Copro-Molecular Identification of Tapeworms in Introduced Invasive Carnivores in Poland
Abstract
:1. Introduction
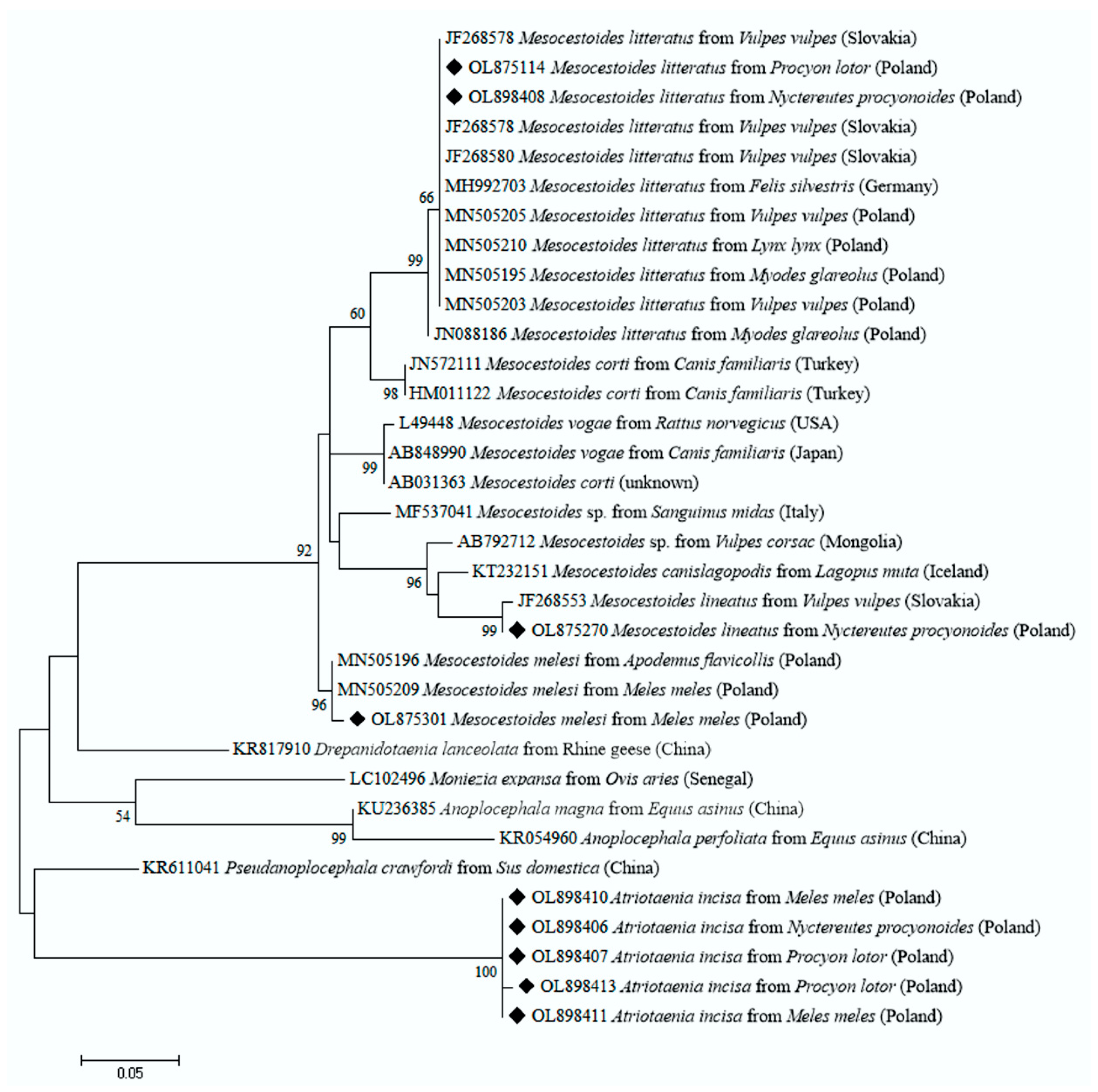
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. DNA Extraction, PCR Amplification, Sequencing and Phylogenetic Analyses
4.3. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salgado, I. Is the raccoon (Procyon lotor) out of control in Europe? Biodivers. Conserv. 2018, 27, 2243–2256. [Google Scholar] [CrossRef] [Green Version]
- Kochmann, J.; Cunze, S.; Klimpel, S. Climatic niche comparison of raccoons Procyon lotor and raccoon dogs Nyctereutes procyonoides in their native and non-native ranges. Mammal Rev. 2021, 51, 585–595. [Google Scholar] [CrossRef]
- Chueca, L.J.; Kochmann, J.; Schell, T.; Greve, C.; Janke, A.; Pfenninger, M.; Klimpel, S. De novo genome sssembly of the raccoon dog (Nyctereutes procyonoides). Front. Genet. 2021, 12, 658256. [Google Scholar] [CrossRef] [PubMed]
- Karamon, J.; Kochanowski, M.; Cencek, T.; Bartoszewicz, M.; Kusyk, P. Gastrointestinal helminths of raccoons (Procyon lotor) in western Poland (Lubuskie province)—With particular regard to Baylisascaris procyonis. Bull. Vet. Inst. Pulawy. 2014, 58, 4. [Google Scholar] [CrossRef] [Green Version]
- Laurimaa, L.; Süld, K.; Davison, J.; Moks, E.; Valdmann, H.; Saarma, U. Alien species and their zoonotic parasites in native and introduced ranges: The raccoon dog example. Vet. Parasitol. 2016, 219, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Leśniańska, K.; Perec-Matysiak, A.; Hildebrand, J.; Buńkowska-Gawlik, K.; Piróg, A.; Popiołek, M. Cryptosporidium spp. and Enterocytozoon bieneusi in introduced raccoons (Procyon lotor)—First evidence from Poland and Germany. Parasitol. Res. 2016, 115, 4535–4541. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, J.; Buńkowska-Gawlik, K.; Adamczyk, M.; Gajda, E.; Merta, D.; Popiołek, M.; Perec-Matysiak, A. The occurrence of Anaplasmataceae in European populations of invasive carnivores. Ticks Tick-Borne Dis. 2018, 9, 934–937. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Popiołek, M.; Zalewski, A. Host-parasite interactions in non-native invasive species are dependent on the levels of standing genetic variation at the immune locus. BMC Evol. Biol. 2020, 20, 43. [Google Scholar] [CrossRef]
- Kjær, L.J.; Jensen, L.M.; Chriél, M.; Bødker, R.; Petersen, H.H. The raccoon dog (Nyctereutes procyonoides) as a reservoir of zoonotic diseases in Denmark. Int. J. Parasitol. Parasites Wildl. 2021, 16, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Perec-Matysiak, A.; Leśniańska, K.; Buńkowska-Gawlik, K.; Merta, D.; Popiołek, M.; Hildebrand, J. Zoonotic genotypes of Enterocytozoon bieneusi in wild living invasive and native carnivores in Poland. Pathogens 2021, 10, 1478. [Google Scholar] [CrossRef]
- Kern, P.; Bardonnet, K.; Renner, E.; Auer, H.; Pawlowski, Z.; Ammann, R.W.; Vuitton, D.A.; Kern, P.; the European Echinococcosis Registry. European echinococcosis registry: Human alveolar echinococcosis, Europe, 1982–2000. Emerg. Infect. Dis. 2003, 9, 343–349. [Google Scholar] [CrossRef]
- Oksanen, A.; Siles-Lucas, M.; Karamon, J.; Possenti, A.; Conraths, F.J.; Romig, T.; Wysocki, P.; Mannocci, A.; Mipatrini, D.; La Torre, G.; et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: A systematic review and meta-analysis. Parasites Vectors 2016, 9, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Thiess, A.; Schuster, R.; Nöckler, K.; Mix, H. Helminth findings in indigenous raccoon dogs Nyctereutes procyonoides (Gray, 1843). Berl. Und Munch. Tierarztl. Wochenschr. 2001, 114, 273–276. [Google Scholar]
- Hurníková, Z.; Miterpakova, M.; Chovancová, B. The important zoonoses in the protected areas of the Tatra National Park (TANAP). Wiad. Parazytol. 2009, 55, 395–398. [Google Scholar]
- Bružinskaitė-Schmidhalter, R.; Šarkūnas, M.; Malakauskas, A.; Mathis, A.; Torgerson, P.R.; Deplazes, P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 2011, 139, 120–127. [Google Scholar] [CrossRef]
- Al-Sabi, M.N.S.; Chriél, M.; Jensen, T.H.; Enemark, H.L. Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009–2012—A comparative study. Int. J. Parasitol. Parasites Wildl. 2013, 2, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, S.; Sutor, A.; Staubach, C.; Mattis, R.; Tackmann, K.; Conraths, F.J. Estimated prevalence of Echinococcus multilocu-laris in raccoon dogs Nyctereutes procyonoides in northern Brandenburg, Germany. Curr. Zool. 2011, 57, 655–661. [Google Scholar] [CrossRef]
- Laurimaa, L.; Süld, K.; Moks, E.; Valdmann, H.; Umhang, G.; Knapp, J.; Saarma, U. First report of the zoonotic tapeworm Echinococcus multilocularis in raccoon dogs in Estonia, and comparisons with other countries in Europe. Vet. Parasitol. 2015, 212, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Machnicka-Rowińska, B.; Rocki, B.; Dziemian, E.; Kołodziej-Sobocińska, M. Raccoon dog (Nyctereutes procyonoides)--the new host of Echinococcus multilocularis in Poland. Wiad. Parazytol. 2002, 48, 65–68. [Google Scholar] [PubMed]
- Rocki, B.; Kołodziej-Sobocińska, M.; Machnicka, B.; Dziemian, E. Detection of Echinococcus multilocularis antigens in faeces by ELISA. Parasitol. Res. 2003, 91, 491–496. [Google Scholar] [CrossRef]
- Sato, H.; Suzuki, K. Gastrointestinal Helminths of Feral Raccoons (Procyon lotor) in Wakayama Prefecture, Japan. J. Vet. Med Sci. 2006, 68, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Waindok, P.; Raue, K.; Grilo, M.L.; Siebert, U.; Strube, C. Predators in northern Germany are reservoirs for parasites of One Health concern. Parasitol. Res. 2021, 120, 4229–4239. [Google Scholar] [CrossRef]
- Chelladurai, J.R.J.; Brewer, M.T. Global prevalence of Mesocestoides infections in animals—A systematic review and meta-analysis. Veter. Parasitol. 2021, 298, 109537. [Google Scholar] [CrossRef] [PubMed]
- Széll, Z.; Tolnai, Z.; Sréter, T. Environmental determinants of the spatial distribution of Mesocestoides spp. and sensitivity of flotation method for the diagnosis of mesocestoidosis. Vet. Parasitol. 2015, 212, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Hrčkova, G.; Miterpakova, M.; O’connor, A.N.N.E.; Šnábel, V.; Olson, P.D. Molecular and morphological circumscription of Mesocestoides tapeworms from red foxes (Vulpes vulpes) in central Europe. Parasitology 2011, 138, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skirnisson, K.; Jouet, D.; Ferté, H.; Nielsen, K. Occurrence of Mesocestoides canislagopodis (Rudolphi, 1810) (Krabbe, 1865) in mammals and birds in Iceland and its molecular discrimination within the Mesocestoides species complex. Parasitol. Res. 2016, 115, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Bajer, A.; Alsarraf, M.; Dwużnik, D.; Mierzejewska, E.J.; Kołodziej-Sobocińska, M.; Behnke-Borowczyk, J.; Banasiak, Ł.; Grzybek, M.; Tołkacz, K.; Kartawik, N.; et al. Rodents as intermediate hosts of cestode parasites of mammalian carnivores and birds of prey in Poland, with the first data on the life-cycle of Mesocestoides melesi. Parasites Vectors 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Duscher, T.; Hodžić, A.; Glawischnig, W.; Duscher, G.G. The raccoon dog (Nyctereutes procyonoides) and the raccoon (Procyon lotor)-their role and impact of maintaining and transmitting zoonotic diseases in Austria, Central Europe. Parasitol. Res. 2017, 116, 1411–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, D.J.; Snyder, D.E.; Owen, W.B. Helminth Parasites of the Raccoon (Procyon lotor) from North-Central Arkansas. J. Parasitol. 1992, 78, 163. [Google Scholar] [CrossRef]
- Borchert, E.J.; Leaphart, J.C.; Bryan, A.L., Jr.; Beasley, J.C. Ecotoxicoparasitology of mercury and trace elements in semi-aquatic mammals and their endoparasite communities. Sci. Total Environ. 2019, 679, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puerta, L.A.; Ticona, D.S.; Lopez-Urbina, M.T.; Gonzalez, A.E. A new species of Atriotaenia (Cestoda: Anoploce-phalidae) from the hog-nosed skunk Conepatus chinga (Carnivora: Mephitidae) in Peru. J. Parasitol. 2012, 98, 806–809. [Google Scholar] [CrossRef]
- Priemer, J.; Lux, E. Atriotaenia incisa (Cestoda), a parasite of the badger, Meles meles, and the raccoon, Procyon lotor, in Brandenburg, Germany. Can. J. Zool. 1994, 72, 1848–1853. [Google Scholar] [CrossRef]
- Karamon, J.; Samorek-Pieróg, M.; Moskwa, B.; Różycki, M.; Bilska-Zając, E.; Zdybel, J.; Włodarczyk, M. Intestinal helminths of raccoon dogs (Nyctereutes procyonoides) and red foxes (Vulpes vulpes) from the Augustów Primeval Forest (north-eastern Po-land). J. Vet. Res. 2016, 60, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, G.D. Handbook of Tapeworm Identification; CRC Press: Boca Raton, FL, USA, 1984; pp. 1–266. [Google Scholar]
- Nickol, B.B.; Khalil, L.F.; Jones, A.; Bray, R.A. Keys to the Cestode Parasites of Vertebrates. J. Parasitol. 1995, 81, 929. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M. Factors affecting the spread of parasites in populations of wild European terrestrial mammals. Mammal Res. 2019, 64, 301–318. [Google Scholar] [CrossRef] [Green Version]
- Mathis, A.; Deplazes, P. Copro-DNA tests for diagnosis of animal taeniid cestodes. Parasitol. Int. 2006, 55, S87–S90. [Google Scholar] [CrossRef]
- Dehghani, M.; Mohammadi, M.A.; Hemmati, S.; Nasibi, S.; Rostami, S.; Harandi, M.F. Cystic echinococcosis of camels: 12S rRNA gene variation revealed changing pattern of genetic dfiversity within Echinococcus granulosus sensu lato in the Middle East and North/Sub-Saharan Africa. Front. Vet. Sci. 2020, 7, 618. [Google Scholar] [CrossRef] [PubMed]
- Literák, I.; Tenora, F.; Letková, V.; Goldová, M.; Torres, J.; Olson, P.D. Mesocestoides litteratus (Batsch, 1786) (Cestoda: Cy-clophyllidea: Mesocestoididae) from the red fox: Morphological and 18S rDNA characterization of European isolates. Hel-Minthologia. 2006, 43, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Karamon, J.; Dąbrowska, J.; Kochanowski, M.; Samorek-Pieróg, M.; Sroka, J.; Różycki, M.; Bilska-Zając, E.; Zdybel, J.; Cencek, T. Prevalence of intestinal helminths of red foxes (Vulpes vulpes) in central Europe (Poland): A significant zoonotic threat. Parasites Vectors 2018, 11, 436. [Google Scholar] [CrossRef]
- Millán, J.; Sevilla, I.; Gerrikagoitia, X.; García-Pérez, A.L.; Barral, M. Helminth parasites of the Eurasian badger (Meles meles L.) in the Basque Country (Spain). Eur. J. Wildl. Res. 2004, 50, 37–40. [Google Scholar] [CrossRef]
- Veit, P.; Bilger, B.; Schad, V.; Schäfer, J.; Frank, W.; Lucius, R. Influence of environmental factors on the infectivity of Echi-nococcus multilocularis eggs. Parasitology 1995, 110, 79–86. [Google Scholar] [CrossRef]
- Goldberg, T.L.; Gendron-Fitzpatrick, A.; Deering, K.M.; Wallace, R.S.; Clyde, V.L.; Lauck, M.; Rosen, G.E.; Bennett, A.J.; Greiner, E.C.; O’Connor, D.H. Fatal Metacestode Infection in Bornean Orangutan Caused by Unknown Versteria Species. Emerg. Infect. Dis. 2014, 20, 109–113. [Google Scholar] [CrossRef]
- von Nickisch-Rosenegk, M.; Lucius, R.; Loos-Frank, B. Contributions to the Phylogeny of the Cyclophyllidea (Cestoda) Inferred from Mitochondrial 12S rDNA. J. Mol. Evol. 1999, 48, 586–596. [Google Scholar] [CrossRef]
- Dyachenko, V.; Beck, E.; Pantchev, N.; Bauer, C. Cost-effective method of DNA extraction from taeniid eggs. Parasitol. Res. 2008, 102, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Szostakowska, B.; Myjak, P.; Korzeniewski, K. The first detection of Echinococcus multilocularis DNA in environ-mental fruit, vegetable, and mushroom samples using nested PCR. Parasitol. Res. 2015, 114, 4023–4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Infecting Cestode | PCR Positive Fecal Samples/Number of Screened/(%; 95% CI) | ||
|---|---|---|---|
| Carnivore Species | |||
| Procyon lotor | Nyctereutes procyonoides | Meles meles | |
| Mesocestoides melesi | 0/103 (0) | 0/111 (0) | 6/47 (12.8; 95%CI 2.9–22.7) |
| Mesocestoides litteratus | 2/103 (1.9; 95%CI 0–4.6) | 6/111 (5.4; 95%CI 1.1–9.7) | 0/47 (0) |
| Mesocestoides lineatus | 0/103 (0) | 2/111 (1.8; 95%CI 0–4.3) | 0/47 (0) |
| Atriotaenia incisa | 17/103 (16.5; 95%CI 9.2–23.8) | 1/111 (0.9; 95%CI 0–2.7) | 16/47 (34.0; 95%CI 20.0–48.1) |
| Unidentified cestode species | 0/103 (0) | 4/111 (3.6; 95%CI 0–7.1) | 1/47 (2.1; 95%CI 0–6.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buńkowska-Gawlik, K.; Hildebrand, J.; Popiołek, M.; Merta, D.; Perec-Matysiak, A. Copro-Molecular Identification of Tapeworms in Introduced Invasive Carnivores in Poland. Pathogens 2022, 11, 110. https://doi.org/10.3390/pathogens11020110
Buńkowska-Gawlik K, Hildebrand J, Popiołek M, Merta D, Perec-Matysiak A. Copro-Molecular Identification of Tapeworms in Introduced Invasive Carnivores in Poland. Pathogens. 2022; 11(2):110. https://doi.org/10.3390/pathogens11020110
Chicago/Turabian StyleBuńkowska-Gawlik, Katarzyna, Joanna Hildebrand, Marcin Popiołek, Dorota Merta, and Agnieszka Perec-Matysiak. 2022. "Copro-Molecular Identification of Tapeworms in Introduced Invasive Carnivores in Poland" Pathogens 11, no. 2: 110. https://doi.org/10.3390/pathogens11020110
APA StyleBuńkowska-Gawlik, K., Hildebrand, J., Popiołek, M., Merta, D., & Perec-Matysiak, A. (2022). Copro-Molecular Identification of Tapeworms in Introduced Invasive Carnivores in Poland. Pathogens, 11(2), 110. https://doi.org/10.3390/pathogens11020110






