Re-Emergence of Circulation of Seasonal Influenza during COVID-19 Pandemic in Russia and Receptor Specificity of New and Dominant Clade 3C.2a1b.2a.2 A(H3N2) Viruses in 2021–2022
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation and Influenza Diagnostics
2.2. Hemagglutination Inhibition Assay
2.3. Phenotypic Analysis of Neuraminidase Inhibition by Oseltamivir and Zanamivir
2.4. Analysis of Herd Immunity
2.5. Sequence Analysis of Influenza Viruses
2.6. Molecular Modeling of HA Receptor Specificity to Human Type Alpha 2,6 Sialoside Receptor
2.7. Preparation of Inactivated Viral Stocks for Receptor-Binding Assay
2.8. Virus Purification for Receptor-Binding Assay
2.9. Receptor Binding Assay
3. Results
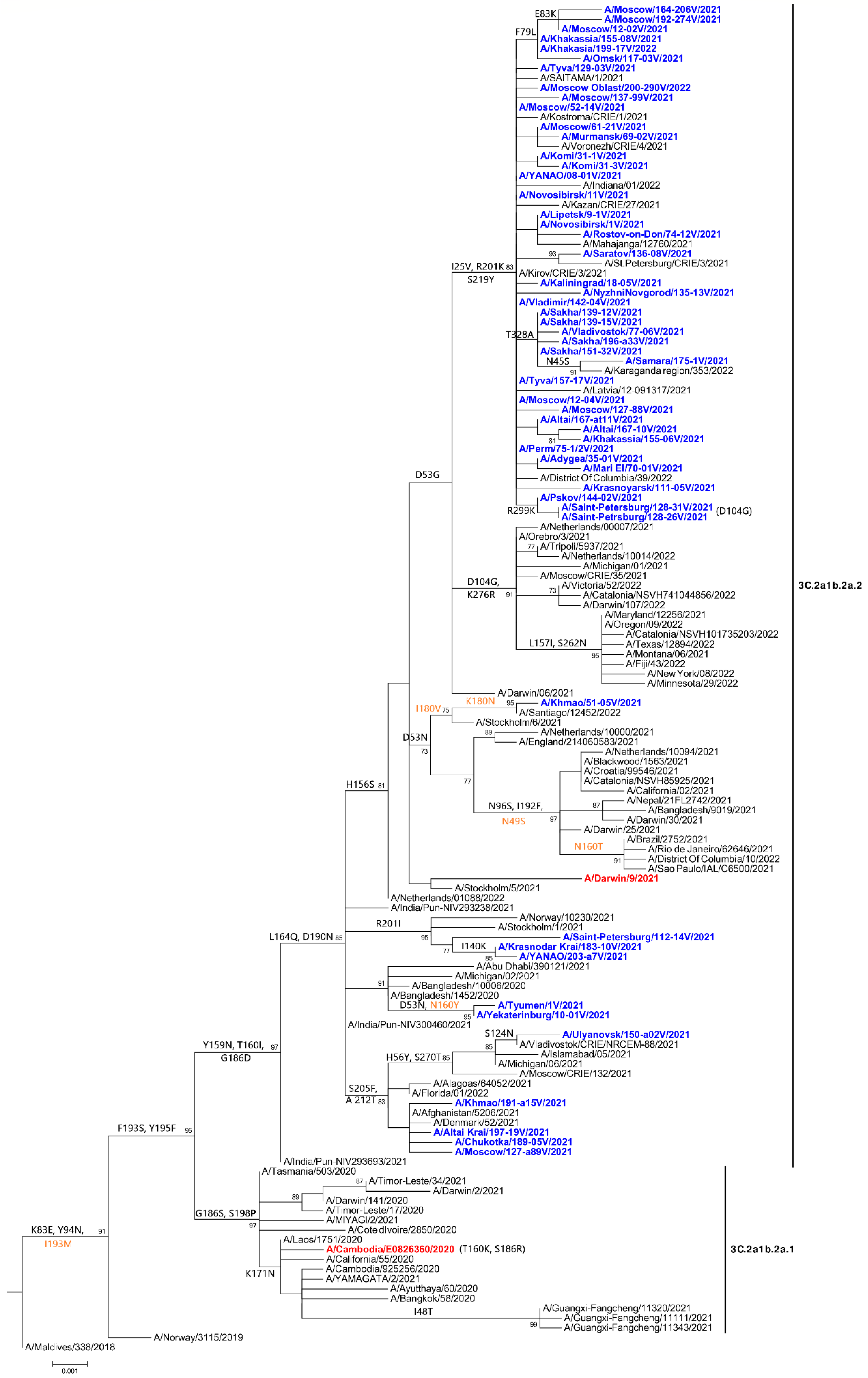
3.1. Genetic and Virological Analysis of Circulating Viruses in Russia in 2020–2022
3.2. Drug Susceptibility
3.3. Investigation of Herd Immunity
3.4. Receptor Specificity Analysis of A(H3N2) Using Molecular Modeling
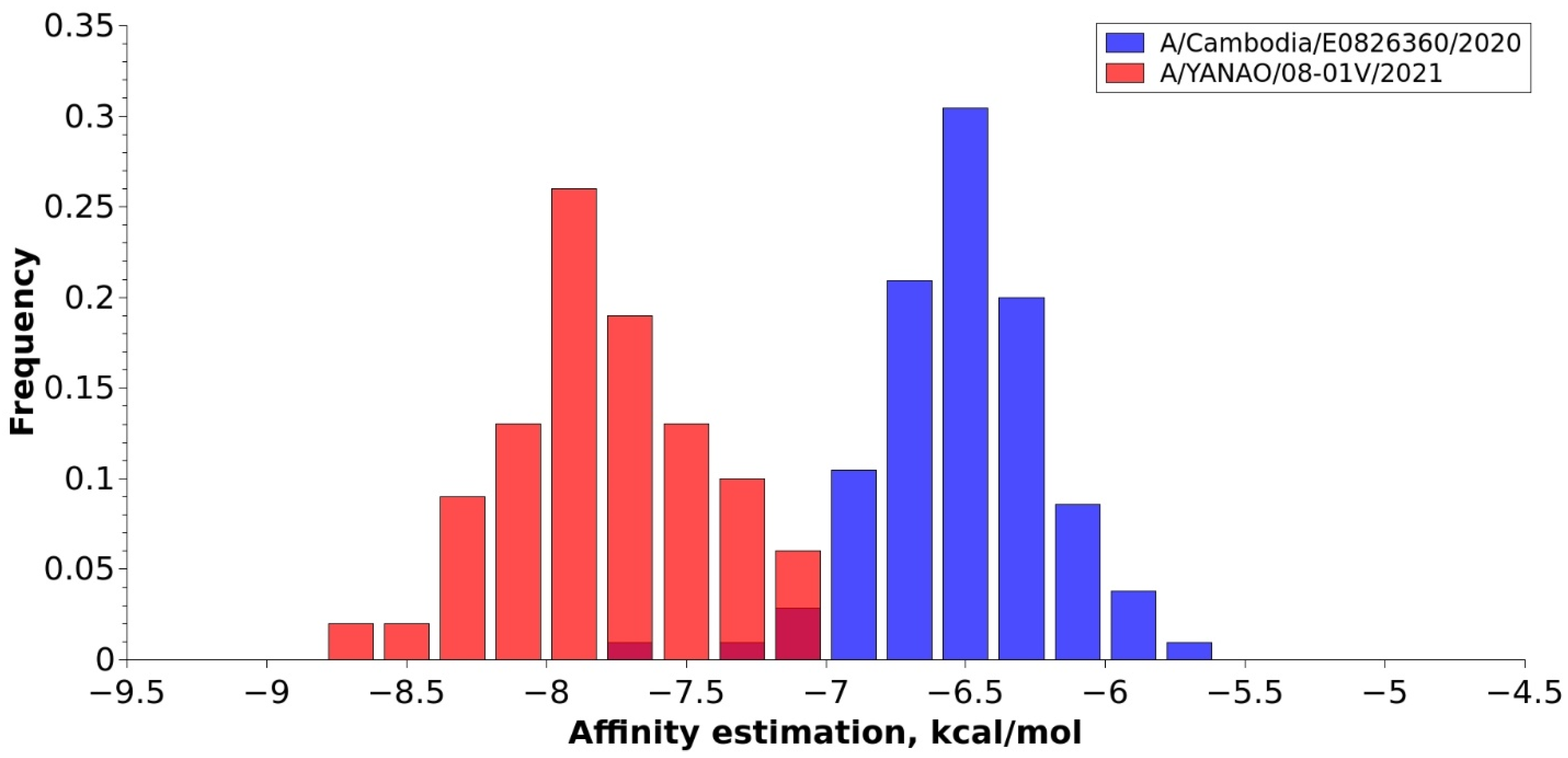
3.5. In Vitro Receptor Specificity Analysis
4. Discussion
4.1. Reemergence of Influenza Circulation in Russia and around the World in 2021–2022
4.2. Genetic and Virological Analysis of H3N2
4.3. Genetic Analysis of A(H1N1)pdm09
4.4. Genetic and Virological Analysis of Type B Viruses
4.5. Population Immunity and Vaccine Effectiveness as Factors Associated with the Emergent and Dominant Circulation of A(H3N2) in the 2021–2022 Season
4.6. Receptor Specificity of Circulating Clade 3C.2a1b.2a.2 A(H3N2) Viruses in Russia in 2021–2022
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Groves, H.E.; Piche-Renaud, P.; Peci, A.; Farrar, D.S.; Buckrell, S.; Bancej, C.; Sevenhuysen, C.; Campigotto, A.; Gubbay, J.B.; Morris, S. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg. Health Am. 2021, 1, 100015. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Mook, P.; Lamb, F.; Ferland, L.; Melidou, A.; Amato-Gauci, A.J.; Pebody, R.; The European Influenza Surveillance Network. Very little influenza in the WHO European Region during the 2020/21 season, weeks 40 2020 to 8 2021. Euro Surveill. 2021, 26, 2100221. [Google Scholar] [CrossRef] [PubMed]
- Spantideas, N.; Bougea, A.M.; Drosou, E.G.; Khanderia, N.; Rai, S. COVID-19 and Seasonal Influenza: No Room for Two. Cureus 2021, 13, e18007. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Cherry, S. Viral Interference: The Case of Influenza Viruses. J. Infect. Dis. 2015, 212, 1690–1691. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Mihaylova, V.T.; Landry, M.L.; Foxman, E.F. Interference between rhinovirus and influenza A virus: A clinical data analysis and experimental infection study. Lancet Microbe 2020, 1, e254–e262. [Google Scholar] [CrossRef]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Murcia, P.R. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza a Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef]
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2021–2022 northern hemisphere influenza season. Wkly. Epidemiol. Rec. 2021, 96, 77–88. [Google Scholar]
- Recommended Composition of Influenza Virus Vaccines for Use in the 2022 Southern Hemisphere Influenza Season. Available online: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2022-southern-hemisphere-influenza-season (accessed on 1 September 2022).
- Recommended Composition of Influenza Virus Vaccines for Use in the 2022–2023 Northernrhemisphere Influenza Season. Available online: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2022–2023-northern-hemisphere-influenza-season (accessed on 1 September 2022).
- Borau, M.S.; Stertz, S. Entry of influenza A virus into host cells—Recent progress and remaining challenges. Curr. Opin. Virol. 2021, 48, 23–29. [Google Scholar] [CrossRef]
- De Graaf, M.; Fouchier, R.A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014, 33, 823–841. [Google Scholar] [CrossRef]
- Lin, Y.P.; Xiong, X.; Wharton, S.A.; Martin, S.R.; Coombs, P.J.; Vachieri, S.G.; Christodoulou, E.; Walker, P.A.; Liu, J.; Skehel, J.J.; et al. Evolution of the Receptor Binding Properties of the Influenza A(H3N2) Hemagglutinin. Proc. Natl. Acad. Sci. USA 2012, 109, 21474–21479. [Google Scholar] [CrossRef]
- Bolton, M.J.; Ort, J.T.; McBride, R.; Swanson, N.J.; Wilson, J.; Awofolaju, M.; Furey, C.; Greenplate, A.R.; Drapeau, E.M.; Pekosz, A.; et al. Antigenic and Virological Properties of an H3N2 Variant That Continues to Dominate the 2021-22 Northern Hemisphere Influenza Season. Cell Rep. 2022, 39, 110897. [Google Scholar] [CrossRef]
- World Health Organization. Surveillance Network: Manual for the LABORATORY Diagnosis and Virological Surveillance of Influenza; WHO Press: Geneva, Switzerland, 2011. [Google Scholar]
- Leang, S.-K.; Hurt, A.C. Fluorescence-Based Neuraminidase Inhibition Assay to Assess the Susceptibility of Influenza Viruses to The Neuraminidase Inhibitor Class of Antivirals. J. Vis. Exp. 2017, 122, e55570. [Google Scholar] [CrossRef]
- Kolosova, N.P.; Ilyicheva, T.N.; Danilenko, A.V.; Bulanovich, J.A.; Svyatchenko, S.V.; Durymanov, A.G.; Goncharova, N.I.; Gudymo, A.S.; Shvalov, A.N.; Susloparov, I.M.; et al. Severe Cases of Seasonal Influenza in Russia in 2017–2018. PLoS ONE 2019, 14, e0220401. [Google Scholar] [CrossRef]
- St George, K. Methods in molecular biology. In Influenza Virus: Methods and Protocols; Kawaoka, Y., Neumann, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Deng, Y.M.; Spirason, N.; Iannello, P.; Jelley, L.; Lau, H.; Barr, I.G. A simplified sanger sequencing method for full genome sequencing of multiple subtypes of human influenza A viruses. J. Clin. Virol. 2015, 68, 43–48. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA—MEM. arXiv 2022, arXiv:1303.3997v2. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Amaro, R.E.; Baudry, J.; Chodera, J.; Demir, Ö.; McCammon, J.A.; Miao, Y.; Smith, J.C. Ensemble docking in drug discovery. Biophys. J. 2018, 114, 2271–2278. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Eswar, N.; Eramian, D.; Webb, B.; Shen, M.-Y.; Sali, A. Protein Structure Modeling with MODELLER. Methods Mol. Biol. 2008, 426, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A Generalizable Biomolecular Force Field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from Ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable Molecular Dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Feller Scott, E.; Zhang, Y.; Pastor, R.W. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Amadei, A.; Linssem, A.B.M.; Berendsen, H.J.C. Essential dynamics of proteins. Proteins Struc. Func. Gen. 1993, 17, 412–425. [Google Scholar] [CrossRef]
- Roe, D.R.; Thomas, E. Cheatham III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Tvaroŝka, I.; Bleha, T. Anomeric and exo-anomeric effects in carbohydrate chemistry. Adv. Carbohydr. Chem. Biochem. 1989, 47, 45–123. [Google Scholar]
- Sapay, N.; Nurisso, A.; Imberty, A. Simulation of carbohydrates, from molecular docking to dynamics in water. Biomol. Simul. 2013, 924, 469–483. [Google Scholar]
- Zhang, Y.; Forli, S.; Omelchenko, A.; Sanner, M.F. AutoGridFR: Improvements on AutoDock Affinity Maps and Associated Software Tools. J. Comput. Chem. 2019, 40, 2882–2886. [Google Scholar] [CrossRef]
- Fei, Y.; Sun, Y.-S.; Li, Y.; Yu, H.; Lau, K.; Landry, J.P.; Luo, Z.; Baumgarth, N.; Chen, X.; Zhu, X. Characterization of receptor binding profiles of influenza a viruses using an ellipsometry-based label-free glycan microarray assay platform. Biomolecules 2015, 5, 1480–1498. [Google Scholar] [CrossRef]
- Recommended Composition of Influenza Virus Vaccines for Use in the 2023 Southern Hemisphere Influenza Season. Available online: https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-southern-hemisphere-recommendation-2023/202209_recommendation.pdf?sfvrsn=83a26d50_3&download=true. (accessed on 1 October 2022).
- Laboratory Methodologies for Testing the Antiviral Susceptibility of Influenza Viruses. Available online: https://www.who.int/teams/global-influenza-programme/laboratory-network/quality-assurance/antiviral-susceptibility-influenza (accessed on 1 September 2022).
- World Health Organization. Meetings of the WHO working group on surveillance of influenza antiviral susceptibility—Geneva, November 2011 and June 2012. Wkly Epidemiol Rec. 2012, 87, 369–374. [Google Scholar]
- Rhodes, G. Other kinds of macromolecular models. In Crystallography Made Crystal Clear: A Guide for Users of Macromolecular Models; Elsevier: Amsterdam, The Netherlands, 2006; pp. 237–267. [Google Scholar]
- Melidou, A.; Ködmön, C.; Nahapetyan, K.; Kraus, A.; Alm, E.; Adlhoch, C.; Mooks, P.; Dave, N.; Carvalho, C.; Meslé, M.M.; et al. Influenza Returns with a Season Dominated by Clade 3C.2a1b.2a.2 A(H3N2) Viruses, WHO European Region, 2021/22. Eur. J. Infect. Dis. Surveill. Epidemiol. Prev. Control. 2022, 27, 2200255. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef]
- Merced-Morales, A.; Daly, P.; Abd Elal, A.I.; Ajayi, N.; Annan, E.; Budd, A.; Barnes, J.; Colon, A.; Cummings, C.N.; Iuliano, A.D. Influenza Activity and Composition of the 2022–23 Influenza Vaccine—United States, 2021–22 Season. MMWR Morb. Mortal Wkly Rep. 2022, 71, 913–919. [Google Scholar] [CrossRef]
- Report Prepared for the WHO Annual Consultation on the Composition of Influenza Vaccines for the Southern Hemisphere 2023. 19–22 September 2022. Worldwide Influenza Centre WHO CC for Reference and Research on Influenza. The Francis Crick Institute. Available online: https://www.crick.ac.uk/sites/default/files/2022-10/Crick%20report%20Sep2022%20for%20SH2023_to%20post.pdf (accessed on 1 September 2022).
- Influenza Virus Characterization: Summary Report, Europe, May 2022. Copenhagen: World Health Organization Regional Office for Europe and European Centre for Disease Prevention and Control; Copenhagen and Stockholm. 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/influenza-characterisation-report-may-2022.pdf (accessed on 1 September 2022).
- Interim US Flu Vaccine Effectiveness (VE) Data for 2021–2022. Available online: https://www.cdc.gov/flu/vaccines-work/2021–2022.html (accessed on 1 September 2022).
- Chung, J.R.; Kim, S.S.; Kondor, R.J.; Smith, C.; Budd, A.P.; Tartof, S.Y.; Florea, A.; Talbot, H.K.; Grijalva, C.G.; Wernli, K.J. Interim Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness—United States, February 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 365–370. [Google Scholar] [CrossRef]
- Kim, S.; Chuang Erica, S.Y.; Sabaiduc, S.; Olsha, R.; Kaweski, S.E.; Zelyas, N.; Gubbay, J.B.; Jassem, A.N.; Charest, H.; De Serres, G.; et al. Influenza vaccine effectiveness against A(H3N2) during the delayed 2021/22 epidemic in Canada. Euro Surveill. 2022, 27, 2200720. [Google Scholar] [CrossRef]
- McLean, H.Q.; Belongia, E.A. Influenza Vaccine Effectiveness: New Insights and Challenges. Cold Spring Harb. Perspect. Med. 2021, 11, a038315. [Google Scholar] [CrossRef]
- On the Course of Immunization of the Population Against Influenza, on the Epidemiological Situation in the Incidence of Acute Respiratory Viral Infections in the World and in the Russian Federation. Available online: https://www.rospotrebnadzor.ru/about/info/news/news_details.php?ELEMENT_ID=16800&sphrase_id=4306263 (accessed on 1 September 2022).
- On the Epidemiological Situation on the Incidence of Influenza and SARS and the Course of Immunization of the Population Against Influenza in the Russian Federation. Available online: https://www.rospotrebnadzor.ru/about/info/news/news_details.php?ELEMENT_ID=20530&sphrase_id=4306263 (accessed on 1 September 2022).
- Ilyicheva, T.N.; Kolosova, N.P.; Durymanov, A.G.; Torzhkova Pyu Svyatchenko, S.V.; Bulanovich, Y.u.A.; Ivanova, E.V.; Ivanova, K.I.; Ryzhikov, A.B. 2019–2020 herd immunity to seasonal influenza viruses prior to epidemic season and rate of severe disease cases. Russ. J. Infect. Immun. Infektsiya I Immun. 2021, 11, 927–933. [Google Scholar] [CrossRef]
- Lee, K.; Jalal, H.; Raviotta, J.M.; Krauland, M.G.; Zimmerman, R.K.; Burke, D.S.; Roberts, M.S. Estimating the Impact of Low Influenza Activity in 2020 on Population Immunity and Future Influenza Seasons in the United States. Open Forum Infect. Dis. 2022, 9, ofab607. [Google Scholar] [CrossRef]
- Olsen, S.J.; Winn, A.K.; Budd, A.P.; Prill, M.M.; Steel, J.; Midgley, C.M.; Kniss, K.; Burns, E.; Rowe, T.; Foust, A. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic—United States, 2020–2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1013–1019. [Google Scholar] [CrossRef]
- About Influenza Vaccination in Questions and Answers. Available online: www.rospotrebnadzor.ru/about/info/news/news_details.php?ELEMENT_ID=18963 (accessed on 1 September 2022).
- DeMarco, M.L.; Woods, R.J. Structural Glycobiology: A Game of Snakes and Ladders. Glycobiology 2008, 18, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.J. Predicting the Structures of Glycans, Glycoproteins, and Their Complexes. Chem. Rev. 2018, 118, 8005–8024. [Google Scholar] [CrossRef] [PubMed]
- Jongkon, N.; Mokmak, W.; Chuakheaw, D.; Shaw, P.J.; Tongsima, S.; Sangma, C. Prediction of avian influenza A binding preference to human receptor using conformational analysis of receptor bound to hemagglutinin. BMC Genom. 2009, 10, S24. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.E.; Newhouse, I.; Amaro, R.E.; Pao, H.C.; Cheng, L.S.; Markwick, P.R.L.; McCammon, J.A.; Li, W.W.; Arzberger, W.P. Distinct glycan topology for avian and human sialopentasaccharide receptor analogues upon binding different hemagglutinins: A molecular dynamics perspective. J. Mol. Biol. 2009, 387, 465–491. [Google Scholar] [CrossRef][Green Version]
- Wu, N.C.; Thompson, A.J.; Xie, J.; Lin, C.W.; Nycholat, C.M.; Zhu, X.; Wilson, I.A. A complex epistatic network limits the mutational reversibility in the influenza hemagglutinin receptor-binding site. Nat. Commun. 2018, 9, 1264. [Google Scholar] [CrossRef]
- Ni, F.; Kondrashkina, E.; Wang, Q. Determinant of receptor-preference switch in influenza hemagglutinin. Virology 2018, 513, 98–107. [Google Scholar] [CrossRef]
- Nunthaboot, N.; Rungrotmongkol, T.; Malaisree, M.; Decha, P.; Kaiyawet, N.; Intharathep, P.; Sompornpisut, P.; Poovorawan, Y.; Hannongbua, S. Molecular insights into human receptor binding to 2009 H1N1 influenza A hemagglutinin. Mon. Chem. Chem. Mon. 2010, 141, 801–807. [Google Scholar] [CrossRef]
- Collins, B.E.; Paulson, J.C. Cell surface biology mediated by low affinity multivalent protein–glycan interactions. Curr. Opin. Chem. Biol. 2004, 8, 617–625. [Google Scholar] [CrossRef]
- Jouimyi, M.R.; Bounder, G.; Essaidi, I.E.; Boura, H.; Zerouali, K.; Lebrazi, H.; Kettani, A.; Maachi, F. Molecular docking of a set of flavonoid compounds with Helicobacter pylori virulence factors CagA and VacA. J. Herbmed Pharmacol. 2020, 9, 412–419. [Google Scholar] [CrossRef]
- Muhammad, S.A.; Fatima, N. In Silico Analysis and Molecular Docking Studies of Potential Angiotensin-Converting Enzyme Inhibitor Using Quercetin Glycosides. Pharmacogn. Mag. 2015, 11 (Suppl. S1), S123–S126. [Google Scholar] [CrossRef]
- Richard, M.; Erny, A.; Caré, B.; Traversier, A.; Barthélémy, M.; Hay, A.; Lin, Y.P.; Ferraris, O.; Lina, B. Rescue of a H3N2 Influenza Virus Containing a Deficient Neuraminidase Protein by a Hemagglutinin with a Low Receptor-Binding Affinity. PLoS ONE 2012, 7, e33880. [Google Scholar] [CrossRef]
- Gambaryan, A.S.; Balish, A.; Klimov, A.I.; Tuzikov, A.B.; Chinarev, A.A.; Pazynina, G.V.; Bovin, N.V. Changes in the Receptor-Binding Properties of H3N2 Viruses during Long-Term Circulation in Humans. Biochem. Biokhimiia 2019, 84, 1177–1185. [Google Scholar] [CrossRef]
- Peng, W.; de Vries, R.P.; Grant, O.C.; Thompson, A.J.; McBride, R.; Tsogtbaatar, B.; Lee, P.S.; Razi, N.; Wilson, I.A.; Woods, R.J.; et al. Recent H3N2 Viruses Have Evolved Specificity for Extended, Branched Human-Type Receptors, Conferring Potential for Increased Avidity. Cell Host Microbe 2017, 21, 23–34. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolosova, N.P.; Ilyicheva, T.N.; Unguryan, V.V.; Danilenko, A.V.; Svyatchenko, S.V.; Onhonova, G.S.; Goncharova, N.I.; Kosenko, M.N.; Gudymo, A.S.; Marchenko, V.Y.; et al. Re-Emergence of Circulation of Seasonal Influenza during COVID-19 Pandemic in Russia and Receptor Specificity of New and Dominant Clade 3C.2a1b.2a.2 A(H3N2) Viruses in 2021–2022. Pathogens 2022, 11, 1388. https://doi.org/10.3390/pathogens11111388
Kolosova NP, Ilyicheva TN, Unguryan VV, Danilenko AV, Svyatchenko SV, Onhonova GS, Goncharova NI, Kosenko MN, Gudymo AS, Marchenko VY, et al. Re-Emergence of Circulation of Seasonal Influenza during COVID-19 Pandemic in Russia and Receptor Specificity of New and Dominant Clade 3C.2a1b.2a.2 A(H3N2) Viruses in 2021–2022. Pathogens. 2022; 11(11):1388. https://doi.org/10.3390/pathogens11111388
Chicago/Turabian StyleKolosova, Natalia P., Tatiana N. Ilyicheva, Vasily V. Unguryan, Alexey V. Danilenko, Svetlana V. Svyatchenko, Galina S. Onhonova, Natalia I. Goncharova, Maksim N. Kosenko, Andrey S. Gudymo, Vasiliy Y. Marchenko, and et al. 2022. "Re-Emergence of Circulation of Seasonal Influenza during COVID-19 Pandemic in Russia and Receptor Specificity of New and Dominant Clade 3C.2a1b.2a.2 A(H3N2) Viruses in 2021–2022" Pathogens 11, no. 11: 1388. https://doi.org/10.3390/pathogens11111388
APA StyleKolosova, N. P., Ilyicheva, T. N., Unguryan, V. V., Danilenko, A. V., Svyatchenko, S. V., Onhonova, G. S., Goncharova, N. I., Kosenko, M. N., Gudymo, A. S., Marchenko, V. Y., Shvalov, A. N., Susloparov, I. M., Tregubchak, T. V., Gavrilova, E. V., Maksyutov, R. A., & Ryzhikov, A. B. (2022). Re-Emergence of Circulation of Seasonal Influenza during COVID-19 Pandemic in Russia and Receptor Specificity of New and Dominant Clade 3C.2a1b.2a.2 A(H3N2) Viruses in 2021–2022. Pathogens, 11(11), 1388. https://doi.org/10.3390/pathogens11111388





