. Integrins alpha1beta1 and alpha2beta1 are receptors for the rotavirus enterotoxin. Proc. Natl. Acad. Sci. USA 2008, 105, 8811–8818. [Google Scholar] [CrossRef] [PubMed]
- Sawasvirojwong, S.; Srimanote, P.; Chatsudthipong, V.; Muanprasat, C. An Adult Mouse Model of Vibrio cholerae-induced Diarrhea for Studying Pathogenesis and Potential Therapy of Cholera. PLoS Negl. Trop. Dis. 2013, 7, e2293. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jayaratne, R.; Barrett, K.E. The Role of Ion Transporters in the Pathophysiology of Infectious Diarrhea. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.J. Pathophysiology of acute diarrhea. Am. J. Med. 1990, 88, 2s–4s. [Google Scholar] [CrossRef]
- Binder, H.J. Development and Pathophysiology of Oral Rehydration Therapy for the Treatment for Diarrhea. Dig. Dis. Sci. 2020, 65, 349–354. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Mirza, M.; Tian, Y.; Roussa, E.; Schreiber, R.; Cook, D.I.; Kunzelmann, K. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflug. Arch. 2011, 461, 579–589. [Google Scholar] [CrossRef]
- Halaihel, N.; Liévin, V.; Ball, J.M.; Estes, M.K.; Alvarado, F.; Vasseur, M. Direct inhibitory effect of rotavirus NSP4(114-135) peptide on the Na+-D-glucose symporter of rabbit intestinal brush border membrane. J. Virol. 2000, 74, 9464–9470. [Google Scholar] [CrossRef]
- Rautanen, T.; Kurki, S.; Vesikari, T. Randomised double blind study of hypotonic oral rehydration solution in diarrhoea. Arch. Dis. Child 1997, 76, 272–274. [Google Scholar] [CrossRef]
- Davidson, G.P.; Barnes, G.L. Structural and functional abnormalities of the small intestine in infants and young children with rotavirus enteritis. Acta Paediatr. Scand. 1979, 68, 181–186. [Google Scholar] [CrossRef]
- Ramig, R.F. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 2004, 78, 10213–10220. [Google Scholar] [CrossRef]
- Kato, A.; Romero, M.F. Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol. 2011, 73, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Shcheynikov, N.; Wang, Y.; Park, M.; Ko, S.B.; Dorwart, M.; Naruse, S.; Thomas, P.J.; Muallem, S. Coupling modes and stoichiometry of Cl-/HCO3- exchange by slc26a3 and slc26a6. J. Gen. Physiol. 2006, 127, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Shcheynikov, N.; Ko, S.B.; Zeng, W.; Choi, J.Y.; Dorwart, M.R.; Thomas, P.J.; Muallem, S. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found. Symp. 2006, 273, 177–186, discussion 186–192, 261–174. [Google Scholar] [PubMed]
- Mount, D.B.; Romero, M.F. The SLC26 gene family of multifunctional anion exchangers. Pflug. Arch. 2004, 447, 710–721. [Google Scholar] [CrossRef]
- Chernova, M.N.; Jiang, L.; Friedman, D.J.; Darman, R.B.; Lohi, H.; Kere, J.; Vandorpe, D.H.; Alper, S.L. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: Differences in anion selectivity, regulation, and electrogenicity. J. Biol. Chem. 2005, 280, 8564–8580. [Google Scholar] [CrossRef]
- Coady, M.J.; Chang, M.H.; Charron, F.M.; Plata, C.; Wallendorff, B.; Sah, J.F.; Markowitz, S.D.; Romero, M.F.; Lapointe, J.Y. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J. Physiol. 2004, 557, 719–731. [Google Scholar] [CrossRef]
- Gopal, E.; Fei, Y.J.; Sugawara, M.; Miyauchi, S.; Zhuang, L.; Martin, P.; Smith, S.B.; Prasad, P.D.; Ganapathy, V. Expression of slc5a8 in kidney and its role in Na+-coupled transport of lactate. J. Biol. Chem. 2004, 279, 44522–44532. [Google Scholar] [CrossRef]
- Teramae, H.; Yoshikawa, T.; Inoue, R.; Ushida, K.; Takebe, K.; Nio-Kobayashi, J.; Iwanaga, T. The cellular expression of SMCT2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomed. Res. 2010, 31, 239–249. [Google Scholar] [CrossRef]
- Srinivas, S.R.; Gopal, E.; Zhuang, L.; Itagaki, S.; Martin, P.M.; Fei, Y.J.; Ganapathy, V.; Prasad, P.D. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2). Biochem. J. 2005, 392, 655–664. [Google Scholar] [CrossRef]
- Skou, J.C. Nobel Lecture. The identification of the sodium pump. Biosci. Rep. 1998, 18, 155–169. [Google Scholar] [CrossRef]
- Camilleri, M.; Sellin, J.H.; Barrett, K.E. Pathophysiology, Evaluation, and Management of Chronic Watery Diarrhea. Gastroenterology 2017, 152, 515–532.e2. [Google Scholar] [CrossRef] [PubMed]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Uliyakina, I.; Kongsuphol, P.; Warth, R.; Mirza, M.; Martins, J.R.; Kunzelmann, K. Expression and function of epithelial anoctamins. J. Biol. Chem. 2010, 285, 7838–7845. [Google Scholar] [CrossRef] [PubMed]
- Almaça, J.; Tian, Y.; Aldehni, F.; Ousingsawat, J.; Kongsuphol, P.; Rock, J.R.; Harfe, B.D.; Schreiber, R.; Kunzelmann, K. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J. Biol. Chem. 2009, 284, 28571–28578. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Martins, J.R.; Schreiber, R.; Rock, J.R.; Harfe, B.D.; Kunzelmann, K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J. Biol. Chem. 2009, 284, 28698–28703. [Google Scholar] [CrossRef]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef]
- Romanenko, V.G.; Catalán, M.A.; Brown, D.A.; Putzier, I.; Hartzell, H.C.; Marmorstein, A.D.; Gonzalez-Begne, M.; Rock, J.R.; Harfe, B.D.; Melvin, J.E. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J. Biol. Chem. 2010, 285, 12990–13001. [Google Scholar] [CrossRef]
- Rock, J.R.; O’Neal, W.K.; Gabriel, S.E.; Randell, S.H.; Harfe, B.D.; Boucher, R.C.; Grubb, B.R. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl- secretory channel in mouse airways. J. Biol Chem. 2009, 284, 14875–14880. [Google Scholar] [CrossRef]
- Oh, S.J.; Hwang, S.J.; Jung, J.; Yu, K.; Kim, J.; Choi, J.Y.; Hartzell, H.C.; Roh, E.J.; Lee, C.J. MONNA, a potent and selective blocker for transmembrane protein with unknown function 16/anoctamin-1. Mol. Pharmacol. 2013, 84, 726–735. [Google Scholar] [CrossRef]
- Namkung, W.; Phuan, P.W.; Verkman, A.S. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J. Biol. Chem. 2011, 286, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, D.M.; Kim, S.; Jensen, A.B.; Matchkov, V.M.; Andersson, K.E. New selective inhibitors of calcium-activated chloride channels—T16A(inh)-A01, CaCC(inh)-A01 and MONNA—What do they inhibit? Br. J. Pharmacol. 2015, 172, 4158–4172. [Google Scholar] [CrossRef]
- Fujii, S.; Suzuki, K.; Kawamoto, A.; Ishibashi, F.; Nakata, T.; Murano, T.; Ito, G.; Shimizu, H.; Mizutani, T.; Oshima, S.; et al. PGE(2) is a direct and robust mediator of anion/fluid secretion by human intestinal epithelial cells. Sci. Rep. 2016, 6, 36795. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Shimizu, T.; Oguchi, S.; Sato, M. Prostaglandins in the plasma and stool of children with rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 1989, 9, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Båtshake, B.; Nilsson, C.; Sundelin, J. Molecular characterization of the mouse prostanoid EP1 receptor gene. Eur. J. Biochem. 1995, 231, 809–814. [Google Scholar] [CrossRef]
- Yang, H.; Jan, L.Y. TMEM16 membrane proteins in health and disease. In Ion Channels in Health and Disease; Pitt, G.S., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume Perspectives in Translational Cell Biology; pp. 165–197. [Google Scholar]
- Jin, X.; Shah, S.; Liu, Y.; Zhang, H.; Lees, M.; Fu, Z.; Lippiat, J.D.; Beech, D.J.; Sivaprasadarao, A.; Baldwin, S.A.; et al. Activation of the Cl− channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci. Signal. 2013, 6, ra73. [Google Scholar] [CrossRef]
- Dey, I.; Lejeune, M.; Chadee, K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br. J. Pharmacol. 2006, 149, 611–623. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; Zheng, F.; Guan, Y.; Zhang, X. Prostaglandin E2 in the Regulation of Water Transport in Renal Collecting Ducts. Int. J. Mol. Sci. 2017, 18, 2539. [Google Scholar] [CrossRef]
- Rajagopal, M.; Thomas, S.V.; Kathpalia, P.P.; Chen, Y.; Pao, A.C. Prostaglandin E2 induces chloride secretion through crosstalk between cAMP and calcium signaling in mouse inner medullary collecting duct cells. Am. J. Physiol. Cell Physiol. 2014, 306, C263–C278. [Google Scholar] [CrossRef]
- Breyer, M.D.; Breyer, R.M. G protein-coupled prostanoid receptors and the kidney. Annu. Rev. Physiol. 2001, 63, 579–605. [Google Scholar] [CrossRef]
- Irie, A.; Segi, E.; Sugimoto, Y.; Ichikawa, A.; Negishi, M. Mouse prostaglandin E receptor EP3 subtype mediates calcium signals via Gi in cDNA-transfected Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 1994, 204, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Whipp, S.C.; Kokue, E.; Morgan, R.W.; Rose, R.; Moon, H.W. Functional significance of histologic alterations induced by Escherichia coli pig-specific, mouse-negative, heat-stable enterotoxin (STb). Vet. Res. Commun. 1987, 11, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Kordasti, S.; Sjövall, H.; Lundgren, O.; Svensson, L. Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut 2004, 53, 952–957. [Google Scholar] [CrossRef] [PubMed]
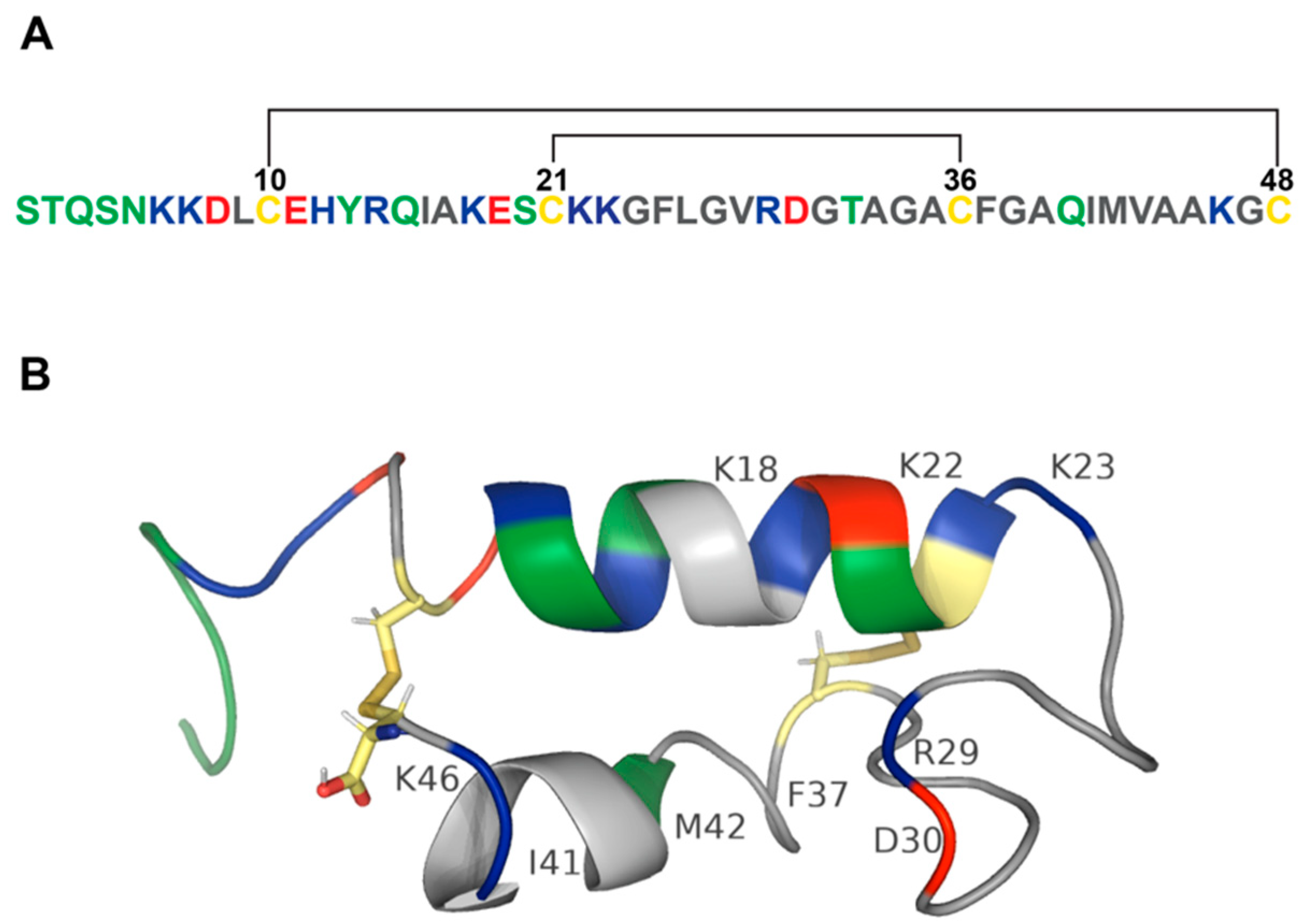

| Amino Acid | Enterotoxicity | Receptor Binding | Oligomerization | Pore Formation | TJ Opening |
|---|---|---|---|---|---|
| C10 | • | • | • | ||
| K18 | • | ||||
| C21 | • | • | • | ||
| K22 | • | • | • | ||
| K23 | • | • | • | ||
| R29 | • | • | |||
| D30 | • | • | • | ||
| C36 | • | • | • | ||
| F37 | • | • | • | ||
| I41 | • | • | • | ||
| M42 | • | • | • | • | |
| K46 | • | ||||
| C48 | • | • | • |
| Cationic Residues | α-Helices | Disulfide Bonds | Chloride Channels | PGE2 Synthesis | Calcium Signaling | TJ Opening | |
|---|---|---|---|---|---|---|---|
| ETEC STb | • | • | • | • | • | • | |
| EBOV delta peptide | • | • | • | • | • |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnik, L.I.; Garry, R.F. Enterotoxigenic Escherichia coli Heat-Stable Toxin and Ebola Virus Delta Peptide: Similarities and Differences. Pathogens 2022, 11, 170. https://doi.org/10.3390/pathogens11020170
Melnik LI, Garry RF. Enterotoxigenic Escherichia coli Heat-Stable Toxin and Ebola Virus Delta Peptide: Similarities and Differences. Pathogens. 2022; 11(2):170. https://doi.org/10.3390/pathogens11020170
Chicago/Turabian StyleMelnik, Lilia I., and Robert F. Garry. 2022. "Enterotoxigenic Escherichia coli Heat-Stable Toxin and Ebola Virus Delta Peptide: Similarities and Differences" Pathogens 11, no. 2: 170. https://doi.org/10.3390/pathogens11020170
APA StyleMelnik, L. I., & Garry, R. F. (2022). Enterotoxigenic Escherichia coli Heat-Stable Toxin and Ebola Virus Delta Peptide: Similarities and Differences. Pathogens, 11(2), 170. https://doi.org/10.3390/pathogens11020170






