Schistosomes Impede ATP-Induced T Cell Apoptosis In Vitro: The Role of Ectoenzyme SmNPP5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parasites and Mice
2.2. T Cell Purification
2.3. Measuring the Impact of Schistosomes and rSmNPP5 in an ATP-Induced Cell Death Assay
2.4. ATP Cleavage by Recombinant SmNPP5
2.5. Statistical Analysis
3. Results
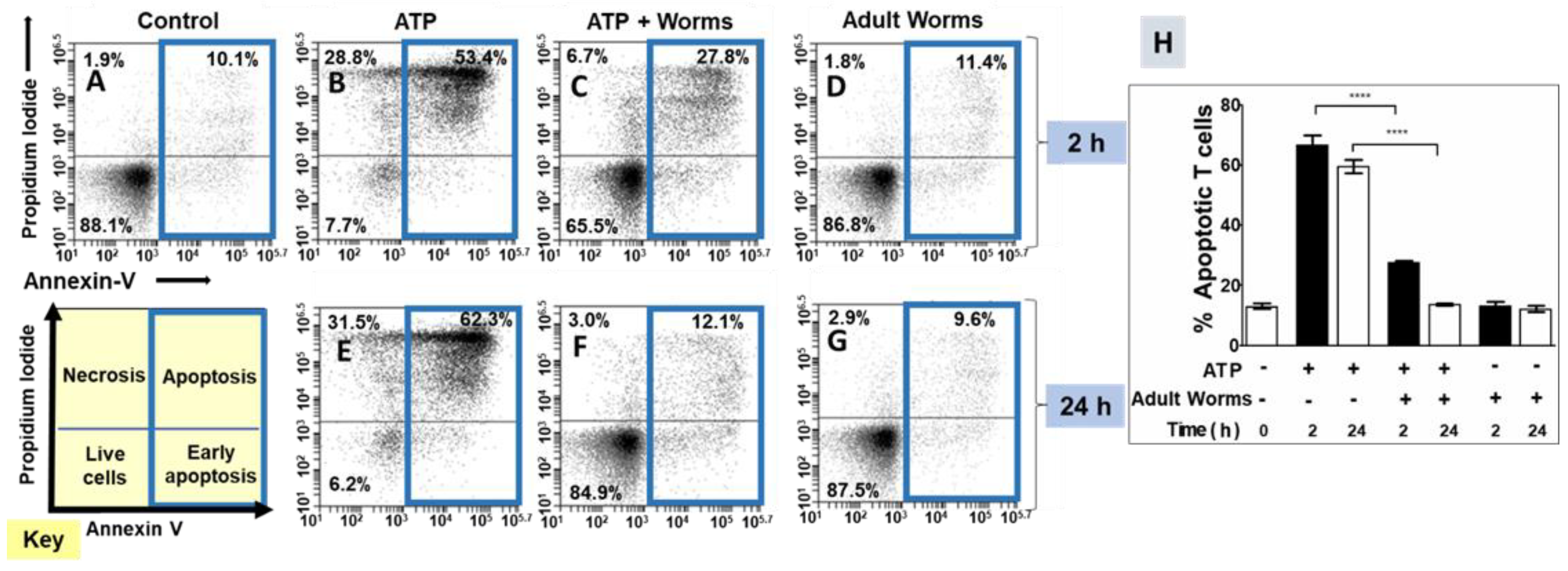
3.1. Adult Schistosomes Prevent ATP-Induced Cell Death In Vitro
3.2. rSmNPP5 Can Degrade ATP
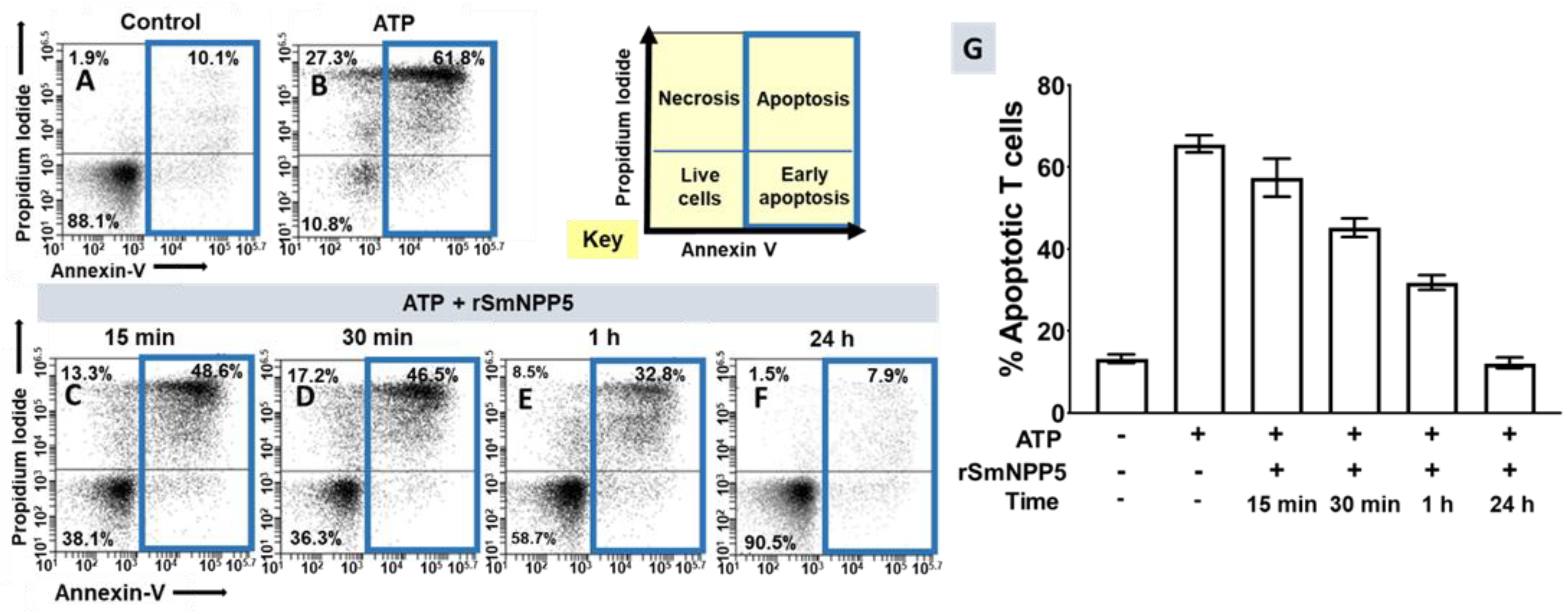
3.3. SmNPP5 Prevents ATP-Induced Cell Death In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Skelly, P. Fighting killer worms. Sci. Am. 2008, 298, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Nation, C.S.; Da’dara, A.A.; Marchant, J.K.; Skelly, P.J. Schistosome migration in the definitive host. PLoS Negl. Trop. Dis. 2020, 14, e0007951. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Skelly, P.J. Purinergic signaling and immune modulation at the schistosome surface? Trends Parasitol. 2009, 25, 256–260. [Google Scholar] [CrossRef]
- Da’dara, A.; Skelly, P.J. Manipulation of vascular function by blood flukes? Blood Rev. 2011, 25, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Angeles, J.M.M.; Mercado, V.J.P.; Rivera, P.T. Behind Enemy Lines: Immunomodulatory Armamentarium of the Schistosome Parasite. Front. Immunol. 2020, 11, 1018. [Google Scholar] [CrossRef]
- Mebius, M.M.; van Genderen, P.J.; Urbanus, R.T.; Tielens, A.G.; de Groot, P.G.; van Hellemond, J.J. Interference with the host haemostatic system by schistosomes. PLoS Pathog. 2013, 9, e1003781. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, B.C.; Da’dara, A.A.; Oliveira, S.C.; Skelly, P.J. Schistosomes Enhance Plasminogen Activation: The Role of Tegumental Enolase. PLoS Pathog. 2015, 11, e1005335. [Google Scholar] [CrossRef]
- Pirovich, D.; Da’dara, A.A.; Skelly, P.J. Why Do Intravascular Schistosomes Coat Themselves in Glycolytic Enzymes? Bioessays 2019, 41, e1900103. [Google Scholar] [CrossRef]
- Skelly, P.; Wilson, R. Making Sense of the Schistosome Surface. Adv. Parasitol. 2006, 63, 185–284. [Google Scholar]
- Wang, Q.; Da’dara, A.A.; Skelly, P.J. The human blood parasite Schistosoma mansoni expresses extracellular tegumental calpains that cleave the blood clotting protein fibronectin. Sci. Rep. 2017, 7, 12912. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Krautz-Peterson, G.; Da’dara, A.; Tzipori, S.; Skelly, P.J. Tegumental Phosphodiesterase SmNPP-5 Is a Virulence Factor for Schistosomes. Infect. Immun. 2011, 79, 4276–4284. [Google Scholar] [CrossRef] [Green Version]
- Rofatto, H.K.; Tararam, C.A.; Borges, W.C.; Wilson, R.A.; Leite, L.C.; Farias, L.P. Characterization of phosphodiesterase-5 as a surface protein in the tegument of Schistosoma mansoni. Mol. Biochem. Parasitol. 2009, 166, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Braschi, S.; Borges, W.C.; Wilson, R.A. Proteomic analysis of the schistosome tegument and its surface membranes. Mem. Inst. Oswaldo Cruz 2006, 101 (Suppl. S1), 205–212. [Google Scholar] [CrossRef] [Green Version]
- Braschi, S.; Wilson, R.A. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol. Cell. Proteom. 2006, 5, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Elzoheiry, M.; Da’dara, A.A.; deLaforcade, A.M.; El-Beshbishi, S.N.; Skelly, P.J. The Essential Ectoenzyme SmNPP5 from the Human Intravascular Parasite Schistosoma mansoni is an ADPase and a Potent Inhibitor of Platelet Aggregation. Thromb. Haemost. 2018, 118, 979–989. [Google Scholar] [CrossRef]
- Nation, C.S.; Da’Dara, A.A.; Skelly, P.J. The essential schistosome tegumental ectoenzyme SmNPP5 can block NAD-induced T cell apoptosis. Virulence 2020, 11, 568–579. [Google Scholar] [CrossRef]
- Rissiek, B.; Haag, F.; Boyer, O.; Koch-Nolte, F.; Adriouch, S. ADP-ribosylation of P2X7: A matter of life and death for regulatory T cells and natural killer T cells. Curr. Top. Microbiol. Immunol. 2015, 384, 107–126. [Google Scholar] [CrossRef]
- Adriouch, S.; Haag, F.; Boyer, O.; Seman, M.; Koch-Nolte, F. Extracellular NAD(+): A danger signal hindering regulatory T cells. Microbes Infect. 2012, 14, 1284–1292. [Google Scholar] [CrossRef]
- Rivas-Yanez, E.; Barrera-Avalos, C.; Parra-Tello, B.; Briceno, P.; Rosemblatt, M.V.; Saavedra-Almarza, J.; Rosemblatt, M.; Acuna-Castillo, C.; Bono, M.R.; Sauma, D. P2X7 Receptor at the Crossroads of T Cell Fate. Int. J. Mol. Sci. 2020, 21, 4937. [Google Scholar] [CrossRef]
- Schenk, U.; Frascoli, M.; Proietti, M.; Geffers, R.; Traggiai, E.; Buer, J.; Ricordi, C.; Westendorf, A.M.; Grassi, F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 2011, 4, ra12. [Google Scholar] [CrossRef]
- Vasconcelos, E.G.; Ferreira, S.T.; Carvalho, T.M.; Souza, W.; Kettlun, A.M.; Mancilla, M.; Valenzuela, M.A.; Verjovski-Almeida, S. Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni. Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J. Biol. Chem. 1996, 271, 22139–22145. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, E.G.; Nascimento, P.S.; Meirelles, M.N.; Verjovski-Almeida, S.; Ferreira, S.T. Characterization and localization of an ATP-diphosphohydrolase on the external surface of the tegument of Schistosoma mansoni. Mol. Biochem. Parasitol. 1993, 58, 205–214. [Google Scholar] [CrossRef]
- van Balkom, B.W.; van Gestel, R.A.; Brouwers, J.F.; Krijgsveld, J.; Tielens, A.G.; Heck, A.J.; van Hellemond, J.J. Mass spectrometric analysis of the Schistosoma mansoni tegumental sub-proteome. J. Proteome Res. 2005, 4, 958–966. [Google Scholar] [CrossRef]
- DeMarco, R.; Kowaltowski, A.T.; Mortara, R.A.; Verjovski-Almeida, S. Molecular characterization and immunolocalization of Schistosoma mansoni ATP-diphosphohydrolase. Biochem. Biophys. Res. Commun. 2003, 307, 831–838. [Google Scholar] [CrossRef]
- Da’dara, A.A.; Bhardwaj, R.; Ali, Y.B.; Skelly, P.J. Schistosome tegumental ecto-apyrase (SmATPDase1) degrades exogenous pro-inflammatory and pro-thrombotic nucleotides. PeerJ 2014, 2, e316. [Google Scholar] [CrossRef] [Green Version]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Elzoheiry, M.; Da’dara, A.A.; Bhardwaj, R.; Wang, Q.; Azab, M.S.; El-Kholy, E.I.; El-Beshbishi, S.N.; Skelly, P.J. Intravascular Schistosoma mansoni Cleave the Host Immune and Hemostatic Signaling Molecule Sphingosine-1-Phosphate via Tegumental Alkaline Phosphatase. Front. Immunol. 2018, 9, 1746. [Google Scholar] [CrossRef] [PubMed]
- Krautz-Peterson, G.; Camargo, S.; Huggel, K.; Verrey, F.; Shoemaker, C.B.; Skelly, P.J. Amino Acid Transport in Schistosomes: Characterization of the Permease Heavy Chain SPRM1hc. J. Biol. Chem. 2007, 282, 21767–21775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krautz-Peterson, G.; Simoes, M.; Faghiri, Z.; Ndegwa, D.; Oliveira, G.; Shoemaker, C.B.; Skelly, P.J. Suppressing glucose transporter gene expression in schistosomes impairs parasite feeding and decreases survival in the mammalian host. PLoS Pathog. 2010, 6, e1000932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faghiri, Z.; Skelly, P.J. The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake. FASEB J. 2009, 23, 2780–2789. [Google Scholar] [CrossRef] [Green Version]
- Elzoheiry, M.; Da’dara, A.A.; Nation, C.S.; El-Beshbishi, S.N.; Skelly, P.J. Schistosomes can hydrolyze proinflammatory and prothrombotic polyphosphate (polyP) via tegumental alkaline phosphatase, SmAP. Mol. Biochem. Parasitol. 2019, 232, 111190. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Coutinho-Silva, R. Purinergic signaling, DAMPs, and inflammation. Am. J. Physiol. Cell Physiol. 2020, 318, C832–C835. [Google Scholar] [CrossRef] [Green Version]
- Venereau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, D.; Chiozzi, P.; Falzoni, S.; Hanau, S.; Di Virgilio, F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 1997, 185, 579–582. [Google Scholar] [CrossRef] [Green Version]
- Savio, L.E.B.; de Andrade Mello, P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Grassi, F. The P2X7 Receptor as Regulator of T Cell Development and Function. Front. Immunol. 2020, 11, 1179. [Google Scholar] [CrossRef]
- Taylor, J.J.; Mohrs, M.; Pearce, E.J. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J. Immunol. 2006, 176, 5839–5847. [Google Scholar] [CrossRef] [Green Version]
- Layland, L.E.; Rad, R.; Wagner, H.; da Costa, C.U. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur. J. Immunol. 2007, 37, 2174–2184. [Google Scholar] [CrossRef]
- Turner, J.D.; Jenkins, G.R.; Hogg, K.G.; Aynsley, S.A.; Paveley, R.A.; Cook, P.C.; Coles, M.C.; Mountford, A.P. CD4+ CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by schistosome infection. PLoS Negl. Trop. Dis. 2011, 5, e1269. [Google Scholar] [CrossRef]
- Tang, C.L.; Lei, J.H.; Wang, T.; Lu, S.J.; Guan, F.; Liu, W.Q.; Li, Y.L. Effect of CD4+ CD25+ regulatory T cells on the immune evasion of Schistosoma japonicum. Parasitol. Res. 2011, 108, 477–480. [Google Scholar] [CrossRef]
- Tang, C.L.; Xie, Y.P.; Yu, W.H.; Jin, L.; Xie, Z.L.; Li, X.R. Effects of regulatory T cells on glyceraldehyde-3-phosphate dehydrogenase vaccine efficacy against Schistosoma japonicum. Acta Trop. 2020, 202, 105239. [Google Scholar] [CrossRef]
- Nausch, N.; Midzi, N.; Mduluza, T.; Maizels, R.M.; Mutapi, F. Regulatory and activated T cells in human Schistosoma haematobium infections. PLoS ONE 2011, 6, e16860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terkeltaub, R. Physiologic and pathologic functions of the NPP nucleotide pyrophosphatase/phosphodiesterase family focusing on NPP1 in calcification. Purinergic Signal. 2006, 2, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Borges, W.; Dowle, A.; Curwen, R.S.; Thomas-Oates, J.; Wilson, R.A. Enzymatic shaving of the tegument surface of live schistosomes for proteomic analysis: A rational approach to select vaccine candidates. PLoS Negl. Trop. Dis. 2011, 5, e993. [Google Scholar] [CrossRef] [PubMed]
- Birk, A.V.; Broekman, M.J.; Gladek, E.M.; Robertson, H.D.; Drosopoulos, J.H.; Marcus, A.J.; Szeto, H.H. Role of extracellular ATP metabolism in regulation of platelet reactivity. J. Lab. Clin. Med. 2002, 140, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Hasko, G. The Purinergic System as a Pharmacological Target for the Treatment of Immune-Mediated Inflammatory Diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nation, C.S.; Da'dara, A.A.; Elzoheiry, M.; Skelly, P.J. Schistosomes Impede ATP-Induced T Cell Apoptosis In Vitro: The Role of Ectoenzyme SmNPP5. Pathogens 2022, 11, 155. https://doi.org/10.3390/pathogens11020155
Nation CS, Da'dara AA, Elzoheiry M, Skelly PJ. Schistosomes Impede ATP-Induced T Cell Apoptosis In Vitro: The Role of Ectoenzyme SmNPP5. Pathogens. 2022; 11(2):155. https://doi.org/10.3390/pathogens11020155
Chicago/Turabian StyleNation, Catherine S., Akram A. Da'dara, Manal Elzoheiry, and Patrick J. Skelly. 2022. "Schistosomes Impede ATP-Induced T Cell Apoptosis In Vitro: The Role of Ectoenzyme SmNPP5" Pathogens 11, no. 2: 155. https://doi.org/10.3390/pathogens11020155
APA StyleNation, C. S., Da'dara, A. A., Elzoheiry, M., & Skelly, P. J. (2022). Schistosomes Impede ATP-Induced T Cell Apoptosis In Vitro: The Role of Ectoenzyme SmNPP5. Pathogens, 11(2), 155. https://doi.org/10.3390/pathogens11020155





