Grass Snakes (Natrix natrix) as a Reservoir of Alaria alata and Other Parasites
Abstract
:1. Introduction
2. Results
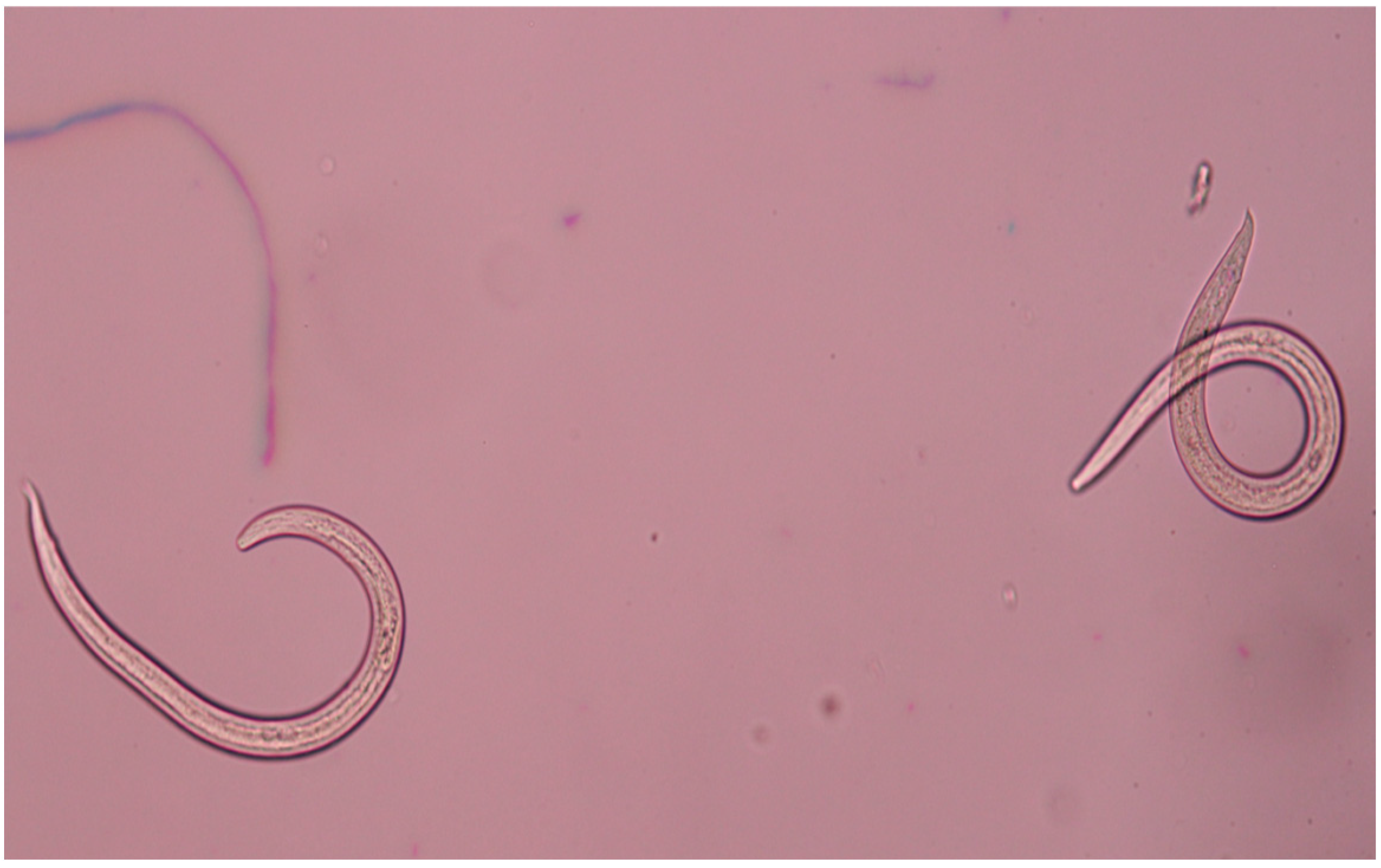
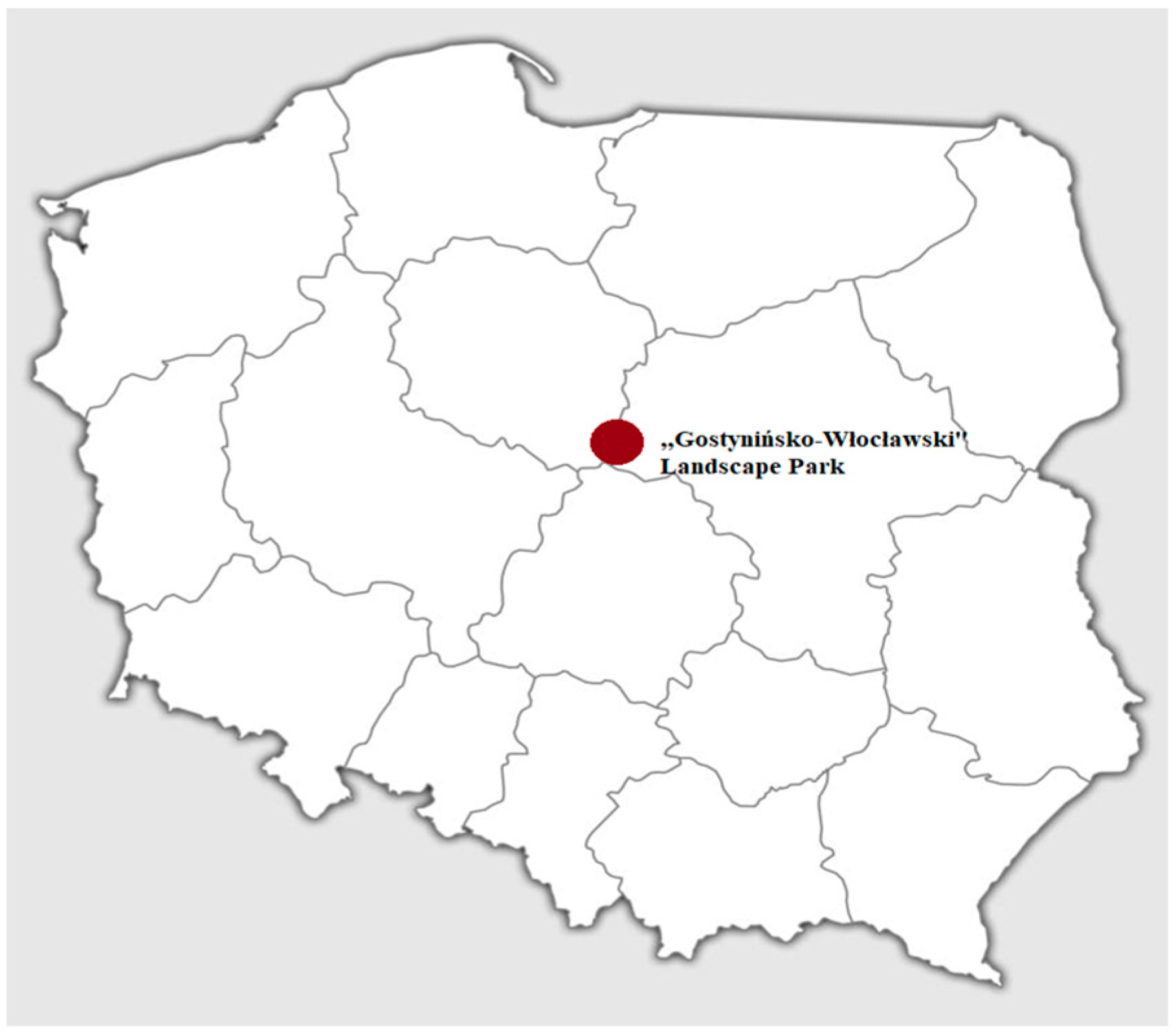
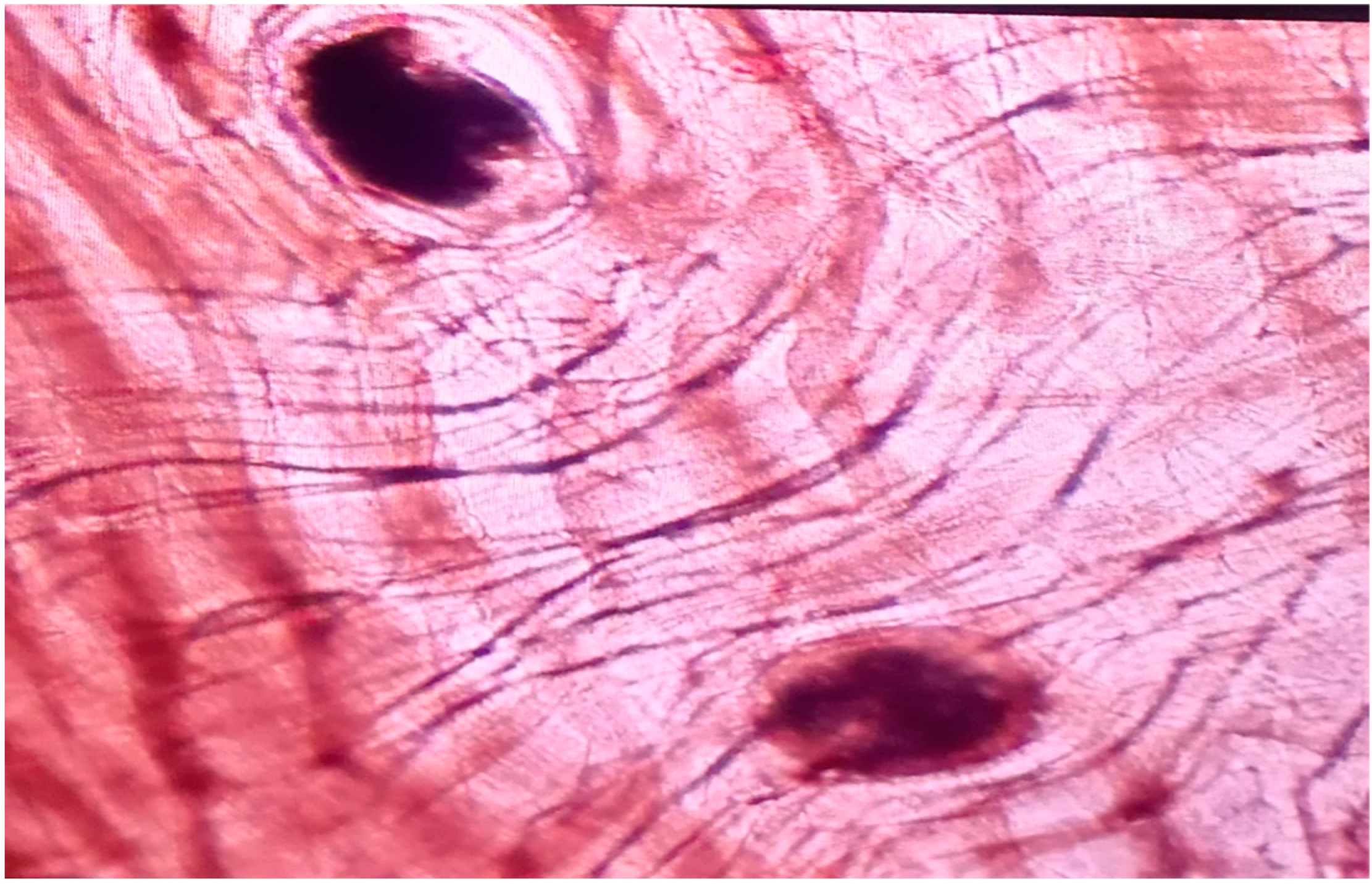
2.1. Prevalence the Parasites in a Sample Population of Grass Snakes in “Gostynińsko-Włocławski Landscape Park”
2.2. Molecular Characterizations of Helminths
3. Discussion
4. Materials and Methods
4.1. Preparing of Samples
4.2. Statistical Analysis
4.3. Species Identification by PCR Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sulgostowska, T. Some parasites of grass snake Natrix natrix (L.) from Warszawa environment. State Sci. Publ. 1971, 19, 195–199. [Google Scholar]
- Borkovcova, M.; Kopřiva, J. Parasitic helminths of reptiles (Reptilia) in south Moravia (Czech Republic). Parasitol. Res. 2005, 95, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Isaac, L.A.; Gregory, P.T. Thermoregulatory behaviour of gravid and non-gravid female grass snakes (Natrix natrix) in a thermally limiting high-latitude environment. J. Zool. 2004, 264, 403–409. [Google Scholar] [CrossRef]
- Mihalca, A.D.; Miclaus, V.; Lefkaditis, M. Pulmonary Lesions caused by the Nematode Rhabdias fuscovenosa in a Grass Snake, Natrix natrix. J. Wildl. Dis. 2010, 46, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.V.; Chizhikova, N.A.; Pavlov, A.V. Microclimatic Conditions of Environment in Termobiology of Natrix natrix. Uchenye Zap. Kazan. Univ.-Seriya EStestvennye Nauk. 2010, 152, 237–250. [Google Scholar]
- Herczek, A.; Gorczyca, J. Płazy i gady Polski. Wydaw. Kubajak 2004, 1, 84–88. [Google Scholar]
- Juszczyk, W. Płazy i gady krajowe. Cz. 3. Gady-Reptilia. Wars. State Sci. Publ. 1987, 1, 70–71. [Google Scholar]
- Erdoğan, D.; Tosunoğlu, M. Plasma Biochemical parameters of Natrix natrix (Linnaeus, 1758; Squamata: Natricidae) population in Çanakkale. Russ. J. Herpetol. 2017, 24, 35–40. [Google Scholar] [CrossRef]
- Brown, P.R. Ecology and Vagility of the Grass Snake, Natrix natrix Helvetica Lacepede. Ph.D. Thesis, University of Southampton, Southampton, UK, 1991. [Google Scholar]
- Filippi, E.; Luiselli, L. Negative effect of the wild boar (Sus scrofa) on the populations of snakes at a protected mountains forest in central Italy. Ecol. Mediterr. 2002, 28, 93–98. [Google Scholar] [CrossRef]
- Möhl, K.; Grosse, K.; Hamedy, A.; Wüste, T.; Kabelitz, P.; Lücker, E. Biology of Alaria spp. and human exposition risk to Alaria mesocercariae—A review. Parasit. Res. 2009, 105, 1–15. [Google Scholar] [CrossRef]
- Beaver, P.C.; Little, M.D.; Tucker, C.F.; Reed, R.J. Mesocercaria in the Skin of Man in Louisiana. Am. J. Trop. Med. Hyg. 1977, 26, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Wasyl, D.; Różycki, M.; Bilska-Zając, E.; Fafiński, Z.; Iwaniak, W.; Krajewska, M.; Hoszowski, A.; Konieczna, O.; Fafińska, P.; et al. Free-Living snakes as a source and possible vector of Salmonella spp. and parasites. Eur. J. Wildl. 2016, 62, 161–166. [Google Scholar] [CrossRef]
- Gradba-Kazubska, B. Parasites of the grass snake Natrix natrix (L.) in Poland. Wiad. Parazytol. 1961, 7, 199–201. [Google Scholar]
- Lewin, J. Parasites of the water snake, Natrix natrix L., in Poland. Act. Parasitol. 1992, 4, 37. [Google Scholar]
- Mihalca, A.D.; Gherman, C.; Ghira, I.; Cozma, V. Severe granulomatous lesions in several organs from Eustrongylides larvae in a free-ranging dice snake, Natrix Tessellata. Vet. Pathol. 2007, 44, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, A.R.; Grygon-Franckiewicz, B.; Zbikowska, E. Current data of Alaria alata (Goeze, 1782) according to own studies. Vet. Med.-Sci. Pract. 2002, 58, 517–519. [Google Scholar]
- Takeuchi-Storm, N. Alaria alata Mesocercariae among feral cats and badgers, Denmark. Emerg. Infect. Dis. 2015, 21, 1872–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korpysa-Dzirba, W.; Różycki, M.; Bilska-Zając, E.; Karamon, J.; Sroka, J.; Bełcik, A.; Wasiak, M.; Cencek, T. Alaria alata in Terms of Risks to Consumers’ Health. Foods 2021, 10, 1614. [Google Scholar] [CrossRef]
- Chmurzyńska, E.; Różycki, M.; Bilska-Zając, E.; Karamon, J.; Cencek, T. Alaria alata-Potential threat for humans, prevalence and diagnostic measures. Vet. Life 2013, 88, 780–784. [Google Scholar]
- Karamon, J.; Sroka, J.; Dąbrowska, J.; Bilska-Zając, E.; Skrzypek, K.; Różycki, M.; Zdybel, J.; Cencek, T. Distribution of Parasitic Helminths in the Small Intestine of the Red Fox (Vulpes vulpes). Pathogens 2020, 9, 477. [Google Scholar] [CrossRef]
- Bilska-Zajac, E.; Marucci, G.; Piróg-Komorowska, A.; Cichocka, M.; Różycki, M.; Karamon, J.; Sroka, J.; Bełcik, A.; Mizak, I.; Cencek, T. Occurrence of Alaria alata in wild boars (Sus scrofa) in Poland and detection of genetic variability between isolates. Parasitol. Res. 2021, 120, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Strokowska, N.; Klich, D.; Bełkot, Z.; Wiśniewski, J.; Didkowska, A.; Chyla, P.; Anusz, K. The occurrence of Alaria alata mesocercariae in wild boars (Sus scrofa) in north-eastern Poland. Int. J. Parasitol.-Parasites Wildl. 2020, 12, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Riehn, K.; Hamedy, A.; Grosse, K.; Zeitler, L.; Lücker, E. A novel detection method for Alaria alata mesocercariae in meat. Parasit. Res. 2010, 107, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.J.; Cooper, J.D.; Cullen, J.B.; Freeman, R.S.; Ritchie, A.C.; Scott, A.A.; Stuart, P.F. Systemic infection with Alaria Am. (Trematoda). Can. Med. Assoc. J. 1976, 115, 1111–1114. [Google Scholar]
- McDonald, H.R.; Kazacos, K.R.; Schatz, H.; Johnson, R.N. Two cases of intraocular infection with Alaria mesocercaria (Trematoda). Am. J. Ophthalmol. 1994, 117, 447–455. [Google Scholar] [CrossRef]
- Lewin, J.; Grabda-Kazubska, B. Parasites of Vipera berus L. in Poland. Act. Parasitol. 1997, 42, 92–96. [Google Scholar]
- Shimalov, V.; Shimalov, V. Helminth fauna of snakes (Reptilia, Serpentes) in Belorussian Polesye. Parasitol. Res. 2000, 86, 340–341. [Google Scholar] [CrossRef]
- Kirillov, A.; Kirillova, N.Y. Helminth fauna of reptiles in the National Park «Smolny», Russia. Nat. Conserv. Res. 2021, 6, 19–22. [Google Scholar] [CrossRef]
- Komorova, P.; Sitko, J.; Špakulová, M.; Hurníková, Z. Intestinal and liver flukes of birds of prey (Accipitriformes, Falconiformes, Strigiformes) from Slovakia: Uniform or diverse compound? Parasitol. Res. 2016, 115, 2837–2844. [Google Scholar] [CrossRef]
- Sitko, J.Z. Trematodes of birds of prey (Falconiformes) in Czech Republic. Helminthology 1998, 35, 131–146. [Google Scholar]
- Taft, S.J.; Suchow, K.; Van Horn, M. Helminths from some Minnesota and Wisconsin raptors. Proc. Helminthol. Soc. Wash 1993, 60, 260–263. [Google Scholar]
- Borgsteede, F.H.M.; Okulewicz, A.; Zoun, P.E.F.; Okulewicz, J. The helminth fauna of birds of prey (Accipitriformes, Falconiformes and Strigiformes) in the Netherlands. ACTA Parasitol. 2003, 48, 200–207. [Google Scholar]
- Sanmartin, M.L.; Alvarez, F.; Barreiro, G.; Leiro, J. Helminth fauna of Falconiform and Strigiform birds of prey in Galicia, Northwest Spain. Parasitol. Res. 2004, 92, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Mortier, J.R.; Fina, C.J.; Edery, E.; White, C.L.; Dhumeaux, M.P. Computed tomographic findings in three dogs naturally infected with Crenosoma vulpis. Vet. Radiol. Ultrasound 2018, 59, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Tolnai, Z.; Széll, Z.; Sréter, T. Environmental determinants of the spatial distribution of Angiostrongylus vasorum, Crenosoma vulpis and Eucoleus aerophilus in Hungary. Vet. Parasitol. 2015, 207, 355–358. [Google Scholar] [CrossRef]
- Morandi, B.; Bertaso, S.; Conboy, G.; Gustinelli, A.; Galuppi, R.; Tosi, G.; Poglayen, G. Crenosoma vulpis in red foxes (Vulpes vulpes) in Northern Italy. Parasitol. Res. 2019, 118, 1981–1985. [Google Scholar] [CrossRef]
- Bihr, T.P. Crenosoma vulpis and the Domestic Dog: A Study of Prevalence on Prince Edward Island and of New Diagnostic Approaches; Univerity of Prince Edward Island: Charlottetown, PE, Canada, 1998. [Google Scholar]
- Santoro, M.; Tkach, V.V.; Mattiucci, S.; Kinsella, J.M.; Nascetti, G. Renifer aniarum (Digenea: Reniferidae), an introduced North American parasite in grass snakes Natrix natrix in Calabria, southern Italy. Dis. Aquat. Org. 2011, 95, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Ammann, M.; Chambrier, A. Ophiotaenia gilberti sp. n. (Eucestoda: Proteocephalidea), a parasite of Thamnodynastes pallidus (Serpentes: Colubridae) from Paraguay. Rev. Suisse Zool. Ann. Soc. Zool. Suisse Mus. D’histoire Nat. Genève 2008, 115, 541–551. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2020/1478 of 14 October 2020 Amending Implementing Regulation (EU) 2015/1375 as Regards Sampling, the Reference Method for Detection and Import Conditions Related to Trichinella Control (Text with EEA Relevance) C/2020/6922. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2020.338.01.0007.01.ENG&toc=OJ%3AL%3A2020%3A338%3ATOC (accessed on 7 December 2021).
- Blaxter, M.L.; De Ley, P.; Garey, J.R.; Liu, L.X.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; Frisse, L.M.; et al. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef]
- Allen, S.; Greig, C.; Rowson, B.; Gasser, R.B.; Jabbar, A.; Morelli, S.; Morgan, E.R.; Wood, M.; Forman, D. DNA Footprints: Using Parasites to Detect Elusive Animals, Proof of Principle in Hedgehogs. Animals 2020, 10, 1420. [Google Scholar] [CrossRef]
- Olson, P.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef]
- Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome (accessed on 23 November 2021).



| Class of Helminth | Species of Helminth | No. of Infected Snakes [Prevalence (%)] | [Median; Intensity * (Range)] |
|---|---|---|---|
| Trematodes | A. alata | 25/51 [49] | [65; 13–319] |
| A. alata + C. spathula | 2/51 [3.9] | [46; 42–50] | |
| A. alata + C. spathula + S. falconis | 1/51 [1.9] | [125; 4–125] | |
| A. alata + S. falconis | 2/51 [3.9] | [218; 70–366] | |
| C. spathula | 5/51 [9.8] | [162; 5–320] | |
| C. spathula + N. attenuatum | 1/51 [1.9] | [10; 1–10] | |
| C. spathula + S. falconis | 7/51 [13.7] | [34; 3–335] | |
| S. falconis | 2/5 [13.9] | [9.5; 4–15] | |
| N. attenuatum | 1/51 [1.9] | [7.5; 1] | |
| Unidentified | 4/51 [7.8] | [7.5; 1] | |
| Nematodes | C. vulpis | 4/51 [7.8] | [3.5; 1–17] |
| Cestodes | Ophiotaenia | 1/51 [1.9] | [1; 1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bełcik, A.; Różycki, M.; Korpysa-Dzirba, W.; Marucci, G.; Fafiński, Z.; Fafińska, P.; Karamon, J.; Kochanowski, M.; Cencek, T.; Bilska-Zając, E. Grass Snakes (Natrix natrix) as a Reservoir of Alaria alata and Other Parasites. Pathogens 2022, 11, 156. https://doi.org/10.3390/pathogens11020156
Bełcik A, Różycki M, Korpysa-Dzirba W, Marucci G, Fafiński Z, Fafińska P, Karamon J, Kochanowski M, Cencek T, Bilska-Zając E. Grass Snakes (Natrix natrix) as a Reservoir of Alaria alata and Other Parasites. Pathogens. 2022; 11(2):156. https://doi.org/10.3390/pathogens11020156
Chicago/Turabian StyleBełcik, Aneta, Mirosław Różycki, Weronika Korpysa-Dzirba, Gianluca Marucci, Zbigniew Fafiński, Patrycja Fafińska, Jacek Karamon, Maciej Kochanowski, Tomasz Cencek, and Ewa Bilska-Zając. 2022. "Grass Snakes (Natrix natrix) as a Reservoir of Alaria alata and Other Parasites" Pathogens 11, no. 2: 156. https://doi.org/10.3390/pathogens11020156
APA StyleBełcik, A., Różycki, M., Korpysa-Dzirba, W., Marucci, G., Fafiński, Z., Fafińska, P., Karamon, J., Kochanowski, M., Cencek, T., & Bilska-Zając, E. (2022). Grass Snakes (Natrix natrix) as a Reservoir of Alaria alata and Other Parasites. Pathogens, 11(2), 156. https://doi.org/10.3390/pathogens11020156






