Extracellular Vesicles Derived from Allergen Immunotherapy-Treated Mice Suppressed IL-5 Production from Group 2 Innate Lymphoid Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of SCIT on the Development of AHR and IL-5 Production in BALF
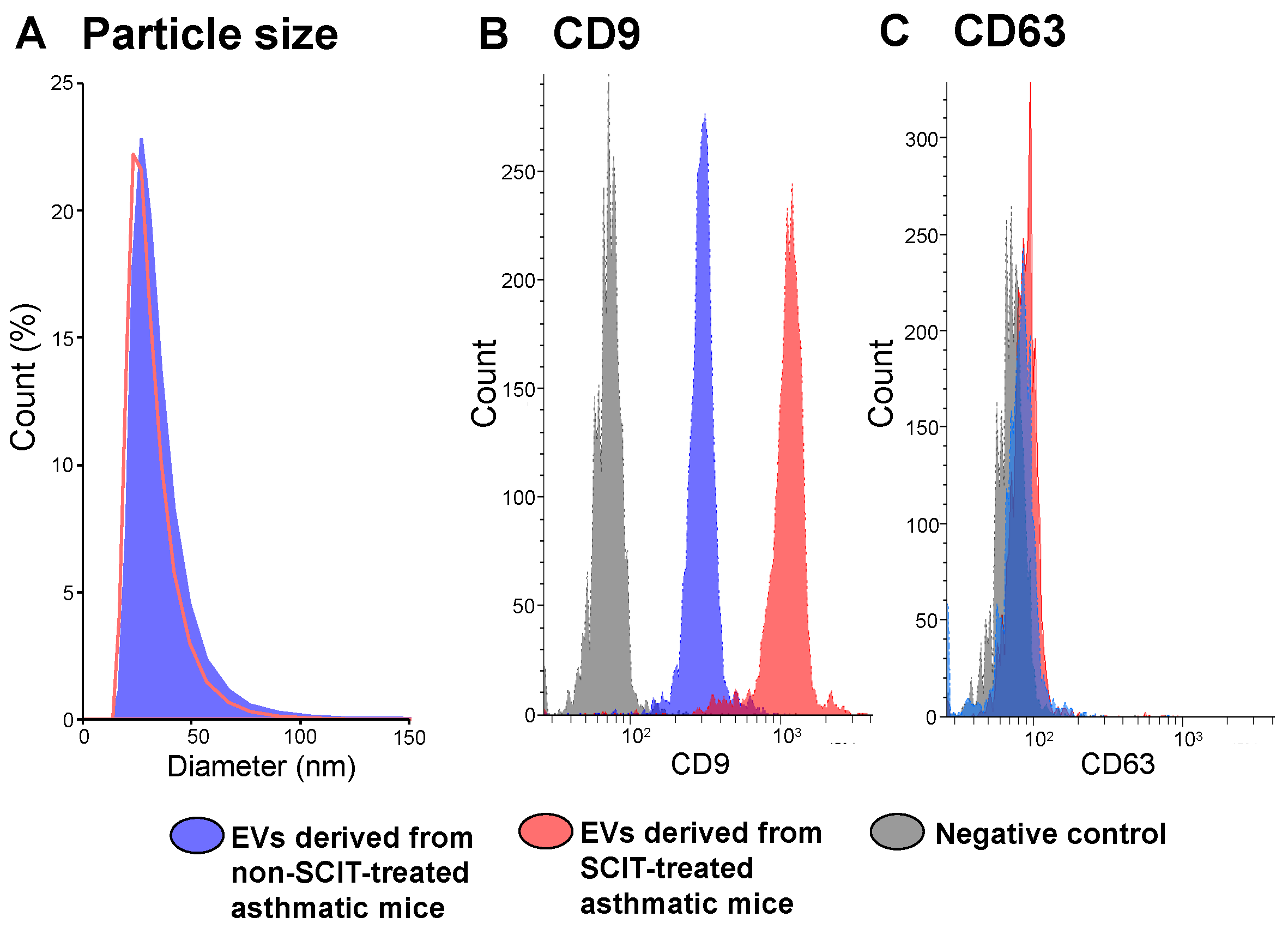
2.2. Phenotypes of EVs Derived from Sera of Non-SCIT-Treated and SCIT-Treated Asthmatic Mice
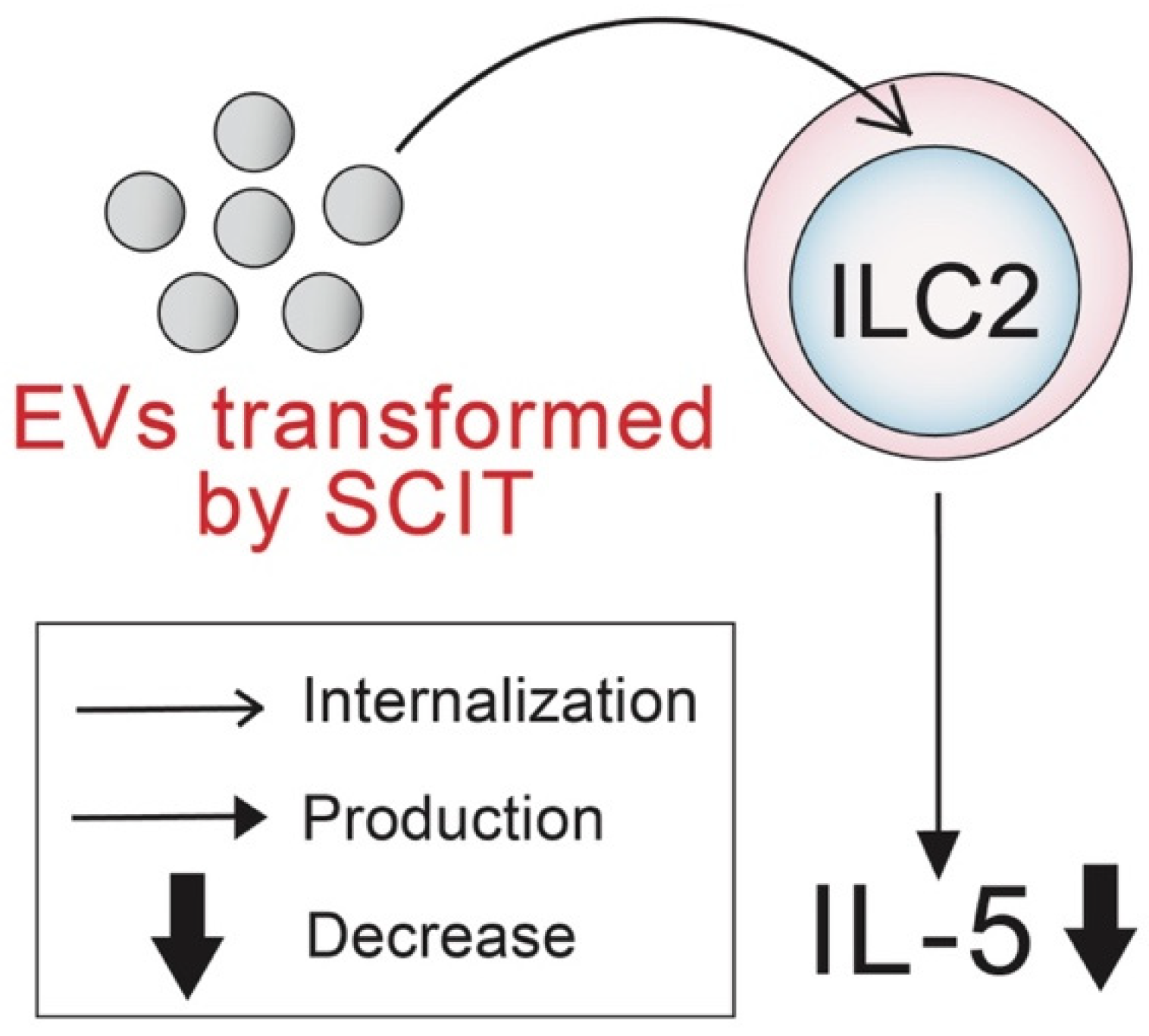
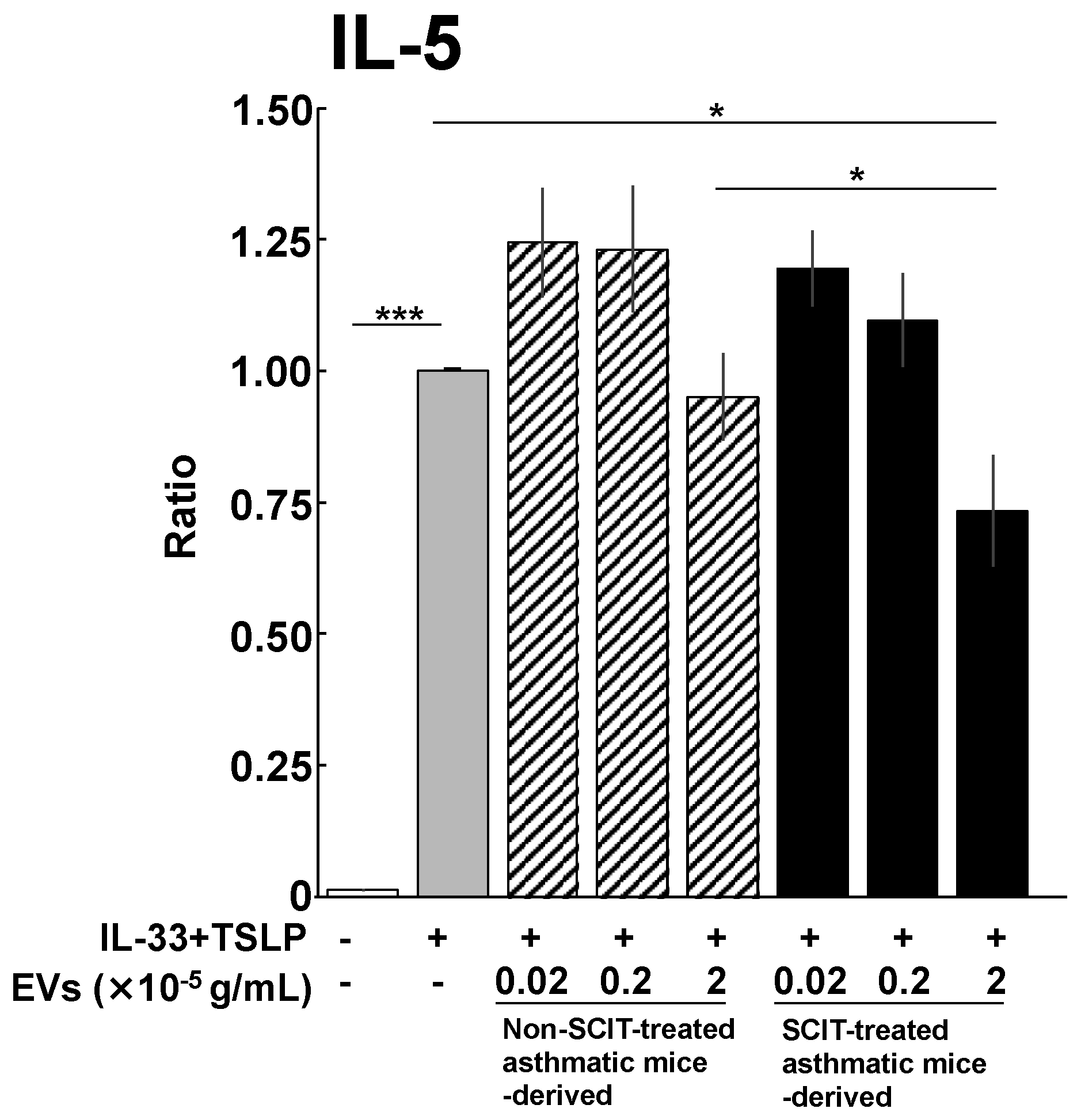
2.3. Effects of EVs on IL-5 Production from ILC2s
3. Discussion
4. Materials and Methods
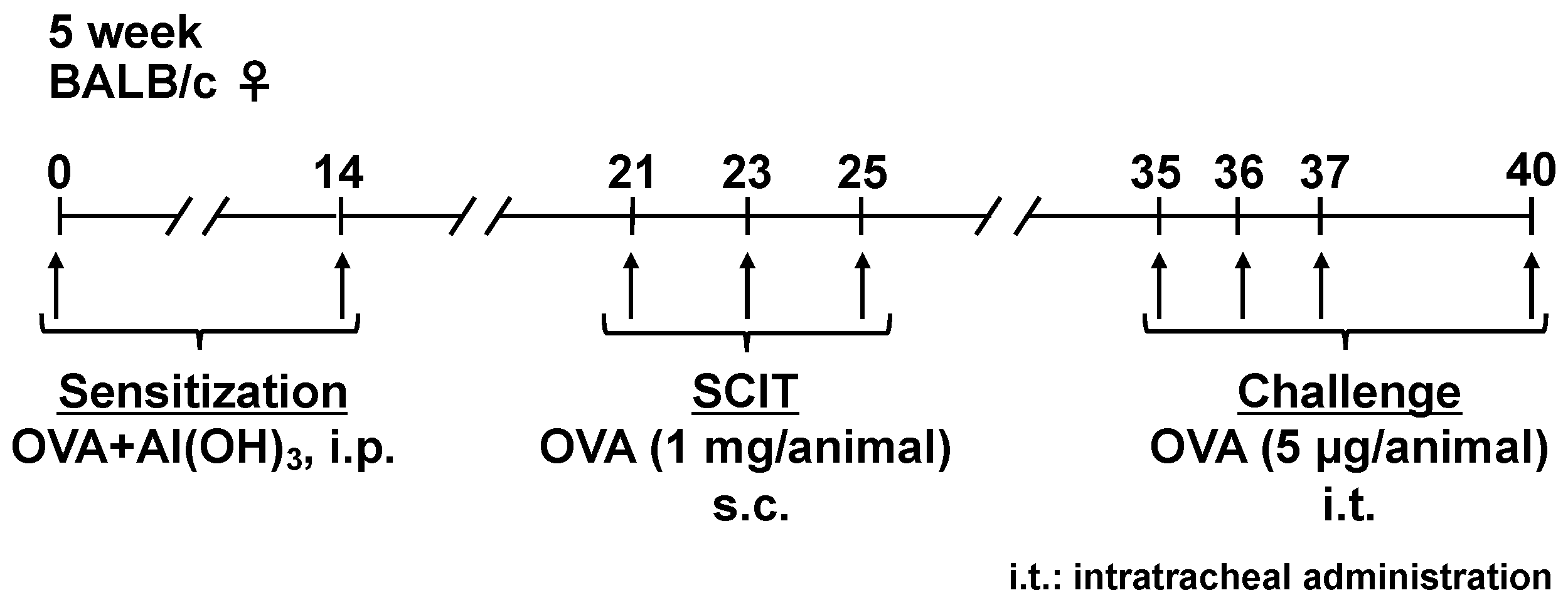
4.1. Sensitization, SCIT, and Challenges
4.2. Measurement of AHR
4.3. Quantitative Analysis of IL-5 in BALF
4.4. Isolation of EVs
4.5. Analyses of CD9 and CD63 Expression Levels and Particle Sizes of EVs
4.6. Effect of EVs on IL-5 Production from ILC2s
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savouré, M.; Bousquet, J.; Jaakkola, J.J.K.; Jaakkola, M.S.; Jacquemin, B.; Nadif, R. Worldwide Prevalence of Rhinitis in Adults: A Review of Definitions and Temporal Evolution. Clin. Transl. Allergy 2022, 12, e12130. [Google Scholar] [CrossRef]
- Gotoh, M.; Suzuki, H.; Okubo, K. Delay of Onset of Symptoms of Japanese Cedar Pollinosis by Treatment with a Leukotriene Receptor Antagonist. Allergol. Int. 2011, 60, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Yamada, T.; Kubo, S.; Osawa, Y.; Kimura, Y.; Oh, M.; Susuki, D.; Takabayashi, T.; Okamoto, M.; Fujieda, S. Efficacy of Oral Olopatadine Hydrochloride for the Treatment of Seasonal Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Study. Allergy Asthma Proc. 2010, 31, 296–303. [Google Scholar] [CrossRef]
- Takahashi, G.; Matsuzaki, Z.; Okamoto, A.; Ito, E.; Matsuoka, T.; Nakayama, T.; Masuyama, K. A Randomized Control Trail of Stepwise Treatment with Fluticasone Propionate Nasal Spray and Fexofenadine Hydrochloride Tablet for Seasonal Allergic Rhinitis. Allergol. Int. 2012, 61, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. SIRIUS Investigators Oral Glucocorticoid-Sparing Effect of Mepolizumab in Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Dávila, I. Dupilumab: A New Paradigm for the Treatment of Allergic Diseases. J. Investig. Allergol. Clin. Immunol. 2018, 28, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Jutel, M.; Agache, I.; Bonini, S.; Burks, A.W.; Calderon, M.; Canonica, W.; Cox, L.; Demoly, P.; Frew, A.J.; O’Hehir, R.; et al. International Consensus on Allergy Immunotherapy. J. Allergy Clin. Immunol. 2015, 136, 556–568. [Google Scholar] [CrossRef] [Green Version]
- Noon, L. Prophylactic Inoculation against Hay Fever. Lancet 1911, 177, 1572–1573. [Google Scholar] [CrossRef] [Green Version]
- Calderon, M.A.; Alves, B.; Jacobson, M.; Hurwitz, B.; Sheikh, A.; Durham, S. Allergen Injection Immunotherapy for Seasonal Allergic Rhinitis. Cochrane Database Syst. Rev. 2007, CD001936. [Google Scholar] [CrossRef]
- Radulovic, S.; Calderon, M.A.; Wilson, D.; Durham, S. Sublingual Immunotherapy for Allergic Rhinitis. Cochrane Database Syst. Rev. 2010, CD002893. [Google Scholar] [CrossRef]
- Abramson, M.J.; Puy, R.M.; Weiner, J.M. Injection Allergen Immunotherapy for Asthma. Cochrane Database Syst. Rev. 2010, CD001186. [Google Scholar] [CrossRef]
- Normansell, R.; Kew, K.M.; Bridgman, A.-L. Sublingual Immunotherapy for Asthma. Cochrane Database Syst. Rev. 2015, CD011293. [Google Scholar] [CrossRef]
- Fortescue, R.; Kew, K.M.; Leung, M.S.T. Sublingual Immunotherapy for Asthma. Cochrane Database Syst. Rev. 2020, 9, CD011293. [Google Scholar] [CrossRef]
- Durham, S.R.; Penagos, M. Sublingual or Subcutaneous Immunotherapy for Allergic Rhinitis? J. Allergy Clin. Immunol. 2016, 137, 339–349.e10. [Google Scholar] [CrossRef]
- Marogna, M.; Spadolini, I.; Massolo, A.; Canonica, G.W.; Passalacqua, G. Long-Lasting Effects of Sublingual Immunotherapy According to Its Duration: A 15-Year Prospective Study. J. Allergy Clin. Immunol. 2010, 126, 969–975. [Google Scholar] [CrossRef]
- Scadding, G.W.; Calderon, M.A.; Shamji, M.H.; Eifan, A.O.; Penagos, M.; Dumitru, F.; Sever, M.L.; Bahnson, H.T.; Lawson, K.; Harris, K.M.; et al. Effect of 2 Years of Treatment With Sublingual Grass Pollen Immunotherapy on Nasal Response to Allergen Challenge at 3 Years Among Patients With Moderate to Severe Seasonal Allergic Rhinitis: The GRASS Randomized Clinical Trial. JAMA 2017, 317, 615–625. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Mansueto, P.; Pacor, M.L.; Rizzo, M.; Castello, F.; Martinelli, N.; Ditta, V.; Lo Bianco, C.; Leto-Barone, M.S.; D’Alcamo, A.; et al. Evaluation of Serum S-IgE/Total IgE Ratio in Predicting Clinical Response to Allergen-Specific Immunotherapy. J. Allergy Clin. Immunol. 2009, 123, 1103–1110.e4. [Google Scholar] [CrossRef] [Green Version]
- Manzotti, G.; Lombardi, C. Allergen Immunotherapy as a Drug: The New Deal of Grass Allergen Tablets from Clinical Trials to Current Practice. Eur. Ann. Allergy Clin. Immunol. 2013, 45, 34–42. [Google Scholar]
- Bernstein, D.I.; Wanner, M.; Borish, L.; Liss, G.M. Immunotherapy Committee, American Academy of Allergy, Asthma and Immunology Twelve-Year Survey of Fatal Reactions to Allergen Injections and Skin Testing: 1990–2001. J. Allergy Clin. Immunol. 2004, 113, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Larenas-Linnemann, D.; Lockey, R.F.; Passalacqua, G. Speaking the Same Language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J. Allergy Clin. Immunol. 2010, 125, 569–574.e7. [Google Scholar] [CrossRef] [PubMed]
- Bartemes, K.R.; Kita, H. Roles of Innate Lymphoid Cells (ILCs) in Allergic Diseases: The 10-Year Anniversary for ILC2s. J. Allergy Clin. Immunol. 2021, 147, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.-I.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate Production of T(H)2 Cytokines by Adipose Tissue-Associated c-Kit(+)Sca-1(+) Lymphoid Cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Kabata, H.; Moro, K.; Koyasu, S. The Group 2 Innate Lymphoid Cell (ILC2) Regulatory Network and Its Underlying Mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Matsuda, M.; Tanaka, Y.; Shimora, H.; Takemoto, N.; Nomura, M.; Terakawa, R.; Hashimoto, K.; Sakae, H.; Kanda, A.; Iwai, H.; et al. Pathogenic Changes in Group 2 Innate Lymphoid Cells (ILC2s) in a Steroid-Insensitive Asthma Model of Mice. Eur. J. Pharmacol. 2022, 916, 174732. [Google Scholar] [CrossRef]
- Golebski, K.; Layhadi, J.A.; Sahiner, U.; Steveling-Klein, E.H.; Lenormand, M.M.; Li, R.C.Y.; Bal, S.M.; Heesters, B.A.; Vilà-Nadal, G.; Hunewald, O.; et al. Induction of IL-10-Producing Type 2 Innate Lymphoid Cells by Allergen Immunotherapy Is Associated with Clinical Response. Immunity 2021, 54, 291–307.e7. [Google Scholar] [CrossRef] [PubMed]
- van Rijt, L.S.; Logiantara, A.; Canbaz, D.; van Ree, R. Birch Pollen-Specific Subcutaneous Immunotherapy Reduces ILC2 Frequency but Does Not Suppress IL-33 in Mice. Clin. Exp. Allergy 2018, 48, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Popowski, K.; Lutz, H.; Hu, S.; George, A.; Dinh, P.-U.; Cheng, K. Exosome Therapeutics for Lung Regenerative Medicine. J. Extracell. Vesicles 2020, 9, 1785161. [Google Scholar] [CrossRef] [PubMed]
- Buzás, E.I.; Tóth, E.Á.; Sódar, B.W.; Szabó-Taylor, K.É. Molecular Interactions at the Surface of Extracellular Vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Torregrosa Paredes, P.; Esser, J.; Admyre, C.; Nord, M.; Rahman, Q.K.; Lukic, A.; Rådmark, O.; Grönneberg, R.; Grunewald, J.; Eklund, A.; et al. Bronchoalveolar Lavage Fluid Exosomes Contribute to Cytokine and Leukotriene Production in Allergic Asthma. Allergy 2012, 67, 911–919. [Google Scholar] [CrossRef]
- Cañas, J.A.; Sastre, B.; Rodrigo-Muñoz, J.M.; Fernández-Nieto, M.; Barranco, P.; Quirce, S.; Sastre, J.; Del Pozo, V. Eosinophil-Derived Exosomes Contribute to Asthma Remodelling by Activating Structural Lung Cells. Clin. Exp. Allergy 2018, 48, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, M.C.; McKenzie, C.I.; Varese, N.; Rolland, J.M.; O’Hehir, R.E. Recent Developments and Highlights in Immune Monitoring of Allergen Immunotherapy. Allergy 2019, 74, 2342–2354. [Google Scholar] [CrossRef] [Green Version]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of Allergen-Specific Immunotherapy and Allergen Tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef]
- Matsuda, M.; Morie, Y.; Oze, H.; Doi, K.; Tsutsumi, T.; Hamaguchi, J.; Inaba, M.; Nabe, T. Phenotype Analyses of IL-10-Producing Foxp3- CD4+ T Cells Increased by Subcutaneous Immunotherapy in Allergic Airway Inflammation. Int. Immunopharmacol. 2018, 61, 297–305. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin Functions and Associated Microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [Green Version]
- Ha, C.T.; Waterhouse, R.; Wessells, J.; Wu, J.A.; Dveksler, G.S. Binding of Pregnancy-Specific Glycoprotein 17 to CD9 on Macrophages Induces Secretion of IL-10, IL-6, PGE2, and TGF-Beta1. J. Leukoc. Biol. 2005, 77, 948–957. [Google Scholar] [CrossRef] [Green Version]
- Kabuto, M.; Fujimoto, N.; Takahashi, T.; Tanaka, T. Decreased Level of Interleukin-10-Producing B Cells in Patients with Pemphigus but Not in Patients with Pemphigoid. Br. J. Dermatol. 2017, 176, 1204–1212. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, I.; Takeda, Y.; He, P.; Minami, S.; Iwasaki, T.; Kida, H.; Goya, S.; Kijima, T.; Yoshida, M.; et al. Tetraspanin CD9 Negatively Regulates Lipopolysaccharide-Induced Macrophage Activation and Lung Inflammation. J. Immunol. 2009, 182, 6485–6493. [Google Scholar] [CrossRef] [Green Version]
- Moro, K.; Kabata, H.; Tanabe, M.; Koga, S.; Takeno, N.; Mochizuki, M.; Fukunaga, K.; Asano, K.; Betsuyaku, T.; Koyasu, S. Interferon and IL-27 Antagonize the Function of Group 2 Innate Lymphoid Cells and Type 2 Innate Immune Responses. Nat. Immunol. 2016, 17, 76–86. [Google Scholar] [CrossRef]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Rigas, D.; Lewis, G.; Aron, J.L.; Wang, B.; Banie, H.; Sankaranarayanan, I.; Galle-Treger, L.; Maazi, H.; Lo, R.; Freeman, G.J.; et al. Type 2 Innate Lymphoid Cell Suppression by Regulatory T Cells Attenuates Airway Hyperreactivity and Requires Inducible T-Cell Costimulator-Inducible T-Cell Costimulator Ligand Interaction. J. Allergy Clin. Immunol. 2017, 139, 1468–1477.e2. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Terada, T.; Tsujimoto, N.; Morie, Y.; Ishida, T.; Takahashi, H.; Hamaguchi, J.; Tabuchi, Y.; Doi, K.; Noro, K.; et al. Regulatory T and B Cells in Peripheral Blood of Subcutaneous Immunotherapy-Treated Japanese Cedar Pollinosis Patients. Immunotherapy 2019, 11, 473–482. [Google Scholar] [CrossRef]
- Shamji, M.H.; Kappen, J.; Abubakar-Waziri, H.; Zhang, J.; Steveling, E.; Watchman, S.; Kouser, L.; Eifan, A.; Switzer, A.; Varricchi, G.; et al. Nasal Allergen-Neutralizing IgG4 Antibodies Block IgE-Mediated Responses: Novel Biomarker of Subcutaneous Grass Pollen Immunotherapy. J. Allergy Clin. Immunol. 2019, 143, 1067–1076. [Google Scholar] [CrossRef]
- Brosseau, C.; Durand, M.; Colas, L.; Durand, E.; Foureau, A.; Cheminant, M.-A.; Bouchaud, G.; Castan, L.; Klein, M.; Magnan, A.; et al. CD9+ Regulatory B Cells Induce T Cell Apoptosis via IL-10 and Are Reduced in Severe Asthmatic Patients. Front. Immunol. 2018, 9, 3034. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Suo, L.; Hu, S.; Liu, H.; Wang, X.; Xiao, X.; Liu, J.; Zeng, X.; Hong, J.; Guan, L.; et al. Characterization of the Immune Regulatory Property of CD22+ CD9+ B Cells. Immunology 2022, 167, 328–339. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Ni, Y.; Li, C.; Xu, X.; Chang, H.; Xu, Z.; Hou, M.; Ji, M. Helminth-Induced CD9+ B-Cell Subset Alleviates Obesity-Associated Inflammation via IL-10 Production. Int. J. Parasitol. 2022, 52, 111–123. [Google Scholar] [CrossRef]
- Schuler, P.J.; Saze, Z.; Hong, C.-S.; Muller, L.; Gillespie, D.G.; Cheng, D.; Harasymczuk, M.; Mandapathil, M.; Lang, S.; Jackson, E.K.; et al. Human CD4+ CD39+ Regulatory T Cells Produce Adenosine upon Co-Expression of Surface CD73 or Contact with CD73+ Exosomes or CD73+ Cells. Clin. Exp. Immunol. 2014, 177, 531–543. [Google Scholar] [CrossRef]
- Elashiry, M.; Elashiry, M.M.; Elsayed, R.; Rajendran, M.; Auersvald, C.; Zeitoun, R.; Rashid, M.H.; Ara, R.; Meghil, M.M.; Liu, Y.; et al. Dendritic Cell Derived Exosomes Loaded with Immunoregulatory Cargo Reprogram Local Immune Responses and Inhibit Degenerative Bone Disease in Vivo. J. Extracell. Vesicles 2020, 9, 1795362. [Google Scholar] [CrossRef]
- Misawa, T.; Toyoshima, M.; Kitatani, K.; Ishibashi, M.; Hasegawa-Minato, J.; Shigeta, S.; Yaegashi, N. Involvement of Small Extracellular Vesicle-Derived TIE-1 in the Chemoresistance of Ovarian Cancer Cells. Cancer Treat. Res. Commun. 2021, 27, 100364. [Google Scholar] [CrossRef]
- Kang, M.; Choi, J.K.; Jittayasothorn, Y.; Egwuagu, C.E. Interleukin 35-Producing Exosomes Suppress Neuroinflammation and Autoimmune Uveitis. Front. Immunol. 2020, 11, 1051. [Google Scholar] [CrossRef]
- Gonzalez, M.; Doña, I.; Palomares, F.; Campo, P.; Rodriguez, M.J.; Rondon, C.; Gomez, F.; Fernandez, T.D.; Perkins, J.R.; Escribese, M.M.; et al. Dermatophagoides Pteronyssinus Immunotherapy Changes the T-Regulatory Cell Activity. Sci. Rep. 2017, 7, 11949. [Google Scholar] [CrossRef] [Green Version]
- Xian, M.; Feng, M.; Dong, Y.; Wei, N.; Su, Q.; Li, J. Changes in CD4+CD25+FoxP3+ Regulatory T Cells and Serum Cytokines in Sublingual and Subcutaneous Immunotherapy in Allergic Rhinitis with or without Asthma. Int. Arch. Allergy Immunol. 2020, 181, 71–80. [Google Scholar] [CrossRef]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-Containing T-Regulatory-Cell-Derived Exosomes Suppress Pathogenic T Helper 1 Cells. Immunity 2014, 41, 503. [Google Scholar] [CrossRef] [Green Version]
- Aiello, S.; Rocchetta, F.; Longaretti, L.; Faravelli, S.; Todeschini, M.; Cassis, L.; Pezzuto, F.; Tomasoni, S.; Azzollini, N.; Mister, M.; et al. Extracellular Vesicles Derived from T Regulatory Cells Suppress T Cell Proliferation and Prolong Allograft Survival. Sci. Rep. 2017, 7, 11518. [Google Scholar] [CrossRef] [Green Version]
- Tung, S.L.; Boardman, D.A.; Sen, M.; Letizia, M.; Peng, Q.; Cianci, N.; Dioni, L.; Carlin, L.M.; Lechler, R.; Bollati, V.; et al. Regulatory T Cell-Derived Extracellular Vesicles Modify Dendritic Cell Function. Sci. Rep. 2018, 8, 6065. [Google Scholar] [CrossRef] [Green Version]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine Generation Catalyzed by CD39 and CD73 Expressed on Regulatory T Cells Mediates Immune Suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Csóka, B.; Németh, Z.H.; Duerr, C.U.; Fritz, J.H.; Pacher, P.; Haskó, G. Adenosine Receptors Differentially Regulate Type 2 Cytokine Production by IL-33-Activated Bone Marrow Cells, ILC2s, and Macrophages. FASEB J. 2018, 32, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Han, X.; Liu, G.; Zhou, D.; Zhang, L.; He, J.; Xu, H.; Zhou, P.; Yang, Q.; Chen, J.; et al. Adenosine Restrains ILC2-Driven Allergic Airway Inflammation via A2A Receptor. Mucosal Immunol. 2022, 15, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Specjalski, K.; Maciejewska, A.; Romantowski, J.; Pawłowski, R.; Jassem, E.; Niedoszytko, M. MiRNA Profiles Change during Grass Pollen Immunotherapy Irrespective of Clinical Outcome. Immunotherapy 2022, 14, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Jakwerth, C.A.; Chaker, A.M.; Guerth, F.; Oelsner, M.; Pechtold, L.; Zur Bonsen, L.S.; Ullmann, J.T.; Krauss-Etschmann, S.; Erb, A.; Kau, J.; et al. Sputum MicroRNA-Screening Reveals Prostaglandin EP3 Receptor as Selective Target in Allergen-Specific Immunotherapy. Clin. Exp. Allergy 2021, 51, 1577–1591. [Google Scholar] [CrossRef]
- Specjalski, K.; Maciejewska, A.; Pawłowski, R.; Zieliński, M.; Trzonkowski, P.; Pikuła, M.; Jassem, E. Changing MicroRNA Expression during Three-Month Wasp Venom Immunotherapy. Immunol. Investig. 2019, 48, 835–843. [Google Scholar] [CrossRef]
- Karpinski, P.; Kahraman, M.; Ludwig, N.; Skiba, P.; Kosinska, M.; Rosiek-Biegus, M.; Królewicz, E.; Panaszek, B.; Nittner-Marszalska, M.; Blin, N.; et al. Limited Long-Term Impact of Insect Venom Immunotherapy on the Micro-RNA Landscape in Whole Blood. J. Investig. Allergol. Clin. Immunol. 2019, 29, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Ahmad, T.; Sharma, A.; Mabalirajan, U.; Kulshreshtha, A.; Agrawal, A.; Ghosh, B. Let-7 MicroRNA-Mediated Regulation of IL-13 and Allergic Airway Inflammation. J. Allergy Clin. Immunol. 2011, 128, 1077–1085.e1-10. [Google Scholar] [CrossRef]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct Effects of Interleukin-13 on Epithelial Cells Cause Airway Hyperreactivity and Mucus Overproduction in Asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, R.; Zhu, C.; Cheng, J.; Wang, H.; Wu, J. MicroRNA-143 Downregulates Interleukin-13 Receptor Alpha1 in Human Mast Cells. Int. J. Mol. Sci. 2013, 14, 16958–16969. [Google Scholar] [CrossRef]
- Kaur, D.; Hollins, F.; Woodman, L.; Yang, W.; Monk, P.; May, R.; Bradding, P.; Brightling, C.E. Mast Cells Express IL-13R Alpha 1: IL-13 Promotes Human Lung Mast Cell Proliferation and Fc Epsilon RI Expression. Allergy 2006, 61, 1047–1053. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Tantilipikorn, P.; Ruxrungtham, K.; Pradubpongsa, P.; Mitthamsiri, W.; Piedvache, A.; Thantiworasit, P.; Sirivichayakul, S.; Jacquet, A.; Suratannon, N.; et al. IL-10-Producing Innate Lymphoid Cells Increased in Patients with House Dust Mite Allergic Rhinitis Following Immunotherapy. J. Allergy Clin. Immunol. 2021, 147, 1507–1510.e8. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Doi, K.; Tsutsumi, T.; Inaba, M.; Hamaguchi, J.; Terada, T.; Kawata, R.; Kitatani, K.; Nabe, T. Adoptive Transfer of Type 1 Regulatory T Cells Suppressed the Development of Airway Hyperresponsiveness in Ovalbumin-Induced Airway Inflammation Model Mice. J. Pharmacol. Sci. 2019, 141, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Doi, K.; Tsutsumi, T.; Fujii, S.; Kishima, M.; Nishimura, K.; Kuroda, I.; Tanahashi, Y.; Yuasa, R.; Kinjo, T.; et al. Regulation of Allergic Airway Inflammation by Adoptive Transfer of CD4+ T Cells Preferentially Producing IL-10. Eur. J. Pharmacol. 2017, 812, 38–47. [Google Scholar] [CrossRef]
- Matsuda, M.; Inaba, M.; Hamaguchi, J.; Tomita, H.; Omori, M.; Shimora, H.; Sakae, H.; Kitatani, K.; Nabe, T. Local IL-10 Replacement Therapy Was Effective for Steroid-Insensitive Asthma in Mice. Int. Immunopharmacol. 2022, 110, 109037. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, M.; Shimizu, S.; Kitatani, K.; Nabe, T. Extracellular Vesicles Derived from Allergen Immunotherapy-Treated Mice Suppressed IL-5 Production from Group 2 Innate Lymphoid Cells. Pathogens 2022, 11, 1373. https://doi.org/10.3390/pathogens11111373
Matsuda M, Shimizu S, Kitatani K, Nabe T. Extracellular Vesicles Derived from Allergen Immunotherapy-Treated Mice Suppressed IL-5 Production from Group 2 Innate Lymphoid Cells. Pathogens. 2022; 11(11):1373. https://doi.org/10.3390/pathogens11111373
Chicago/Turabian StyleMatsuda, Masaya, Seito Shimizu, Kazuyuki Kitatani, and Takeshi Nabe. 2022. "Extracellular Vesicles Derived from Allergen Immunotherapy-Treated Mice Suppressed IL-5 Production from Group 2 Innate Lymphoid Cells" Pathogens 11, no. 11: 1373. https://doi.org/10.3390/pathogens11111373
APA StyleMatsuda, M., Shimizu, S., Kitatani, K., & Nabe, T. (2022). Extracellular Vesicles Derived from Allergen Immunotherapy-Treated Mice Suppressed IL-5 Production from Group 2 Innate Lymphoid Cells. Pathogens, 11(11), 1373. https://doi.org/10.3390/pathogens11111373





