Characterizing the Proliferation Patterns of Representative Microsporidian Species Enlightens Future Studies of Infection Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Purification of Microsporidian Spores
2.2. Cell Infections with Spores of N. bombycis and E. helllem
2.3. FISH Probes for Labeling the Microsporidia
2.4. FISH Staining
3. Results
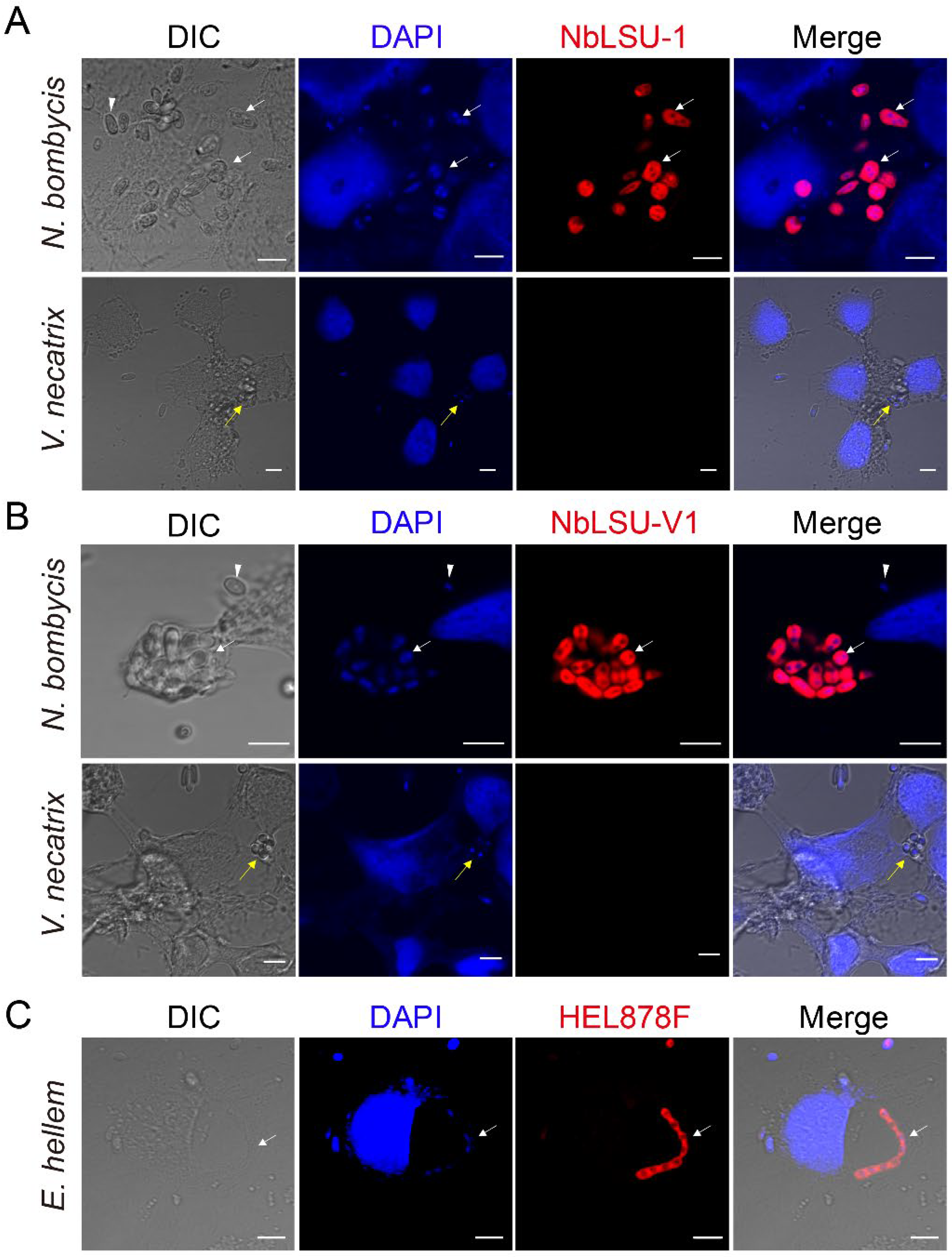
3.1. Pathogen Infections Demonstrated by FISH
3.2. The Proliferation and Life Cycle of N. bombycis in BmE Cells
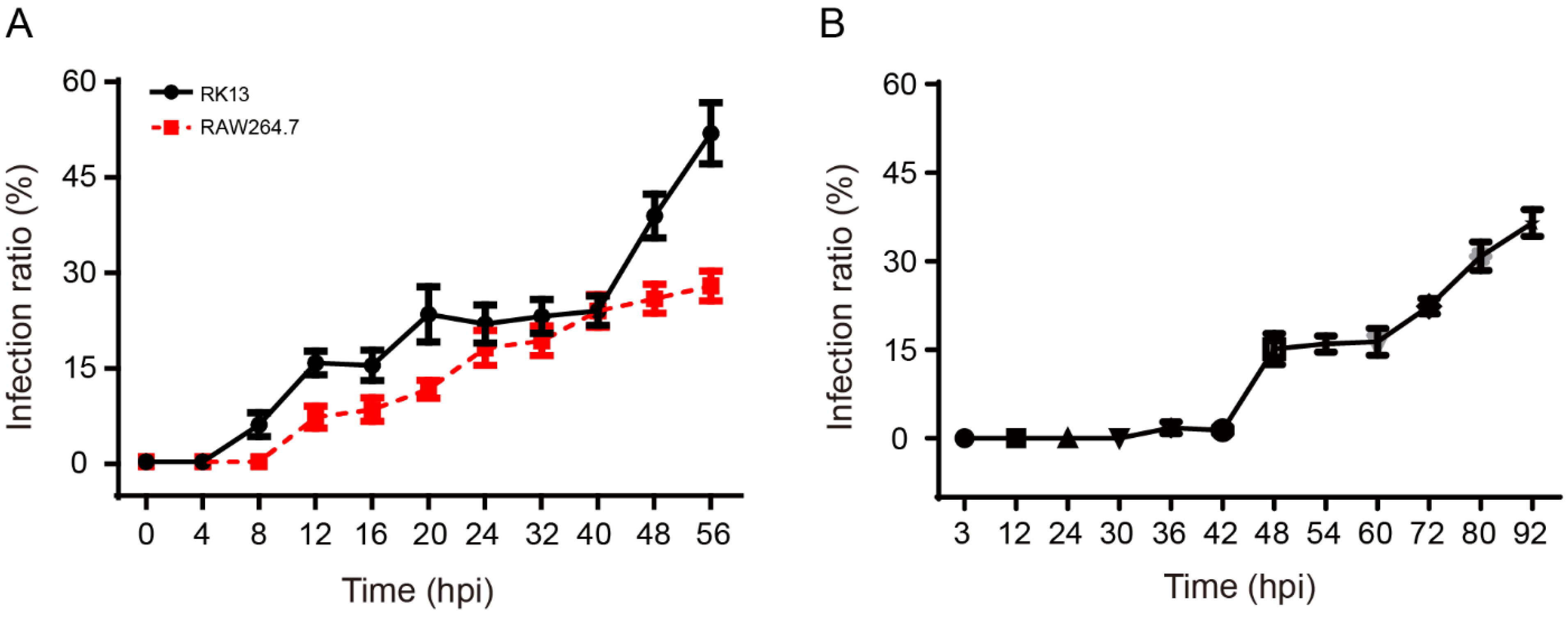
3.3. The Proliferation and Life Cycle of E. hellem in RK13, HEK293, and RAW264.7 Cells
3.4. The Infection Patterns of N. bombycis and E. hellem
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cali, A.; Takvorian, P.M. Developmental morphology and life cycles of the microsporidia. In Microsporidia-Pathogens of Opportunity; Weiss, L.M., Becnel, J.J., Eds.; Wiley: Hoboken, NJ, USA, 2014; Volume 2, pp. 71–133. [Google Scholar]
- Han, M.-S.; Watanabe, H. Transovarial transmission of two microsporidia in the silkworm, Bombyx mori, and disease occurrence in the progeny population. J. Invertebr. Pathol. 1988, 51, 41–45. [Google Scholar] [CrossRef]
- Cai, S.; Lu, X.-m.; Hai-hong, Q.; Ming-qian, L.; Zhen-zhen, F. Phagocytic uptake of Nosema bombycis (Microsporidia) spores by insect cell lines. J. Integr. Agric. 2012, 11, 1321–1326. [Google Scholar] [CrossRef]
- Hinney, B.; Sak, B.; Joachim, A.; Kváč, M. Encephalitozoon more than a rabbit’s tale—Spp. in wild mammals and birds. Int. J. Parasitol. Parasites Wildl. 2016, 5, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.; Longano, A.; Dendle, C.; Polkinghorne, K.; Kanellis, J. Confirmed microsporidial graft infection in a HIV-negative renal transplant recipient: A case report and review of the literature. Transpl. Infect. Dis. 2018, 20, e12888. [Google Scholar] [CrossRef]
- Agashe, R.; Radhakrishnan, N.; Pradhan, S.; Srinivasan, M.; Prajna, V.; Lalitha, P. Clinical and demographic study of microsporidial keratoconjunctivitis in South India: A 3-year study (2013–2015). Br. J. Ophthalmol. 2017, 101, 1436–1439. [Google Scholar] [CrossRef]
- Han, Y.; Gao, H.; Xu, J.; Luo, J.; Han, B.; Bao, J.; Pan, G.; Li, T.; Zhou, Z. Innate and Adaptive Immune Responses Against Microsporidia Infection in Mammals. Front. Microbiol. 2020, 11, 1468. [Google Scholar] [CrossRef]
- Ishihara, R. The life cycle of Nosema bombycis as revealed in tissue culture cells of Bombyx mori. J. Invertebr. Pathol. 1969, 14, 316–320. [Google Scholar] [CrossRef]
- Tatsuo, A.; Kenichi, I.; Yasuhiko, M.; Yohei, H.; Hiroshi, H.; Kazuhisa, S. Niemann-Pick disease type C2 protein induces triglyceride accumulation in silkworm and mammalian cell lines. Biochem. J. 2014, 459, 137–147. [Google Scholar] [CrossRef]
- Weiss, L.M. Microsporidia: Emerging pathogenic protists. Acta Trop. 2001, 78, 89–102. [Google Scholar] [CrossRef]
- Szumowski, S.C.; Troemel, E.R. Microsporidia–host interactions. Curr. Opin. Microbiol. 2015, 26, 10–16. [Google Scholar] [CrossRef]
- Rodríguez-Tovar, L.; Villarreal-Marroquín, A.; Nevárez-Garza, A.; Castillo-Velázquez, U.; Rodríguez-Ramírez, H.; Navarro-Soto, M.; Zárate-Ramos, J.; Hernández-Vidal, G.; Trejo-Chávez, A. Histochemical study of Encephalitozoon cuniculi spores in the kidneys of naturally infected New Zealand rabbits. J. Vet. Diagn. Investig. 2017, 29, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Luallen, R.J.; Reinke, A.W.; Tong, L.; Botts, M.R.; Felix, M.-A.; Troemel, E.R. Discovery of a natural microsporidian pathogen with a broad tissue tropism in Caenorhabditis elegans. PLoS Pathog. 2016, 12, e1005724. [Google Scholar] [CrossRef] [Green Version]
- Sak, B.; Kváč, M.; Květoňová, D.; Albrecht, T.; Piálek, J. The first report on natural Enterocytozoon bieneusi and Encephalitozoon spp. infections in wild East-European House Mice (Mus musculus musculus) and West-European House Mice (M. m. domesticus) in a hybrid zone across the Czech Republic-Germany border. Vet. Parasitol. 2011, 178, 246–250. [Google Scholar] [CrossRef]
- Schwartz, D.; Bryan, R.; Hewan-Lowe, K.; Visvesvara, G.; Weber, R.; Cali, A.; Angritt, P. Disseminated microsporidiosis (Encephalitozoon hellem) and acquired immunodeficiency syndrome. Autopsy evidence for respiratory acquisition. Arch. Pathol. Lab. Med. 1992, 116, 660–668. [Google Scholar]
- van Gool, T.; Snijders, F.; Reiss, P.; Eeftinck Schattenkerk, J.; van den Bergh Weerman, M.; Bartelsman, J.; Bruins, J.; Canning, E.; Dankert, J. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J. Clin. Pathol. 1993, 46, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Moura, H.; Schwartz, D.; Bornay-Llinares, F.; Sodré, F.; Wallace, S.; Visvesvara, G. A new and improved “quick-hot Gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch. Pathol. Lab. Med. 1997, 121, 888–893. [Google Scholar]
- Aurore, D.; Smith, J.E.; Leellen, S.; M Alejandra, P.; Braig, H.R.; Dunn, A.M. Specific detection and localization of microsporidian parasites in invertebrate hosts by using in situ hybridization. Appl. Environ. Microbiol. 2013, 79, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Guo, W.; Dang, X.; Huang, Y.; Liu, F.; Meng, X.; An, Y.; Long, M.; Bao, J.; Zhou, Z. Easy labeling of proliferative phase and sporogonic phase of microsporidia Nosema bombycis in host cells. PLoS ONE 2017, 12, e0179618. [Google Scholar] [CrossRef] [Green Version]
- Graczyk, T.K.; Johansson, M.A.; Leena, T.; Visvesvara, G.S.; Moura, L.S.; Dasilva, A.J.; Girouard, A.S.; Olga, M. Retrospective species identification of microsporidian spores in diarrheic fecal samples from human immunodeficiency virus/AIDS patients by multiplexed fluorescence in situ hybridization. J. Clin. Microbiol. 2007, 45, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Perotti, M.A.; Allen, J.M.; Reed, D.L.; Braig, H.R. Host-symbiont interactions of the primary endosymbiont of human head and body lice. FASEB J. 2007, 21, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Balla, K.M.; Luallen, R.J.; Bakowski, M.A.; Troemel, E.R. Cell-to-cell spread of microsporidia causes Caenorhabditis elegans organs to form syncytia. Nat. Microbiol. 2016, 1, 16144. [Google Scholar] [CrossRef]
- Troemel, E.R.; Felix, M.A.; Whiteman, N.K.; Barriere, A.; Ausubel, F.M. Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol. 2008, 6, e309. [Google Scholar] [CrossRef] [Green Version]
- Cali, A.; Kotler, D.; Orenstein, J. Septata intestinalis N. G., N. Sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J. Eukaryot. Microbiol. 1993, 40, 101–112. [Google Scholar] [CrossRef]
- Schottelius, J.; Schmetz, C.; Kock, N.; Schüler, T.; Sobottka, I.; Fleischer, B. Presentation by scanning electron microscopy of the life cycle of microsporidia of the genus Encephalitozoon. Microbes Infect. 2000, 2, 1401–1406. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, X.; Wang, J.; Huang, J.; Li, H. Observation on the Inoculation and Propagation of the Nosema bombycis in BmN Cell. Acta Sericologica Sin. 2003, 29, 260–263. [Google Scholar]
- Wu, Z.; Li, Y.; Pan, G.; Tan, X.; Hu, J.; Zhou, Z.; Xiang, Z. Proteomic analysis of spore wall proteins and identification of two spore wall proteins from Nosema bombycis (Microsporidia). Proteomics 2010, 8, 2447–2461. [Google Scholar] [CrossRef]
- Luo, J.; He, Q.; Xu, J.; Xu, C.; Han, Y.; Gao, H.; Meng, X.; Pan, G.; Li, T.; Zhou, Z. Microsporidia infection upregulates host energy metabolism but maintains ATP homeostasis. J. Invertebr. Pathol. 2021, 186, 107596. [Google Scholar] [CrossRef]
- Dang, X.; Pan, G.; Li, T.; Lin, L.; Ma, Q.; Geng, L.; He, Y.; Zhou, Z. Characterization of a subtilisin-like protease with apical localization from microsporidian Nosema bombycis. J. Invertebr. Pathol. 2013, 112, 166–174. [Google Scholar] [CrossRef]
- Pan, G.; He, J.; Yang, Y.; Zhao, L.; Liu, J.; Zhou, X.; Dang, X.; Zhou, Z. Histopathological observation of silkworm infected by Nosema bombycis CQ1 isolate. Sci. Seric. 2013, 39, 310–318. [Google Scholar]
- He, Q.; Luo, J.; Xu, J.; Meng, X.; Pan, G.; Li, T.; Zhou, Z. Characterization of Hsp70 gene family provides insight into its functions related to microsporidian proliferation. J. Invertebr. Pathol. 2020, 174, 107394. [Google Scholar] [CrossRef]
- Han, Y.; Gao, H.; Xu, J.; Luo, J.; Chen, J.; Han, B.; Li, T.; Zhou, Z. Microsporidian Encephalitozoon hellem secretes EhPTP4 to regulate host endoplasmic reticulum-associated degradation. Acta Microbiol. Sin. 2022, 61, 357–373. [Google Scholar] [CrossRef]
- Han, B.; Polonais, V.; Sugi, T.; Yakubu, R.; Takvorian, P.; Cali, A.; Maier, K.; Long, M.; Levy, M.; Tanowitz, H.; et al. The role of microsporidian polar tube protein 4 (PTP4) in host cell infection. PLoS Pathog. 2017, 13, e1006341. [Google Scholar] [CrossRef]
- Huang, W.F.; Tsai, S.J.; Lo, C.F.; Soichi, Y.; Wang, C.H. The novel organization and complete sequence of the ribosomal RNA gene of Nosema bombycis. Fungal Genet. Biol. 2004, 41, 473–481. [Google Scholar] [CrossRef]
- Hester, J.; Lindquist, H.; Bobst, A.; Schaefer, F. Fluorescent in situ detection of Encephalitozoon hellem spores with a 6-carboxyfluorescein-labeled ribosomal RNA-targeted oligonucleotide probe. J. Eukaryot. Microbiol. 2000, 47, 299–308. [Google Scholar] [CrossRef]
- Iwano, H.; Ishihara, R. Dimorphism of spores of Nosema spp. in cultured cell. J. Invertebr. Pathol. 1991, 57, 211–219. [Google Scholar] [CrossRef]
- Fuchs, B.; Wallner, G.; Beisker, W.; Schwippl, I.; Ludwig, W.; Amann, R. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 1998, 64, 4973–4982. [Google Scholar] [CrossRef] [Green Version]
- Wasson, K.; Barry, P. Molecular characterization of Encephalitozoon intestinalis (Microspora) replication kinetics in a murine intestinal cell line. J. Eukaryot. Microbiol. 2003, 50, 169–174. [Google Scholar] [CrossRef]
- Takvorian, P.; Weiss, L.; Cali, A. The early events of Brachiola algerae (Microsporidia) infection: Spore germination, sporoplasm structure, and development within host cells. Folia Parasitol. 2005, 52, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Mathews, A.; Hotard, A.; Hale-Donze, H. Innate immune responses to Encephalitozoon species infections. Microbes Infect. 2009, 11, 905–911. [Google Scholar] [CrossRef]
- Texier, C.; Vidau, C.; Viguès, B.; Alaoui, H.E.; Delbac, F. Microsporidia: A model for minimal parasite-host interactions. Curr. Opin. Microbiol. 2010, 13, 443–449. [Google Scholar] [CrossRef]
- Dvorak, J.; Howe, C. Toxoplasma gondii-vertebrate cell interactions. II. The intracellular reproductive phase. J. Protozool. 1979, 26, 114–117. [Google Scholar] [CrossRef]
- Lindsay, D.; Toivio-Kinnucan, M.; Blagburn, B. Ultrastructural determination of cystogenesis by various Toxoplasma gondii isolates in cell culture. J. Parasitol. 1993, 79, 289–292. [Google Scholar] [CrossRef]
- Colley, F.C.; Joe, L.K.; Zaman, V.; Canning, E.U. Light and electron microscopical study of Nosema eurytremae. J. Invertebr. Pathol. 1975, 26, 11–20. [Google Scholar] [CrossRef]
- Hossain, Z.; Gupta, S.K.; Chakrabarty, S.; Saha, A.K.; Bindroo, B.B. Studies on the life cycle of five microsporidian isolates and histopathology of the mid-gut of the silkworm Bombyx mori (Lepidoptera: Bombycidae). Int. J. Trop. Insect Sci. 2012, 32, 203–209. [Google Scholar] [CrossRef]
- Cali, A. Morphogenesis in the genus Nosema. In Proceedings of the IVth International Colloquium in Insect Pathology, College Park, MD, USA, 25–28 August 1970; pp. 431–438. [Google Scholar]
- Gong, C.; Hayasaka, S. Propagation of Nosema bombycis in Antheraea eucalypti cell line. Acta Sericologica Sin. 1999, 25, 92–96. [Google Scholar]
- Kawarabata, T.; Ishihara, R. Infection and development of Nosema bombycis (Microsporida: Protozoa) in a cell line of Antheraea eucalypti. J. Invertebr. Pathol. 1984, 44, 52–62. [Google Scholar] [CrossRef]



| Probe Name | Probe Sequence (5′-3′) |
|---|---|
| NbLSU-1 | GAACATTAGGTTTCTATCCT |
| NbLSU-2 | TTGTATCTTAGGACAACTGTG |
| NbLSU-V1 | GCAATCGTACTCTACATTG |
| NbLSU-V3-D3 | TACTGTCATCTGGTAATCT |
| NbLSU-V4-D13 | CGCCCACTTGAGTATCGT |
| NbLSU-V4-E14 | TAACAACTATCACATCATAT |
| NbLSU-I3 | TCATTCTTACAGTCCCACTC |
| NbSSU | CCTGGTAAATTACCCCGCG |
| NbITS | TTACCCCGCGTTGAGTCAAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Gao, H.; Xu, J.; Xu, C.; Li, T.; Zhou, Z. Characterizing the Proliferation Patterns of Representative Microsporidian Species Enlightens Future Studies of Infection Mechanisms. Pathogens 2022, 11, 1352. https://doi.org/10.3390/pathogens11111352
Luo J, Gao H, Xu J, Xu C, Li T, Zhou Z. Characterizing the Proliferation Patterns of Representative Microsporidian Species Enlightens Future Studies of Infection Mechanisms. Pathogens. 2022; 11(11):1352. https://doi.org/10.3390/pathogens11111352
Chicago/Turabian StyleLuo, Jian, Hailong Gao, Jinzhi Xu, Chen Xu, Tian Li, and Zeyang Zhou. 2022. "Characterizing the Proliferation Patterns of Representative Microsporidian Species Enlightens Future Studies of Infection Mechanisms" Pathogens 11, no. 11: 1352. https://doi.org/10.3390/pathogens11111352
APA StyleLuo, J., Gao, H., Xu, J., Xu, C., Li, T., & Zhou, Z. (2022). Characterizing the Proliferation Patterns of Representative Microsporidian Species Enlightens Future Studies of Infection Mechanisms. Pathogens, 11(11), 1352. https://doi.org/10.3390/pathogens11111352






