Enhanced Serum IgG Detection Potential Using 38KD-MPT32-MPT64, CFP10-Mtb81-EspC Fusion Protein and Lipoarabinomannan (LAM) for Human Tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Study and Sample Collection
2.2. Antigen Preparation
2.3. ELISA
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Characteristics of the Study Subjects
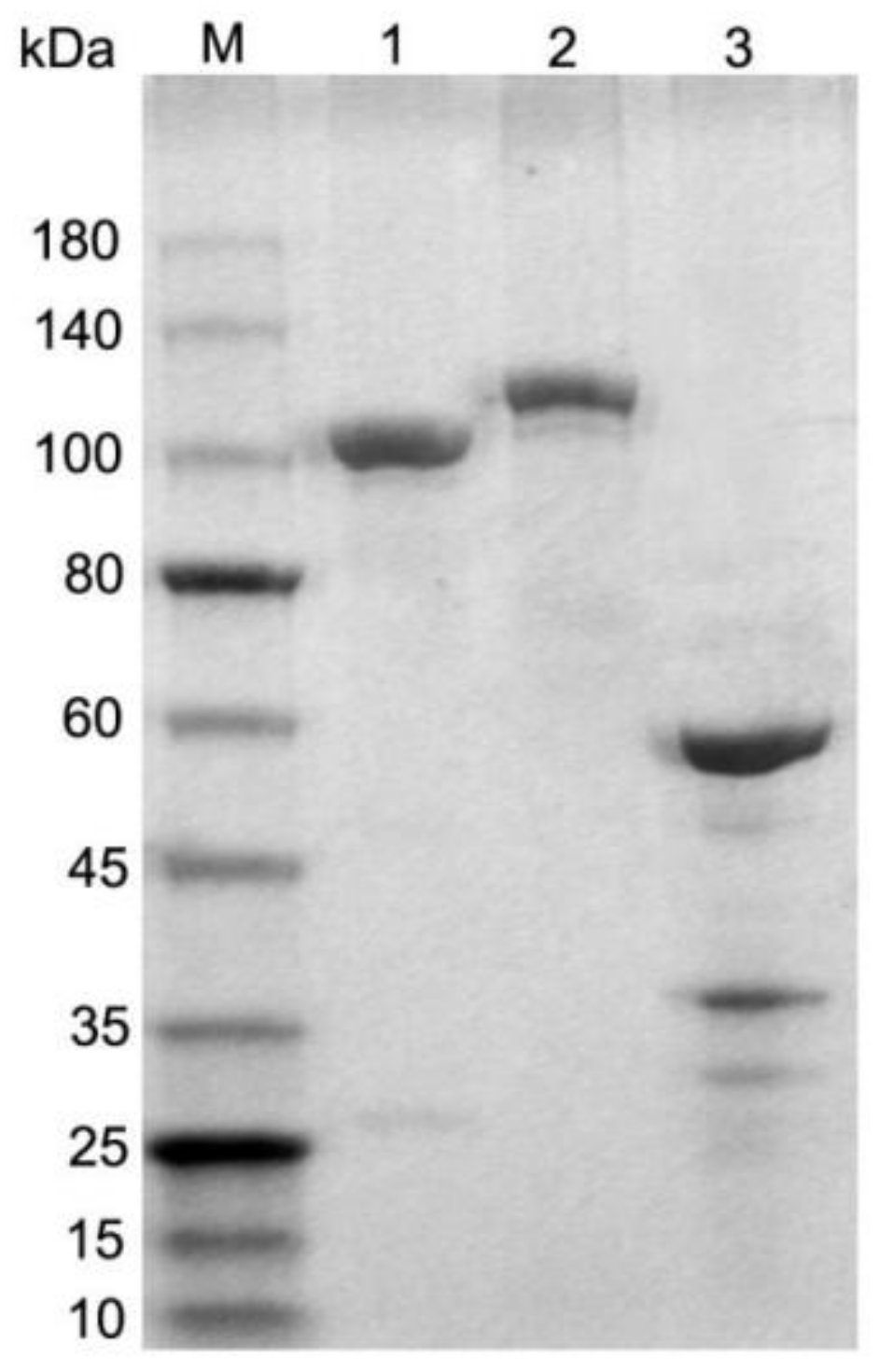
3.2. Generation of Fusion Antigens
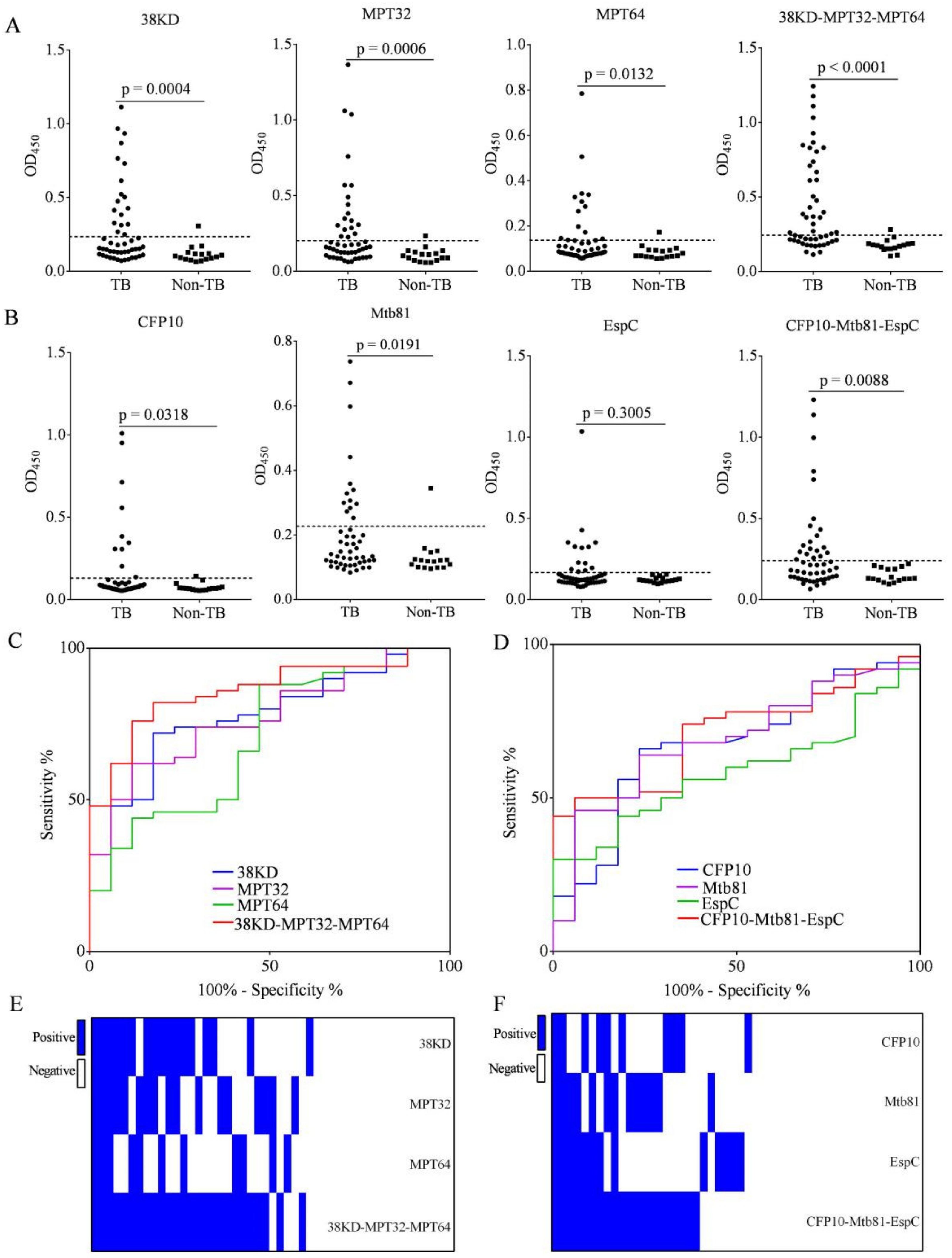
3.3. Increased IgG Detection Ability Using 38KD-MPT32-MPT64 and CFP10-Mtb81-EspC Fusion Proteins Compared with Individual Antigens in Patients with PTB
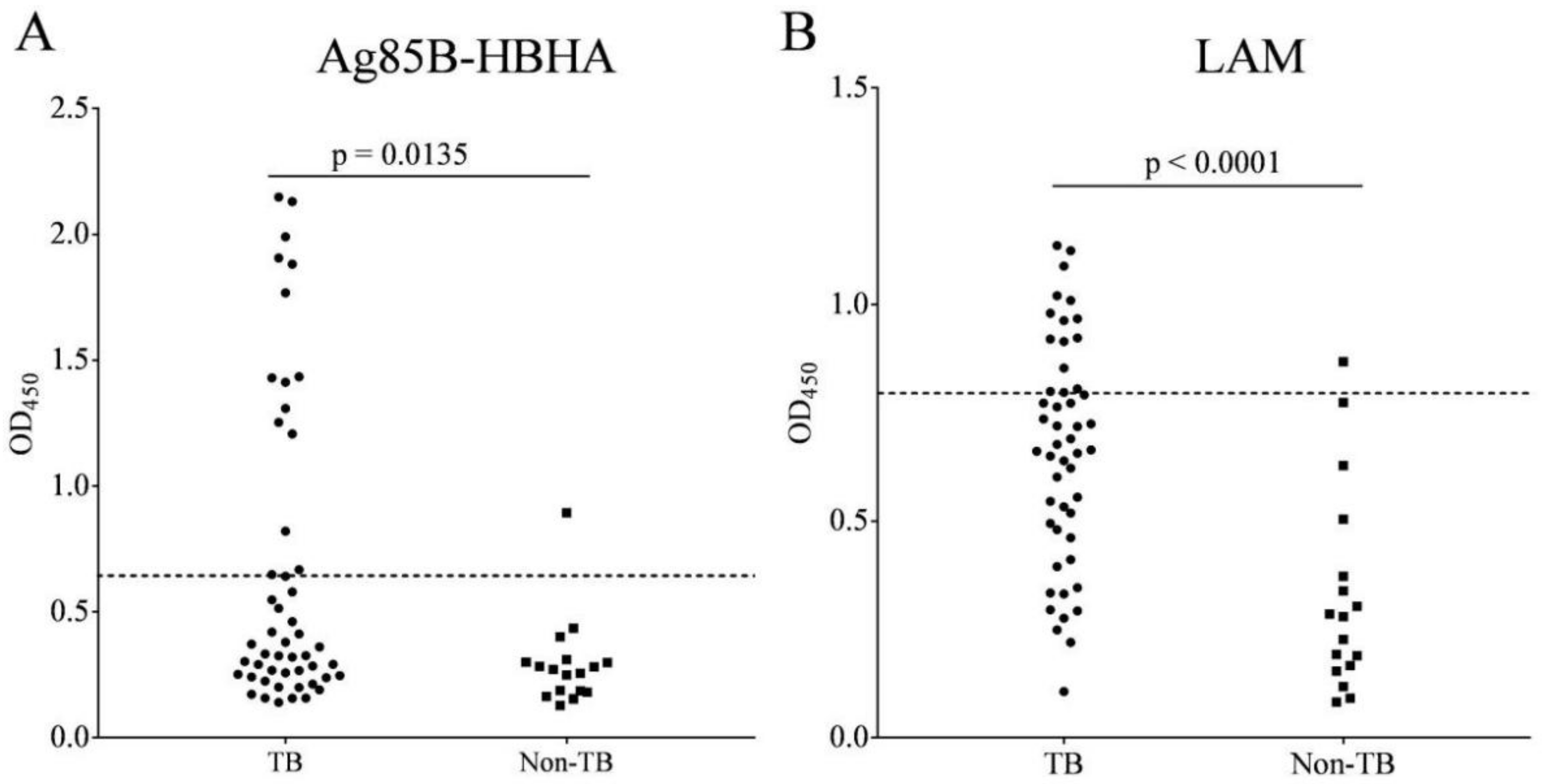
3.4. IgG to Ag85B-HBHA Fusion Protein and LAM Polysaccharide Antigen in Patients with PTB
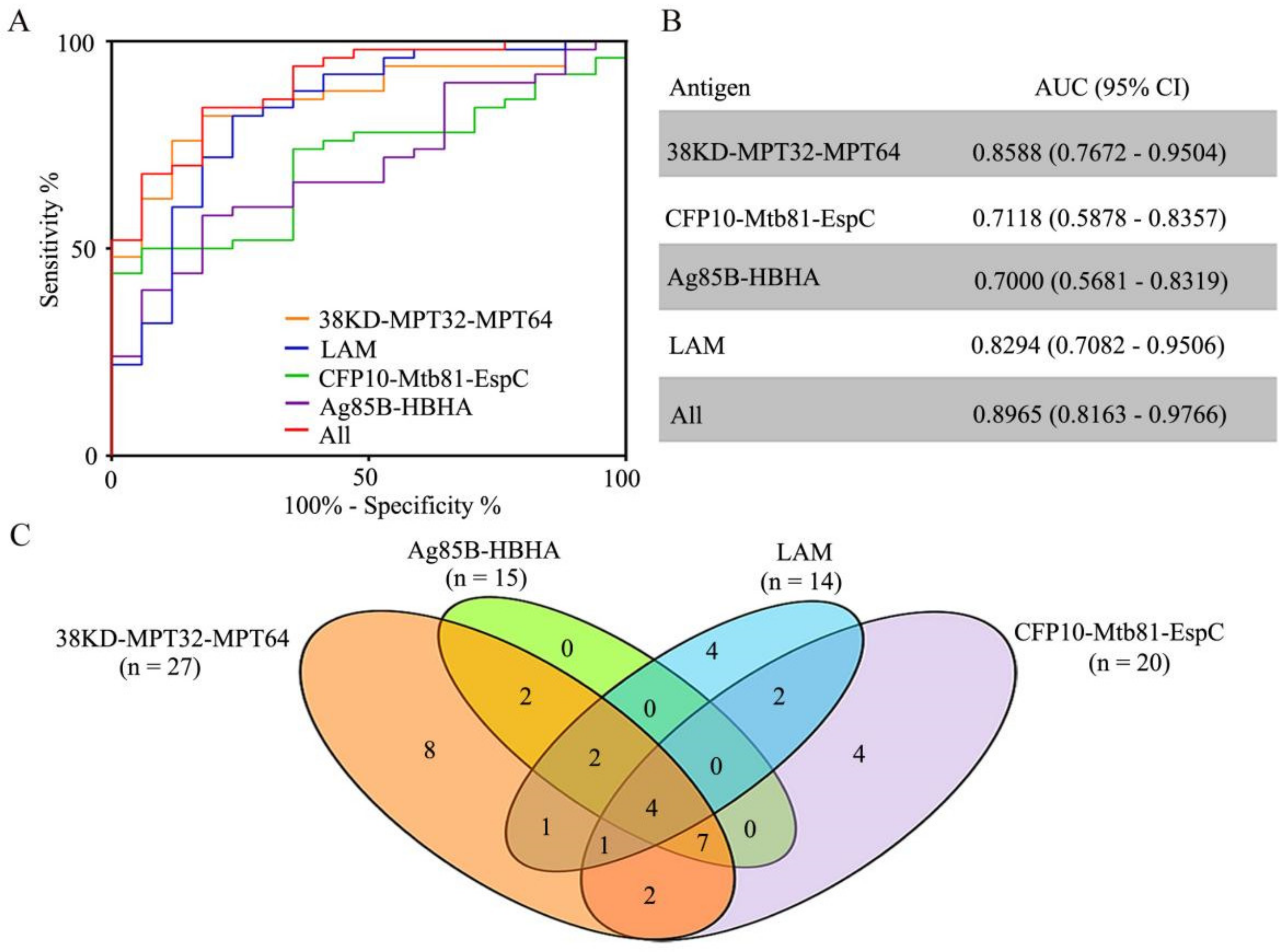
3.5. Characteristics of the IgG Reaction to Antigen Alone, and in Combination, among Patients with PTB
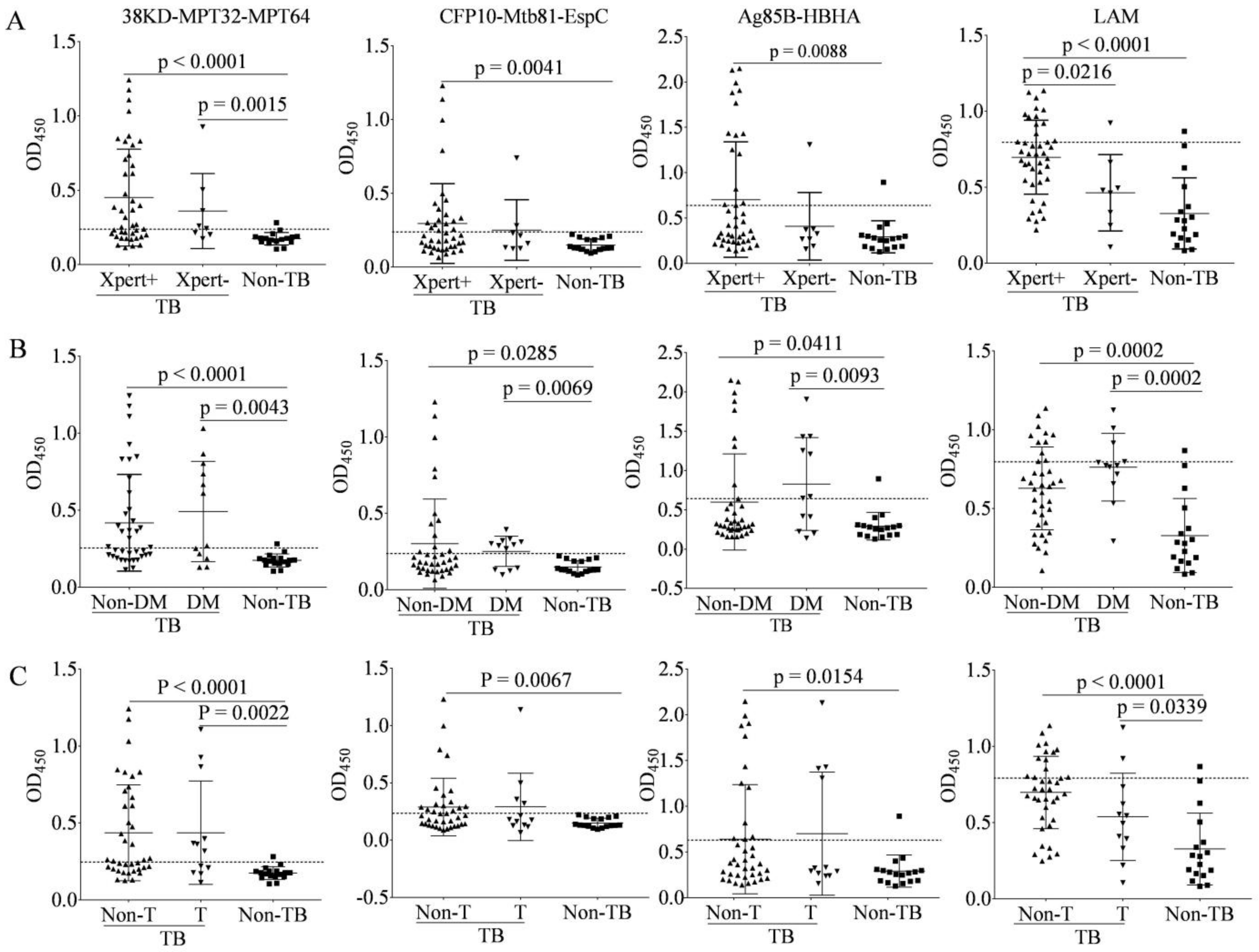
3.6. Characteristics of IgG to Antigens among Multiple Subgroups of TB Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta BioMed. 2020, 91, 157–160. [Google Scholar] [PubMed]
- Larsen, S.E.; Williams, B.D.; Rais, M.; Coler, R.N.; Baldwin, S.L. It Takes a Village: The Multifaceted Immune Response to Mycobacterium tuberculosis Infection and Vaccine-Induced Immunity. Front. Immunol. 2022, 13, 840225. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Acharya, A.; Gautam, S.; Ghimire, S.P.; Mishra, G.; Parajuli, N.; Sapkota, B. Advances in diagnosis of Tuberculosis: An update into molecular diagnosis of Mycobacterium tuberculosis. Mol. Biol. Rep. 2020, 47, 4065–4075. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Kerkhoff, A.D.; Nicol, M.P.; Meintjes, G. Underestimation of the True Specificity of the Urine Lipoarabinomannan Point-of-Care Diagnostic Assay for HIV-Associated Tuberculosis. J. Acquir. Immune Defic. Syndr. 2015, 69, e144-6. [Google Scholar] [CrossRef][Green Version]
- Bjerrum, S.; Schiller, I.; Dendukuri, N.; Kohli, M.; Nathavitharana, R.R.; Zwerling, A.A.; Denkinger, C.M.; Steingart, K.R.; Shah, M. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst. Rev. 2019, 10, CD011420. [Google Scholar] [CrossRef]
- De, P.; Amin, A.G.; Flores, D.; Simpson, A.; Dobos, K.; Chatterjee, D. Structural implications of lipoarabinomannan glycans from global clinical isolates in diagnosis of Mycobacterium tuberculosis infection. J. Biol. Chem. 2021, 297, 101265. [Google Scholar] [CrossRef]
- De, P.; Shi, L.; Boot, C.; Ordway, D.; McNeil, M.; Chatterjee, D. Comparative Structural Study of Terminal Ends of Lipoarabinomannan from Mice Infected Lung Tissues and Urine of a Tuberculosis Positive Patient. ACS Infect. Dis. 2020, 6, 291–301. [Google Scholar] [CrossRef]
- Ren, N.; JinLi, J.; Chen, Y.; Zhou, X.; Wang, J.; Ge, P.; Khan, F.A.; Zhang, L.; Hu, C.; Robertson, I.D.; et al. Identification of new diagnostic biomarkers for Mycobacterium tuberculosis and the potential application in the serodiagnosis of human tuberculosis. Microb. Biotechnol. 2018, 11, 893–904. [Google Scholar] [CrossRef]
- Yan, Z.H.; Yi, L.; Wei, P.J.; Jia, H.Y.; Wang, J.; Wang, X.J.; Yang, B.; Gao, X.; Zhao, Y.L.; Zhang, H.T. Evaluation of panels of Mycobacterium tuberculosis antigens for serodiagnosis of tuberculosis. Int. J. Tuberc. Lung Dis. 2018, 22, 959–965. [Google Scholar] [CrossRef]
- Sulman, S.; Shahid, S.; Khaliq, A.; Ambreen, A.; Khan, I.H.; Cooper, A.M.; Akhtar, M.W. Enhanced serodiagnostic potential of a fusion molecule consisting of Rv1793, Rv2628 and a truncated Rv2608 of Mycobacterium tuberculosis. PLoS ONE 2021, 16, e0258389. [Google Scholar] [CrossRef]
- Ernst, J.D.; Cornelius, A.; Bolz, M. Dynamics of Mycobacterium tuberculosis Ag85B Revealed by a Sensitive Enzyme-Linked Immunosorbent Assay. mBio 2019, 10, e00611-19. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Squeglia, F.; Goletti, D.; Delogu, G. The Mycobacterial HBHA Protein: A Promising Biomarker for Tuberculosis. Curr. Med. Chem. 2019, 26, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Diogo, G.R.; Pepponi, I.; van Dolleweerd, C.; Arias, M.A.; Locht, C.; Rider, C.C.; Sibley, L.; Cutting, S.M.; Loxley, A.; et al. Mucosal delivery of antigen-coated nanoparticles to lungs confers protective immunity against tuberculosis infection in mice. Eur. J. Immunol. 2014, 44, 440–449. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, P.; Zhang, K.; Xiang, J.; Zhang, L.; Robertson, I.D.; Guo, A. Use of Rv0222-Rv2657c-Rv1509 Fusion Protein to Improve the Accuracy of an Antibody ELISA for Extra-Pulmonary Tuberculosis in Humans. Pathogens 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Rijnink, W.F.; Ottenhoff, T.H.M.; Joosten, S.A. B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis. Front. Immunol. 2021, 12, 640168. [Google Scholar] [CrossRef]
- Fletcher, H.A.; Snowden, M.A.; Landry, B.; Rida, W.; Satti, I.; Harris, S.A.; Matsumiya, M.; Tanner, R.; O’Shea, M.K.; Dheenadhayalan, V.; et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat. Commun. 2016, 7, 11290. [Google Scholar] [CrossRef]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A Functional Role for Antibodies in Tuberculosis. Cell 2016, 167, 433–443.e14. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.X.; Wang, B.; Fu, L.; Liu, G.; Lu, Y.; Cao, M.; Huang, H.; Javid, B. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2017, 114, 5023–5028. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fc gamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef]
- Simmons, J.D.; Stein, C.M.; Seshadri, C.; Campo, M.; Alter, G.; Fortune, S.; Schurr, E.; Wallis, R.S.; Churchyard, G.; Mayanja-Kizza, H.; et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 2018, 18, 575–589. [Google Scholar] [CrossRef]
- Qu, M.; Zhou, X.; Li, H. BCG vaccination strategies against tuberculosis: Updates and perspectives. Hum. Vaccin Immunother. 2021, 17, 5284–5295. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.; Villarreal-Ramos, B.; Vordermeier, H.M.; McShane, H. The Humoral Immune Response to BCG Vaccination. Front. Immunol. 2019, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- National Health and Family Planning Commission of the People’s Republic of China. Diagnosis for Pulmonary Tuberculosis (WS 288-2017). Electron. J. Emerg. Infect. Dis. 2018, 1, 59–61. [Google Scholar]
- Belisle, J.T.; Vissa, V.D.; Sievert, T.; Takayama, K.; Brennan, P.J.; Besra, G.S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 1997, 276, 1420–1422. [Google Scholar] [CrossRef]
- Millington, K.A.; Fortune, S.M.; Low, J.; Garces, A.; Hingley-Wilson, S.M.; Wickremasinghe, M.; Kon, O.M.; Lalvani, A. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5730–5735. [Google Scholar] [CrossRef] [PubMed]
- Bulterys, M.A.; Wagner, B.; Redard-Jacot, M.; Suresh, A.; Pollock, N.R.; Moreau, E.; Denkinger, C.M.; Drain, P.K.; Broger, T. Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update. J. Clin. Med. 2019, 9, 111. [Google Scholar] [CrossRef]
- Briken, V.; Porcelli, S.A.; Besra, G.S.; Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol. Microbiol. 2004, 53, 391–403. [Google Scholar] [CrossRef]
- Zhou, K.L.; Li, X.; Zhang, X.L.; Pan, Q. Mycobacterial mannose-capped lipoarabinomannan: A modulator bridging innate and adaptive immunity. Emerg. Microbes Infect. 2019, 8, 1168–1177. [Google Scholar] [CrossRef]
- Gupta-Wright, A.; Corbett, E.L.; van Oosterhout, J.J.; Wilson, D.; Grint, D.; Alufandika-Moyo, M.; Peters, J.A.; Chiume, L.; Flach, C.; Lawn, S.D.; et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): A pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018, 392, 292–301. [Google Scholar] [CrossRef]
- Lowary, T.L.; Achkar, J.M. Tailor made: New insights into lipoarabinomannan structure may improve TB diagnosis. J. Biol. Chem. 2022, 298, 101678. [Google Scholar] [CrossRef]
- Yan, Z.H.; Zhao, B.; Pang, Y.; Wang, X.J.; Yi, L.; Wang, H.L.; Yang, B.; Wei, P.J.; Jia, H.Y.; Li, S.P.; et al. Generation of mycobacterial lipoarabinomannan-specific monoclonal antibodies and their ability to identify mycobacterium isolates. J. Microbiol. Immunol. Infect. 2021, 54, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Hamasur, B.; Haile, M.; Pawlowski, A.; Schroder, U.; Kallenius, G.; Svenson, S.B. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab’) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004, 138, 30–38. [Google Scholar] [CrossRef] [PubMed]
- De Vallière, S.; Abate, G.; Blazevic, A.; Heuertz, R.M.; Hoft, D.F. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect. Immun. 2005, 73, 6711–6720. [Google Scholar] [CrossRef] [PubMed]
- Achkar, J.M.; Prados-Rosales, R. Updates on antibody functions in Mycobacterium tuberculosis infection and their relevance for developing a vaccine against tuberculosis. Curr. Opin. Immunol. 2018, 53, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Aaby, P.; Behr, M.A.; Donald, P.R.; Kaufmann, S.H.; Netea, M.G.; Mandalakas, A.M. 100 years of Mycobacterium bovis bacille Calmette-Guérin. Lancet Infect. Dis. 2022, 22, e2–e12. [Google Scholar] [CrossRef]
- Nziza, N.; Cizmeci, D.; Davies, L.; Irvine, E.B.; Jung, W.; Fenderson, B.A.; de Kock, M.; Hanekom, W.A.; Franken, K.L.; Day, C.L.; et al. Defining Discriminatory Antibody Fingerprints in Active and Latent Tuberculosis. Front. Immunol. 2022, 13, 856906. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236. [Google Scholar] [CrossRef]
| Characteristics | PTB (n = 50) | Non-TB (n = 17) |
|---|---|---|
| Gender | ||
| Male % (no.) | 74.0%(37) | 76.5% (13) |
| Female % (no.) | 26.0% (13) | 23.5% (4) |
| Age, mean ± SD, years | 45.42 ± 18.59 | 39.82 ± 5.60 |
| Clinical category | ||
| Mycobacterial culture test | ||
| Culture-positive % (no.) | 74.0% (37) | – |
| Culture-negative % (no.) | 26.0% (13) | – |
| Xpert MTB/RIF assay | ||
| Xpert Mtb positive % (no.) | 84.0% (42) | – |
| Xpert Mtb negative % (no.) | 16.0% (8) | – |
| DM status | ||
| TB-Non-DM % (no.) | 76.0% (38) | – |
| TB-DM % (no.) | 24.0% (12) | – |
| Initial anti-TB treatment status | ||
| TB-Non-T % (no.) | 76.0% (38) | – |
| TB-T % (no.) | 24.0% (12) | – |
| Sensitivity | Specificity | PPV | NPV | ACC | |
|---|---|---|---|---|---|
| 38KD-MPT32-MPT64 | 54.0% | 94.1% | 96.4% | 41.0% | 64.2% |
| CFP10-Mtb81-EspC | 40.0% | 100.0% | 100% | 36.2% | 55.2% |
| Ag85B-HBHA | 30.0% | 94.1% | 93.8% | 31.4% | 46.3% |
| LAM | 28.0% | 94.1% | 93.3% | 30.8% | 44.4% |
| 38KD-MPT32-MPT64 + CFP10-Mtb81-EspC | 66.0% | 94.1% | 97.1% | 48.5% | 73.1% |
| 38KD-MPT32-MPT64 + CFP10-Mtb81-EspC + LAM | 74.0% | 88.2% | 94.9% | 53.6% | 77.6% |
| Xpert MTB | DM Status | Anti-TB Treatment | ||||
|---|---|---|---|---|---|---|
| Xpert+ (n = 42) %(n) | Xpert− (n = 8) % (n) | TB-Non-DM (n = 38) %(n) | TB-DM (n = 12) %(n) | TB-Non-T (n = 38) %(n) | TB-T (n = 12) %(n) | |
| 38KD-MPT32-MPT64 | 54.8% (23) | 50.0% (4) | 52.6% (20) | 58.3% (7) | 52.6% (20) | 58.3% (7) |
| CFP10-Mtb81-EspC | 42.9% (18) | 25.0% (2) | 31.6% (12) | 66.7% (8) | 42.1% (16) | 33.3% (4) |
| Ag85B-HBHA | 33.3%(14) | 12.5% (1) | 21.1% (8) | 58.3% (7) | 28.9% (11) | 33.3% (4) |
| LAM | 31.0% (13) | 12.5% (1) | 28.9% (11) | 25.0% (3) | 31.6% (12) | 16.7% (2) |
| 38KD-MPT32-MPT64 + CFP10-Mtb81-EspC | 66.7% (28) | 62.5% (5) | 63.2% (24) | 75.0% (9) | 65.8% (25) | 66.7% (8) |
| 38KD-MPT32-MPT64 + CFP10-Mtb81-EspC + LAM | 73.8% (31) | 75.0% (6) | 71.1% (27) | 83.3% (10) | 76.3% (29) | 66.7% (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Wang, X.; Yi, L.; Yang, B.; Wei, P.; Ruan, H.; Wang, J.; Yang, X.; Zhang, H. Enhanced Serum IgG Detection Potential Using 38KD-MPT32-MPT64, CFP10-Mtb81-EspC Fusion Protein and Lipoarabinomannan (LAM) for Human Tuberculosis. Pathogens 2022, 11, 1545. https://doi.org/10.3390/pathogens11121545
Yan Z, Wang X, Yi L, Yang B, Wei P, Ruan H, Wang J, Yang X, Zhang H. Enhanced Serum IgG Detection Potential Using 38KD-MPT32-MPT64, CFP10-Mtb81-EspC Fusion Protein and Lipoarabinomannan (LAM) for Human Tuberculosis. Pathogens. 2022; 11(12):1545. https://doi.org/10.3390/pathogens11121545
Chicago/Turabian StyleYan, Zhuohong, Xiaojue Wang, Ling Yi, Bin Yang, Panjian Wei, Hongyun Ruan, Jinghui Wang, Xinting Yang, and Hongtao Zhang. 2022. "Enhanced Serum IgG Detection Potential Using 38KD-MPT32-MPT64, CFP10-Mtb81-EspC Fusion Protein and Lipoarabinomannan (LAM) for Human Tuberculosis" Pathogens 11, no. 12: 1545. https://doi.org/10.3390/pathogens11121545
APA StyleYan, Z., Wang, X., Yi, L., Yang, B., Wei, P., Ruan, H., Wang, J., Yang, X., & Zhang, H. (2022). Enhanced Serum IgG Detection Potential Using 38KD-MPT32-MPT64, CFP10-Mtb81-EspC Fusion Protein and Lipoarabinomannan (LAM) for Human Tuberculosis. Pathogens, 11(12), 1545. https://doi.org/10.3390/pathogens11121545





