Anisakis Infection in the Spotted Flounder Citharus linguatula (Pleuronectiformes: Citharidae) Caught in the Gulf of Cadiz (Area FAO 27-ICES IXa) Appears to Negatively Affect Fish Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Host and Parasites
2.2. Molecular Identification of Anisakis larvae
2.3. Epidemiological Parameters and Statistical Analysis
3. Results
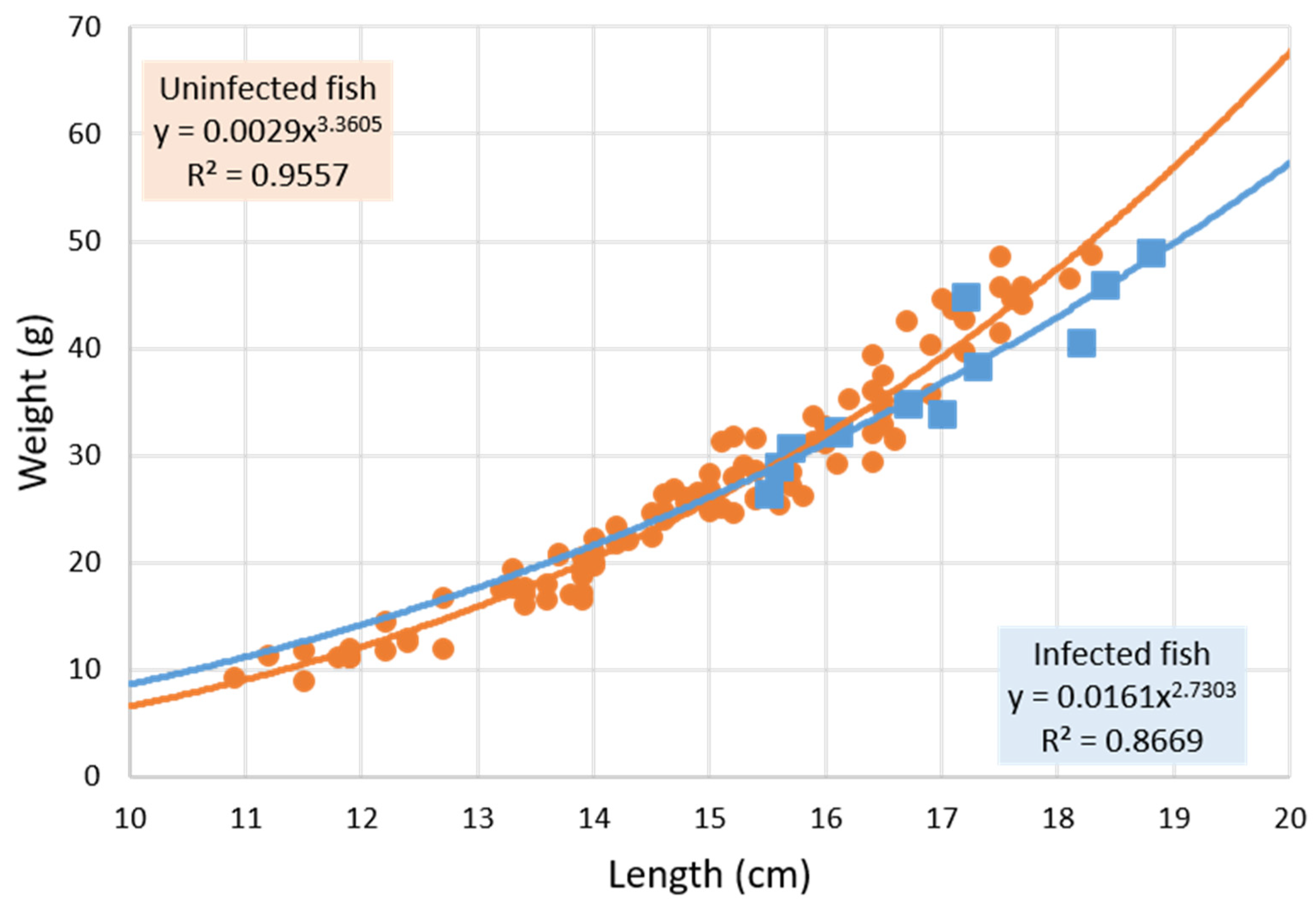
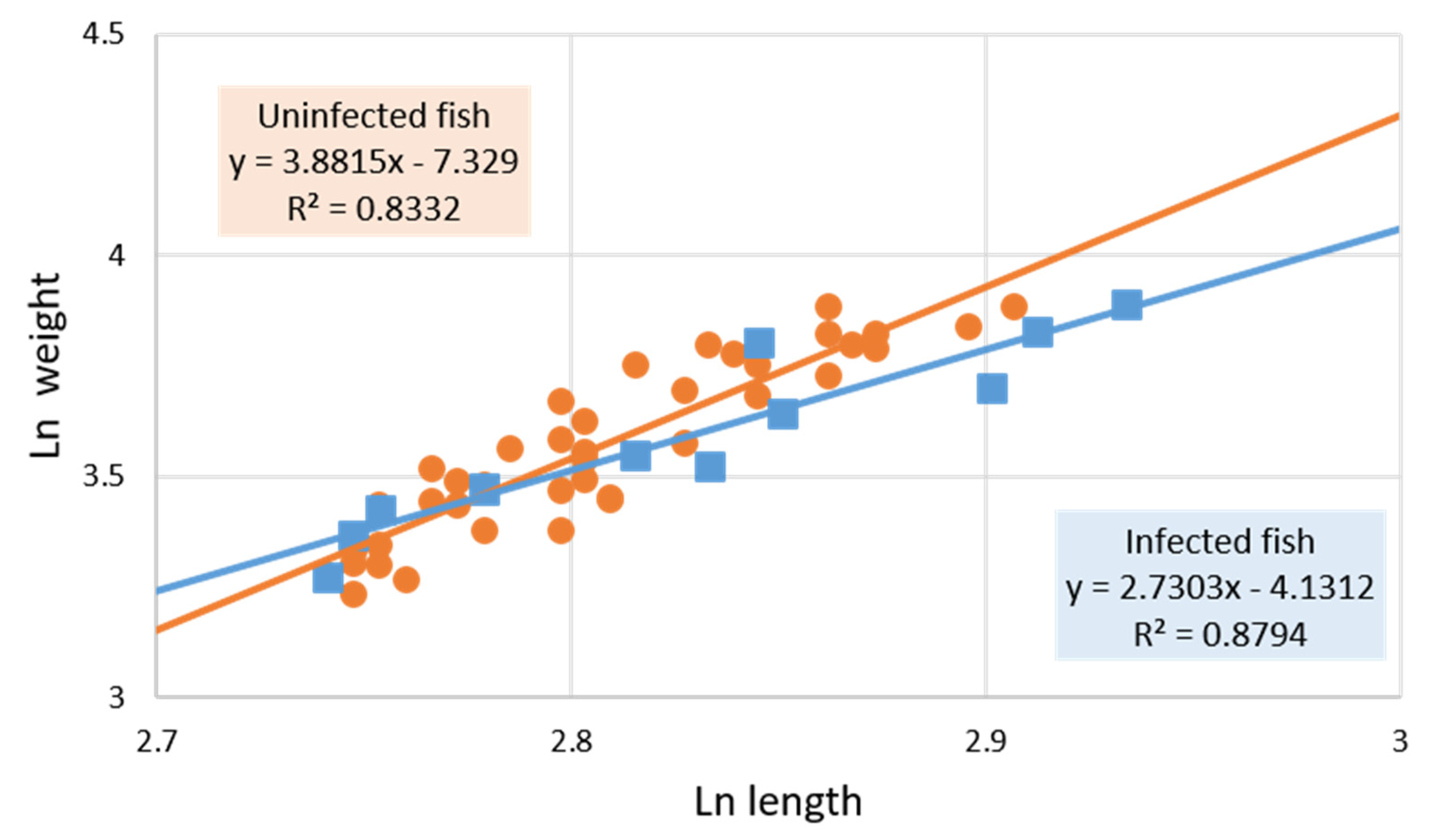
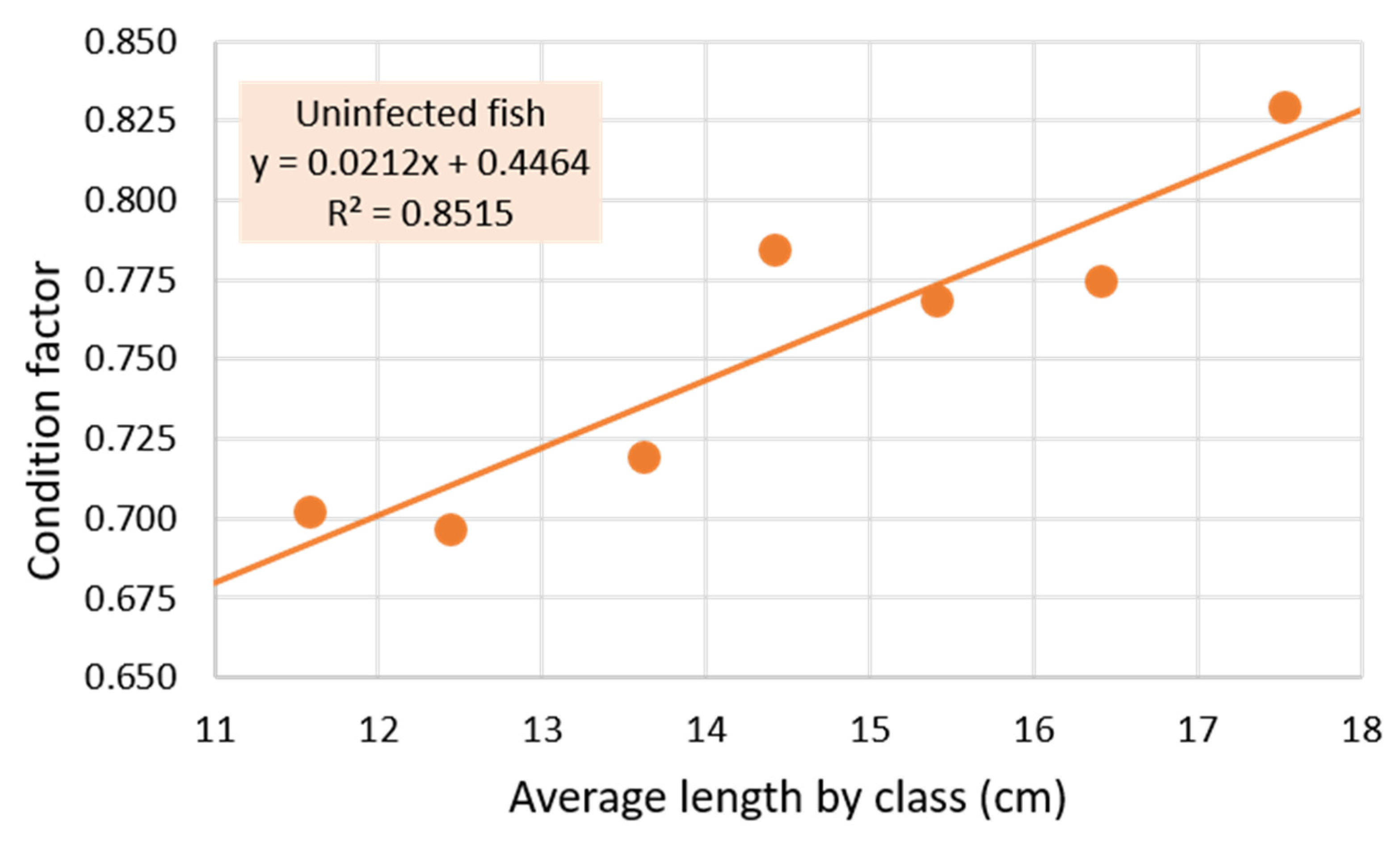
3.1. Parasitized and Non-Parasitized Fish
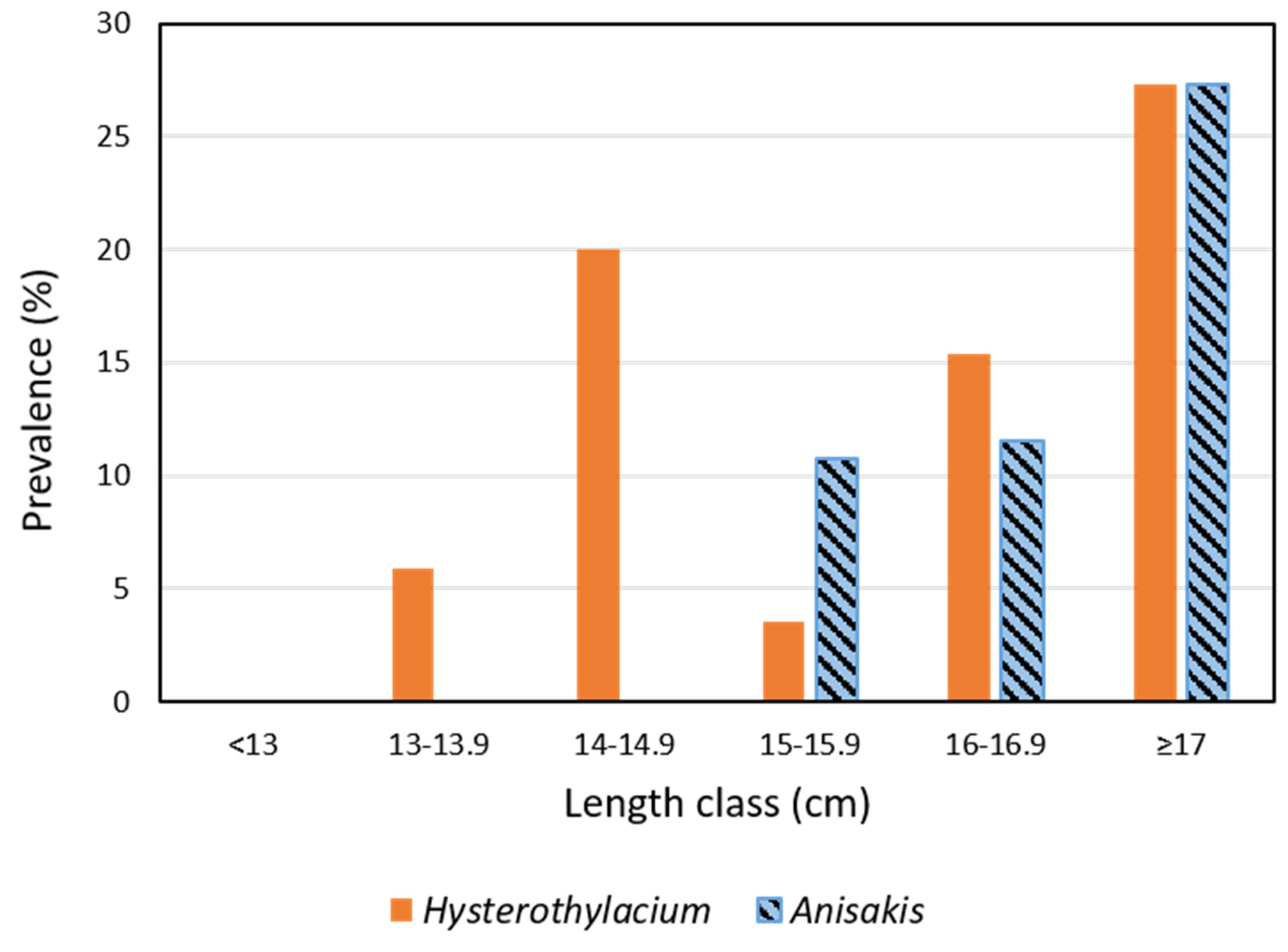
3.2. Parasites
3.3. Molecular Identification of Anisakis larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.G. Citharidae. In FAO Species Identification Sheets for Fishery Purposes. Eastern Central Atlantic; Fishing Areas 34, 47 (in Part); Fischer, W., Bianchi, G., Scott, W.B., Eds.; FAO Species Identification Sheets for Fishery Purposes; FAO: Rome, Italy, 1981; Volume 2, p. Sheet CITH Cith 1. [Google Scholar]
- Fischer, W.; Bauchot, M.-L.; Schneider, M. Fiches FAO D’identification des Espèces pour les Besoins de la Pêche. (Révision 1). Méditerranée et mer Noire. Zone de pêche 37; FAO Species Identification Sheets for Fishery Purposes; FAO: Rome, Italy, 1987; Volume 2, 1529p. [Google Scholar]
- IDAPES Consulta Estadísticas Pesqueras. Primera Venta de Pesca Fresca en Lonja. Available online: https://www.juntadeandalucia.es/agriculturaypesca/idapes/ (accessed on 29 September 2022).
- Gómez-Morales, M.Á.; Castro, C.M.; Lalle, M.; Fernández, R.; Pezzotti, P.; Abollo, E.; Pozio, E. UV-press method versus artificial digestion method to detect Anisakidae L3 in fish fillets: Comparative study and suitability for the industry. Fish. Res. 2018, 202, 22–28. [Google Scholar] [CrossRef]
- Pippy, J.H.C. Use of ultraviolet light to find parasitic nematodes in situ. J. Fish. Res. Board Can. 1970, 27, 963–965. [Google Scholar] [CrossRef]
- Petter, A.J.; Maillard, C. Larves d’ascarides parasites de poissons en Méditerranée occidentale. Bull. Mus. Natl. Hist. Nat. 1988, 10A, 347–369. [Google Scholar]
- Berland, B. Nematodes from some Norwegian marine fishes. Sarsia 1961, 2, 1–50. [Google Scholar] [CrossRef]
- Hartwich, G. Keys to genera of the Ascaridoidea. In CIH Keys to the Nematode Parasites of Vertebrates. No. 2; Anderson, R., Chabaud, A., Willmott, S., Eds.; CAB: Slough, UK, 1974; p. iv + 15 p. [Google Scholar]
- Yoshinaga, T.; Ogawa, K.; Wakabayashi, H. Experimental life cycle of Hysterothylacium aduncum (Nematoda: Anisakidae) in fresh water. Fish Pathol. 1987, 22, 243–251. [Google Scholar] [CrossRef]
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Hysterothylacium aduncum. In Fish Parasites. A Handbook of Protocols for Their Isolation, Culture and Transmission; Sitjà-Bobadilla, A., Bron, J.E., Wiegertjes, G., Piazzon, M.C., Eds.; European Association of Fish Pathologists (EAFP)/5m Books Series; 5M Books Ltd: Great Easton, UK, 2021; pp. 311–329. [Google Scholar]
- Navone, G.T.; Sardella, N.H.; Timi, J.T. Larvae and adults of Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda: Anisakidae) in fishes and crustaceans in the South West Atlantic. Parasite 1998, 5, 127–136. [Google Scholar] [CrossRef]
- Monstad, T. Some aspects of mortality, condition factors and liver state with Anisakis-infection in blue whiting in the North-East Atlantic. In Proceedings of the Fourth Soviet-Norwegian Symposium, Bergen, Norway, 12–16 June 1990; Monstad, T., Ed.; Institute of Marine Research: Bergen, Norway, 1990; pp. 319–339. [Google Scholar]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Buzo-Domínguez, S.; Morales-Yuste, M.; Domingo-Hernández, A.M.; Benítez, R.; Adroher, F.J. Molecular epidemiology of Anisakis spp. in wedge sole, Dicologlossa cuneata (Moreau, 1881), from fishmarkets in Granada (southern Spain), caught in two adjacent NE and CE Atlantic areas. Pathogens 2021, 10, 1302. [Google Scholar] [CrossRef]
- Molina-Fernández, D.; Rubio-Calvo, D.; Adroher, F.J.; Benítez, R. Molecular epidemiology of Anisakis spp. in blue whiting Micromesistius poutassou in eastern waters of Spain, western Mediterranean Sea. Int. J. Food Microbiol. 2018, 282, 49–56. [Google Scholar] [CrossRef]
- Molina-Fernández, D.; Malagón, D.; Gómez-Mateos, M.; Benítez, R.; Martín-Sánchez, J.; Adroher, F.J. Fishing area and fish size as risk factors of Anisakis infection in sardines (Sardina pilchardus) from Iberian waters, southwestern Europe. Int. J. Food Microbiol. 2015, 203, 27–34. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Gasser, R.B.; Podolska, M.; Chilton, N.B. Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int. J. Parasitol. 1998, 28, 1911–1921. [Google Scholar] [CrossRef]
- D’Amelio, S.; Mathiopoulos, K.D.; Santos, C.P.; Pugachev, O.N.; Webb, S.C.; Picanço, M.; Paggi, L. Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase chain reaction-based restriction fragment length polymorphism. Int. J. Parasitol. 2000, 30, 223–226. [Google Scholar] [CrossRef]
- Pontes, T.; D’Amelio, S.; Costa, G.; Paggi, L. Molecular characterization of larval anisakid nematodes from marine fishes of Madeira by a PCR-based approach, with evidence for a new species. J. Parasitol. 2005, 91, 1430–1434. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Reiczigel, J.; Rózsa, L. Quantitative Parasitology 3.0. Available online: https://www.zoologia.hu/qp/ (accessed on 23 February 2022).
- Rózsa, L.; Reiczigel, J.; Majoros, G. Quantifying parasites in samples of hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- R Core Team. A language and Environment for Statistical Computing, R Foundation for Statistical Computing. 2013. Available online: https://www.r-project.org/ (accessed on 20 June 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.Sage Publications: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 20 June 2022).
- Komsta, L. Outliers: Tests for Outliers. 2022. Available online: https://cran.r-project.org/web/packages/outliers/index.html (accessed on 20 June 2022).
- García-Rodríguez, M.; Esteban, A. Contribution to the knowledge of Citharus linguatula (Linnaeus, 1758) (Osteicthyes: Heterosomata) in the Iberian Mediterranean. Demersal Resour. Mediterr. Actes Colloq. IFREMER 2000, 26, 131–140. [Google Scholar]
- Planas, A.; Vives, F. Contribución al estudio de la solleta (Citharus linguatula Günth) del Mediterráneo occidental (Sectores de Vinaroz e islas Columbretes). Investig. Pesq. 1956, 3, 107–131. [Google Scholar]
- Marques, J.F.; Cabral, H.N.; Busi, M.; D’Amelio, S. Molecular identification of Anisakis species from Pleuronectiformes off the Portuguese coast. J. Helminthol. 2006, 80, 47–51. [Google Scholar] [CrossRef]
- Marques, J.F.; Santos, M.J.; Cabral, H.N. Zoogeographical patterns of flatfish (Pleuronectiformes) parasites in the Northeast Atlantic and the importance of the Portuguese coast as a transitional area. Sci. Mar. 2009, 73, 461–471. [Google Scholar] [CrossRef][Green Version]
- Marques, J.F.; Teixeira, C.M.; Cabral, H.N. Differentiation of commercially important flatfish populations along the Portuguese coast: Evidence from morphology and parasitology. Fish. Res. 2006, 81, 293–305. [Google Scholar] [CrossRef]
- Carreras-Aubets, M.; Montero, F.E.; Padrós, F.; Crespo, S.; Carrassón, M. Parasites and hystopathology of Mullus barbatus and Citharus linguatula (Pisces) from two sites in the NW Mediterranean with different degrees of pollution. Sci. Mar. 2010, 75, 369–378. [Google Scholar] [CrossRef]
- Carrassón, M.; Dallarés, S.; Cartes, J.E.; Constenla, M.; Pérez del Olmo, A.; Zucca, L.; Kostadinova, A. Drivers of parasite community structure in fishes of the continental shelf of the Western Mediterranean: The importance of host phylogeny and autecological traits. Int. J. Parasitol. 2019, 49, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Petter, A.J.; Radujković, B.M. Parasites des poissons marins du Montenegro: Nematodes. Acta Adriat. 1989, 30, 195–236. [Google Scholar] [CrossRef]
- Petter, A.J.; Radujković, B.M. Nématodes parasites de poissons de la mer Adriatique. Bull. Mus. Natl. Hist. Nat. 1986, 8, 487–499. [Google Scholar]
- Teixeira, C.M.; Batista, M.I.; Cabral, H.N. Diet, growth and reproduction of four flatfishes on the Portuguese coast. Sci. Mar. 2010, 74, 223–233. [Google Scholar] [CrossRef]
- Redon, M.J.; Morte, M.S.; Sanz-Brau, A. Feeding habits of the spotted flounder Citharus linguatula off the eastern coast of Spain. Mar. Biol. 1994, 120, 197–201. [Google Scholar] [CrossRef]
- Belghyti, D.; Aguesse, P.; Gabrion, C. Éthologie alimentaire de Citharus linguatula et Dicologoglossa cuneata sur les côtes atlantiques du Maroc. Vie Milieu 1993, 43, 95–108. [Google Scholar]
- Klimpel, S.; Rückert, S. Life cycle strategy of Hysterothylacium aduncum to become the most abundant anisakid fish nematode in the North Sea. Parasitol. Res. 2005, 97, 141–149. [Google Scholar] [CrossRef]
- Horbowy, J.; Podolska, M.; Nadolna-Ałtyn, K. Increasing occurrence of anisakid nematodes in the liver of cod (Gadus morhua) from the Baltic Sea: Does infection affect the condition and mortality of fish? Fish. Res. 2016, 179, 98–103. [Google Scholar] [CrossRef]
- Santoro, M.; Mattiucci, S.; Work, T.; Cimmaruta, R.; Nardi, V.; Cipriani, P.; Bellisario, B.; Nascetti, G. Parasitic infection by larval helminths in Antarctic fishes: Pathological changes and impact on the host body condition index. Dis. Aquat. Org. 2013, 105, 139–148. [Google Scholar] [CrossRef]
- Vuić, N.; Čakalić, I.T.; Vlaičević, B.; Piperac, M.S.; Čerba, D. The influence of Contracaecum larvae (Nematoda, Anisakidae) parasitism on the population of Prussian carp (Carassius gibelio) in Lake Sakadaš, Croatia. Pathogens 2022, 11, 600. [Google Scholar] [CrossRef]
- Serrat, A.; Lloret, J.; Frigola-Tepe, X.; Muñoz, M. Trade-offs between life-history traits in a coldwater fish in the Mediterranean Sea: The case of blue whiting Micromesistius poutassou. J. Fish Biol. 2019, 95, 428–443. [Google Scholar] [CrossRef]
- Eltink, A. Anisakis larvae (Nematoda: Ascaridida) in mackerel, (Scomber scombrus L.) in lCES sub-areas IV, VI, VII and VIII in 1970–1971 and 1982–1984. Int. Counc. Explor. Sea 1988, CM1988/H23, 29. [Google Scholar] [CrossRef]
- Richards, J. Preliminary results of the 1977 blue whiting surveys of the west of Scotland. Int. Counc. Explor. Sea 1977, CM1977/H33, 15 p. [Google Scholar]
- Rohde, K. Ecology of marine parasites. Helgoländer Meeresunters. 1984, 37, 5–33. [Google Scholar] [CrossRef]
- Mo, T.A.; Gahr, A.; Hansen, H.; Hoel, E.; Oaland, Ø.; Poppe, T.T. Presence of Anisakis simplex (Rudolphi, 1809 det. Krabbe, 1878) and Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda; Anisakidae) in runts of farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 2014, 37, 135–140. [Google Scholar] [CrossRef]
- Bussmann, B.; Ehrich, S. Investigations on infestation of blue whiting (Micromesistius poutassou) with larval Anisakis sp. (Nematoda: Ascaridida). Arch. Fischereiwiss. 1979, 29, 155–165. [Google Scholar]
- Valero, A.; Martín-Sánchez, J.; Reyes-Muelas, E.; Adroher, F.J. Larval anisakids parasitizing the blue whiting, Micromesistius poutassou, from Motril Bay in the Mediterranean region of southern Spain. J. Helminthol. 2000, 74, 361–364. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Bosi, G.; DePasquale, J.A.; Manera, M.; Giari, L. Fish innate immunity against intestinal helminths. Fish Shellfish. Immunol. 2016, 50, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.N.; Steinel, N.C.; Peng, F.; Shim, K.C.; Lohman, B.K.; Fuess, L.E.; Subramanian, S.; Lisle, S.P.D.; Bolnick, D.I. Evolutionary gain and loss of a pathological immune response to parasitism. Science 2022, 377, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Levsen, A.; Berland, B. Anisakis species. In Fish Parasites: Pathobiology and Protection; Woo, P.T.K., Buchmann, K., Eds.; CAB International: Wallingford, UK, 2012; pp. 298–309. ISBN 9781845938062. [Google Scholar]
- Strømnes, E.; Andersen, K. Distribution of whaleworm (Anisakis simplex, Nematoda, Ascaridoidea) L3 larvae in three species of marine fish; saithe (Pollachius virens (L.)), cod (Gadus morhua L.) and redfish (Sebastes marinus (L.)) from Norwegian waters. Parasitol. Res. 1998, 84, 281–285. [Google Scholar] [CrossRef]
- Mattiucci, S.; Cimmaruta, R.; Cipriani, P.; Abaunza, P.; Bellisario, B. Integrating Anisakis spp. parasites data and host genetic structure in the frame of a holistic approach for stock identification of selected Mediterranean Sea fish species. Parasitology 2015, 142, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Hernández, A.M.; Morales-Yuste, M.; Buzo-Domínguez, S.; Adroher, F.J.; Benítez, R. Anisakis infection in anchovies (Engraulis encrasicolus) from Iberian waters, Southwestern Europe, Post-mortem larval migration. 2022; submitted. [Google Scholar]
- Romero, M.C.; Valero, A.; Navarro-Moll, M.C.; Martín-Sánchez, J. Experimental comparison of pathogenic potential of two sibling species Anisakis simplex s.s. and Anisakis pegreffii in Wistar rat. Trop. Med. Int. Health 2013, 18, 979–984. [Google Scholar] [CrossRef]
- Suzuki, J.; Murata, R.; Hosaka, M.; Araki, J. Risk factors for human Anisakis infection and association between the geographic origins of Scomber japonicus and anisakid nematodes. Int. J. Food Microbiol. 2010, 137, 88–93. [Google Scholar] [CrossRef]
- Oliveira, M.S.B.; Lima Corrêa, L.; Prestes, L.; Neves, L.R.; Brasiliense, A.R.P.; Ferreira, D.O.; Tavares-Dias, M. Comparison of the endoparasite fauna of Hoplias malabaricus and Hoplerythrinus unitaeniatus (Erythrinidae), sympatric hosts in the eastern Amazon region (Brazil). Helminthologia 2018, 55, 157–165. [Google Scholar] [CrossRef]
| Ascaridoidea | Anisakis | Hysterothylacium | |
|---|---|---|---|
| Prevalence (%) (CI 95%) | 20.3 (14.0–28.1) | 9.4 (5.4–15.9) | 12.5 (7.7–19.4) |
| Mean Intensity (range) (CI 95%) | 1.31 (1–3) (1.12–1.50) | 1.42 (1–3) (1.08–1.75) | 1.06 (1–2) (1.00–1.19) |
| Mean Abundance (CI 95%) | 0.27 (0.17–0.37) | 0.13 (0.06–0.23) | 0.13 (0.08–0.20) |
| Host Parameters | Uninfected Fish | Fish Infected with | ||
|---|---|---|---|---|
| Ascaridoidea | Anisakis | Hysterothylacium | ||
| Number of fish | 101 | 26 | 12 | 16 |
| Mean length ± SD (cm) | 14.93 ± 1.72 | 16.47 ± 1.45 ** | 16.88 ± 1.14 ** | 16.24 ± 1.55 * |
| Mean weight ± SD (g) | 26.71 ± 10.01 | 35.02 ± 9.29 ** | 36.50 ± 7.19 * | 34.46 ± 10.49 * |
| Condition factor ± SD | 0.762 ± 0.072 | 0.768 ± 0.071 ns | 0.752 ± 0.054 ns | 0.783 ± 0.085 ns |
| Parasites | Parameters | <15.5 cm | ≥15.5 cm |
|---|---|---|---|
| Fish number | 65 | 62 | |
| Ascaridoidea | Prevalence (%) (CI 95%) | 7.7 (2.5–17.1) | 33.9 ** (22.3–47.0) |
| Mean Intensity (range) (CI 95%) | 1.00 (1) uncertain | 1.38 * (1–3) (1.14–1.62) | |
| Mean Abundance (CI 95%) | 0.08 (0.02–0.14) | 0.47 ** (0.29–0.65) | |
| Anisakis | Prevalence (%) (CI 95%) | 0 (0.00–5.52) | 19.4 ** (10.4–31.4) |
| Mean Intensity (range) (CI 95%) | 0 n.a. | 1.42 ns (1–3) (1.08–1.75) | |
| Mean Abundance (CI 95%) | 0 uncertain | 0.27 * (0.15–0.45) | |
| Hysterothylacium | Prevalence (%) (CI 95%) | 7.7 (2.5–17.1) | 17.7 ns (9.2–29.5) |
| Mean Intensity (range) (CI 95%) | 1.00 (1) uncertain | 1.09 ns (1–2) (1.00–1.27) | |
| Mean Abundance (CI 95%) | 0.08 (0.02–0.14) | 0.19 ns (0.10–0.31) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Yuste, M.; Sánchez-Yebra, W.; Garrido, M.; Benítez, R.; Adroher, F.J. Anisakis Infection in the Spotted Flounder Citharus linguatula (Pleuronectiformes: Citharidae) Caught in the Gulf of Cadiz (Area FAO 27-ICES IXa) Appears to Negatively Affect Fish Growth. Pathogens 2022, 11, 1432. https://doi.org/10.3390/pathogens11121432
Morales-Yuste M, Sánchez-Yebra W, Garrido M, Benítez R, Adroher FJ. Anisakis Infection in the Spotted Flounder Citharus linguatula (Pleuronectiformes: Citharidae) Caught in the Gulf of Cadiz (Area FAO 27-ICES IXa) Appears to Negatively Affect Fish Growth. Pathogens. 2022; 11(12):1432. https://doi.org/10.3390/pathogens11121432
Chicago/Turabian StyleMorales-Yuste, Manuel, Waldo Sánchez-Yebra, Mario Garrido, Rocío Benítez, and Francisco Javier Adroher. 2022. "Anisakis Infection in the Spotted Flounder Citharus linguatula (Pleuronectiformes: Citharidae) Caught in the Gulf of Cadiz (Area FAO 27-ICES IXa) Appears to Negatively Affect Fish Growth" Pathogens 11, no. 12: 1432. https://doi.org/10.3390/pathogens11121432
APA StyleMorales-Yuste, M., Sánchez-Yebra, W., Garrido, M., Benítez, R., & Adroher, F. J. (2022). Anisakis Infection in the Spotted Flounder Citharus linguatula (Pleuronectiformes: Citharidae) Caught in the Gulf of Cadiz (Area FAO 27-ICES IXa) Appears to Negatively Affect Fish Growth. Pathogens, 11(12), 1432. https://doi.org/10.3390/pathogens11121432








