Long-Term Pulmonary Dysfunction by Hyperoxia Exposure during Severe Viral Lower Respiratory Tract Infection in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Virus Strain
2.2. Animal Protocols
2.3. Measurements Viral Load
2.4. Pulmonary Function
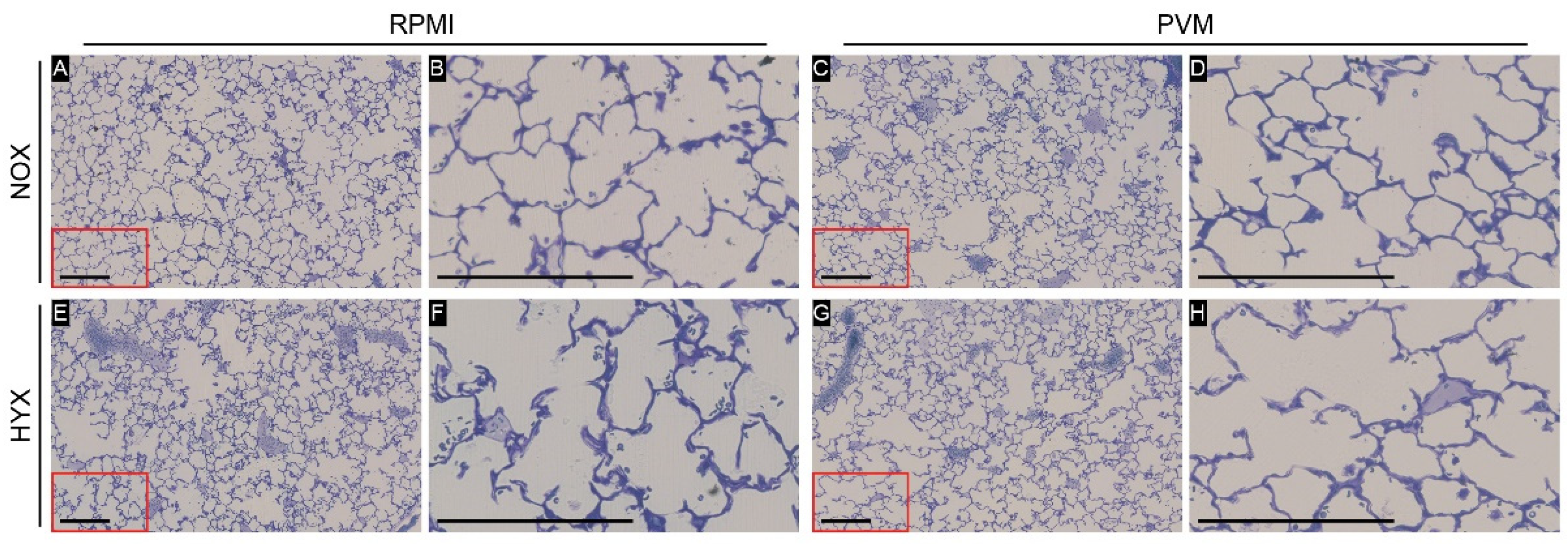
2.5. Alveolar Structural Analysis and Quantification
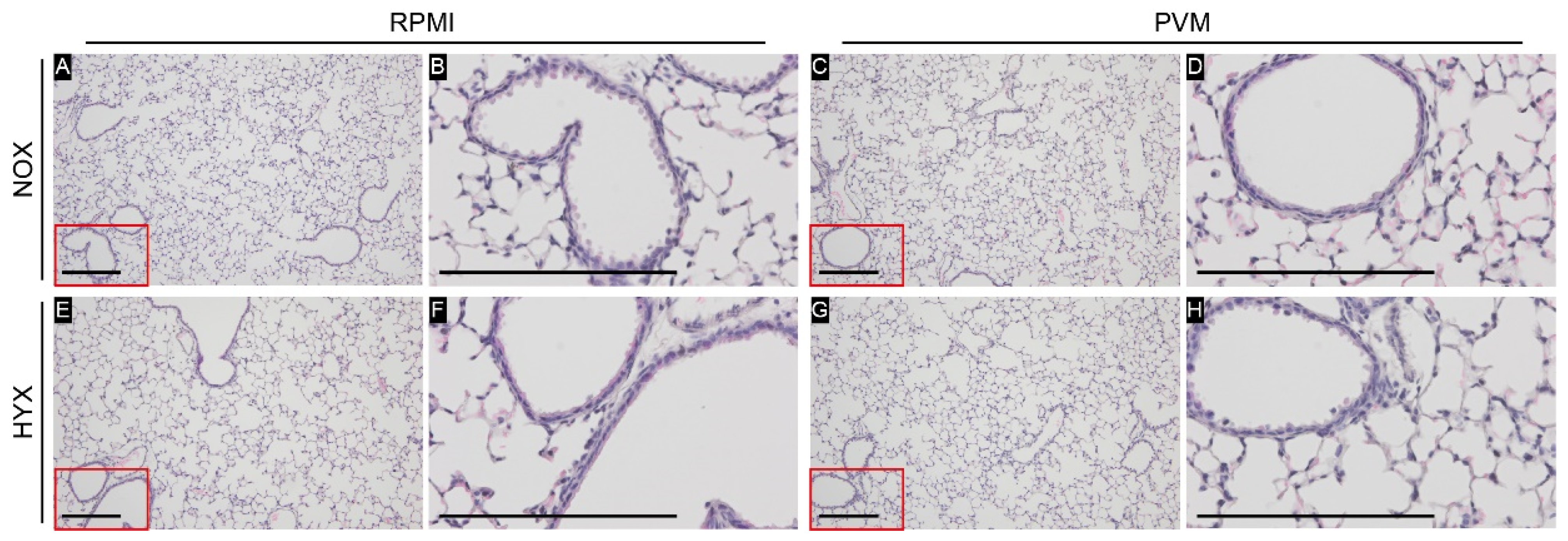
2.6. Lung Histology
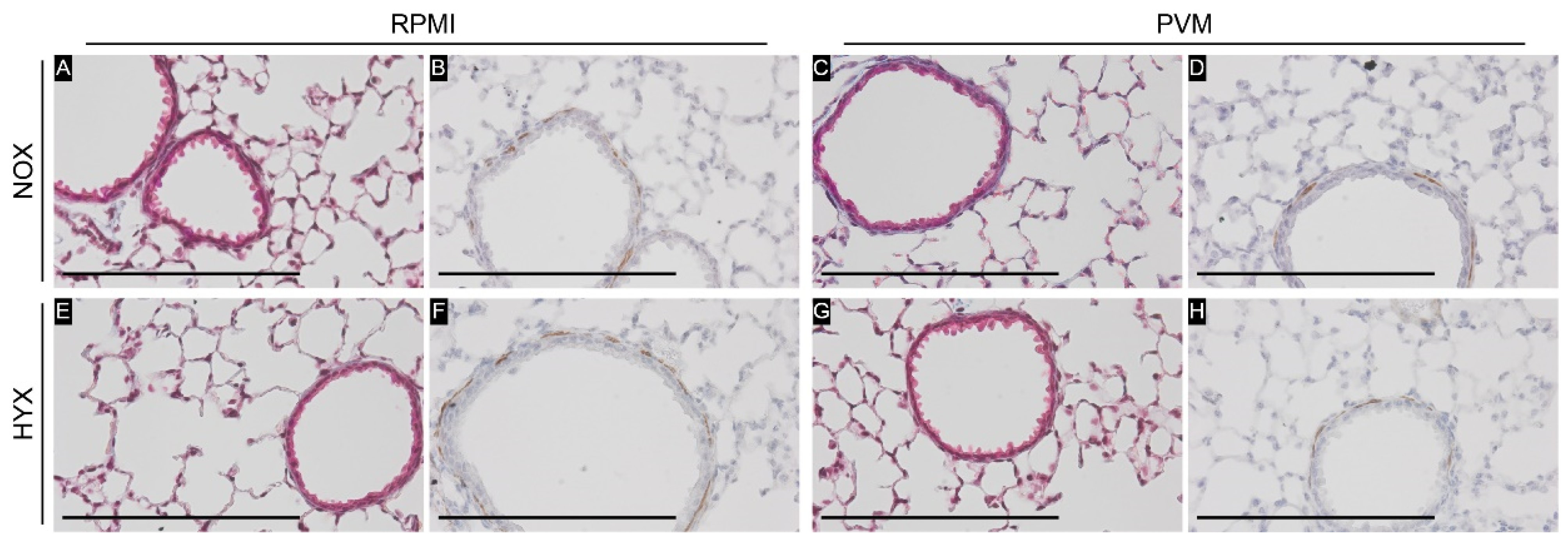
2.7. Immunohistochemistry
2.8. Statistical Analysis
3. Results
3.1. Establishing the Model
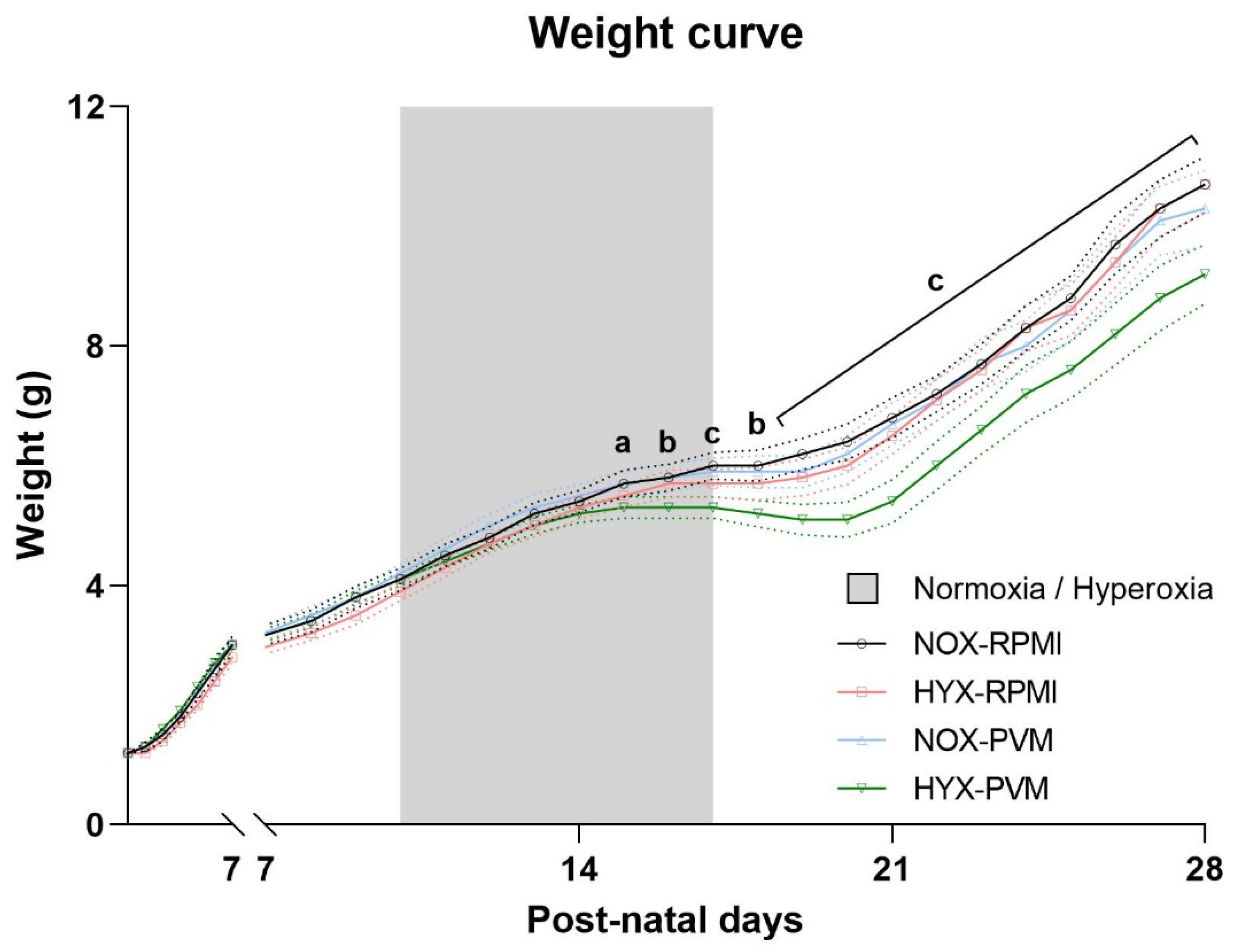
3.2. Exposure to Hyperoxia during PVM Infection Leads to Growth Restriction
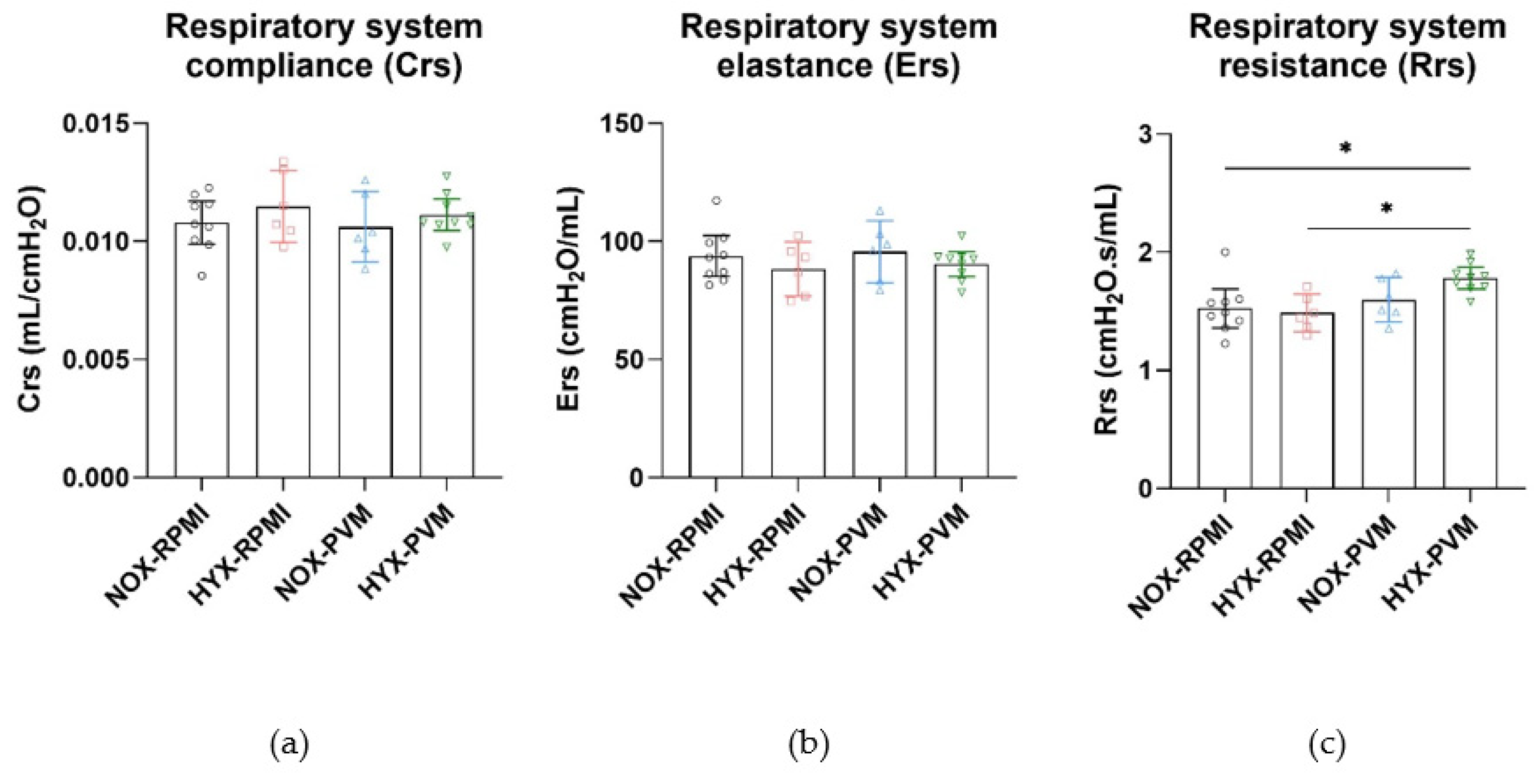
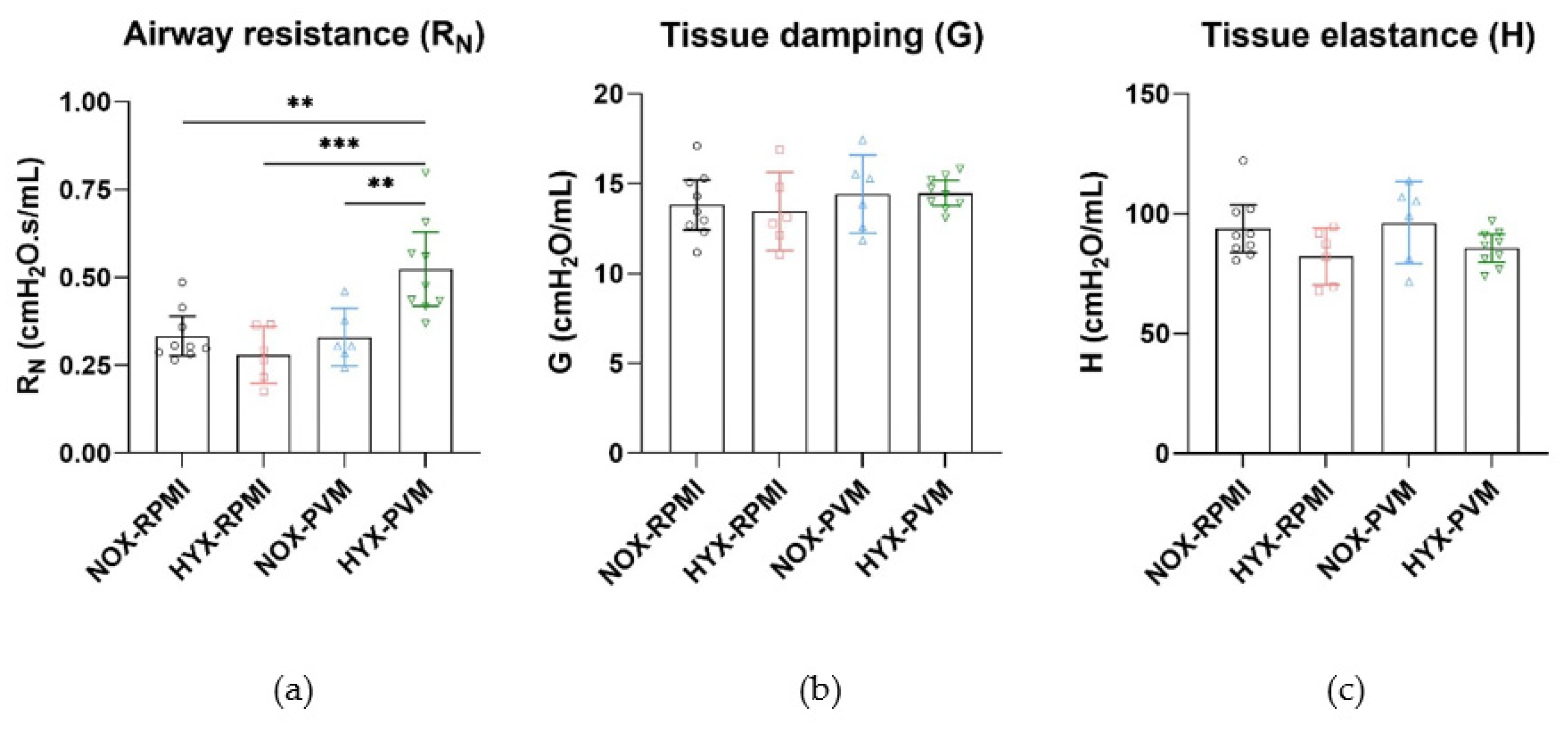
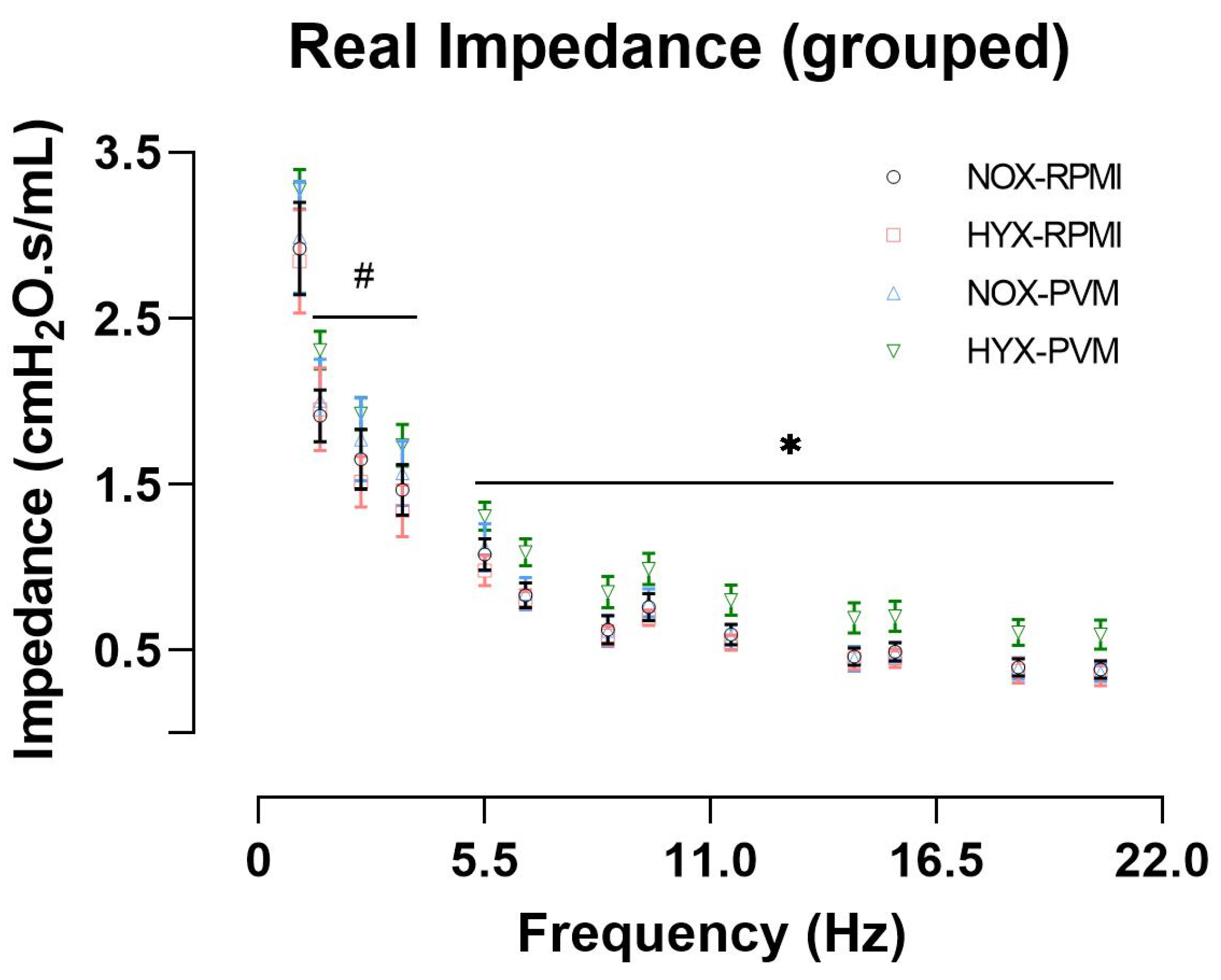
3.3. Hyperoxia Exposure during PVM Infection Alters Long-Term Pulmonary Function
3.4. Alveolar Development Is Not Impaired by Hyperoxia Exposure during PVM Infection
3.5. Hyperoxia Exposure during PVM Infection Does Not Lead to Airway Remodeling
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simoes, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Agusti, A.; Faner, R. Lung function trajectories in health and disease. Lancet Respir. Med. 2019, 7, 358–364. [Google Scholar] [CrossRef]
- Fauroux, B.; Simoes, E.A.F.; Checchia, P.A.; Paes, B.; Figueras-Aloy, J.; Manzoni, P.; Bont, L.; Carbonell-Estrany, X. The Burden and Long-term Respiratory Morbidity Associated with Respiratory Syncytial Virus Infection in Early Childhood. Infect. Dis. Ther. 2017, 6, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Brunwasser, S.M.; Snyder, B.M.; Driscoll, A.J.; Fell, D.B.; Savitz, D.A.; Feikin, D.R.; Skidmore, B.; Bhat, N.; Bont, L.J.; Dupont, W.D.; et al. Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: A systematic review and meta-analysis. Lancet Respir. Med. 2020, 8, 795–806. [Google Scholar] [CrossRef]
- de Sonnaville, E.S.V.; Knoester, H.; Terheggen-Lagro, S.W.J.; Knigs, M.; Oosterlaan, J.; van Woensel, J.B.M. Long-Term Pulmonary Outcomes in Children Mechanically Ventilated for Severe Bronchiolitis. Pediatr. Crit. Care Med. 2022, 23, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Lilien, T.A.; de Sonnaville, E.S.V.; van Woensel, J.B.M.; Bem, R.A. The Local and Systemic Exposure to Oxygen in Children With Severe Bronchiolitis on Invasive Mechanical Ventilation: A Retrospective Cohort Study. Pediatr. Crit. Care Med. 2022. Accepted. [Google Scholar]
- Kallet, R.H.; Matthay, M.A. Hyperoxic acute lung injury. Respir. Care 2013, 58, 123–141. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.F.; Schultz, M.J.; van der Voort, P.H.J.; de Jonge, E.; van Westerloo, D.J. Bench-to-bedside review: The effects of hyperoxia during critical illness. Crit. Care 2015, 19, 284. [Google Scholar] [CrossRef]
- Lilien, T.A.; Groeneveld, N.S.; van Etten-Jamaludin, F.; Peters, M.J.; Buysse, C.M.P.; Ralston, S.L.; van Woensel, J.B.M.; Bos, L.D.J.; Bem, R.A. Association of Arterial Hyperoxia With Outcomes in Critically Ill Children: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2142105. [Google Scholar] [CrossRef]
- Gilfillan, M.; Bhandari, A.; Bhandari, V. Diagnosis and management of bronchopulmonary dysplasia. BMJ 2021, 375, n1974. [Google Scholar] [CrossRef]
- Bem, R.A.; Domachowske, J.B.; Rosenberg, H.F. Animal models of human respiratory syncytial virus disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L148–L156. [Google Scholar] [CrossRef] [PubMed]
- Bem, R.A.; van Woensel, J.B.M.; Lutter, R.; Domachowske, J.B.; Medema, J.P.; Rosenberg, H.F.; Bos, A.P. Granzyme A- and B-Cluster Deficiency Delays Acute Lung Injury in Pneumovirus-Infected Mice. J. Immunol. 2010, 184, 931–938. [Google Scholar] [CrossRef]
- Wolterink-Donselaar, I.G.; Meerding, J.M.; Fernandes, C. A method for gender determination in newborn dark pigmented mice. Lab. Anim. 2009, 38, 35–38. [Google Scholar] [CrossRef]
- Easton, A.J.; Chambers, P. Nucleotide sequence of the genes encoding the matrix and small hydrophobic proteins of pneumonia virus of mice. Virus Res. 1997, 48, 27–33. [Google Scholar] [CrossRef]
- Bem, R.A.; van Woensel, J.B.; Bos, A.P.; Koski, A.; Farnand, A.W.; Domachowske, J.B.; Rosenberg, H.F.; Martin, T.R.; Matute-Bello, G. Mechanical ventilation enhances lung inflammation and caspase activity in a model of mouse pneumovirus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L46–L56. [Google Scholar] [CrossRef] [PubMed]
- Hauber, H.P.; Karp, D.; Goldmann, T.; Vollmer, E.; Zabel, P. Effect of low tidal volume ventilation on lung function and inflammation in mice. BMC Pulm. Med. 2010, 10, 21. [Google Scholar] [CrossRef]
- Hsia, C.C.; Hyde, D.M.; Ochs, M.; Weibel, E.R.; Structure, A.E.J.T.F.o.Q.A.o.L. An official research policy statement of the American Thoracic Society/European Respiratory Society: Standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care Med. 2010, 181, 394–418. [Google Scholar] [CrossRef]
- Muhlfeld, C.; Ochs, M. Quantitative microscopy of the lung: A problem-based approach. Part 2: Stereological parameters and study designs in various diseases of the respiratory tract. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L205–L221. [Google Scholar] [CrossRef]
- Ochs, M.; Muhlfeld, C. Quantitative microscopy of the lung: A problem-based approach. Part 1: Basic principles of lung stereology. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L15–L22. [Google Scholar] [CrossRef]
- Pozarska, A.; Rodríguez-Castillo, J.A.; Surate Solaligue, D.E.; Ntokou, A.; Rath, P.; Mižíková, I.; Madurga, A.; Mayer, K.; Vadász, I.; Herold, S.; et al. Stereological monitoring of mouse lung alveolarization from the early postnatal period to adulthood. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L882–L895. [Google Scholar] [CrossRef]
- Michel, R.P.; Cruz-Orive, L.M. Application of the Cavalieri principle and vertical sections method to lung: Estimation of volume and pleural surface area. J. Microsc. 1988, 150, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.C.; Jarett, L.; Finke, E.H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain. Technol. 1960, 35, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.Z.; Sun, D.; Rawlins, E.L. Human lung development: Recent progress and new challenges. Development 2018, 145, dev163485. [Google Scholar] [CrossRef] [PubMed]
- Brust, V.; Schindler, P.M.; Lewejohann, L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front. Zool. 2015, 12 (Suppl. 1), S17. [Google Scholar] [CrossRef] [PubMed]
- Bozanich, E.M.; Gualano, R.C.; Zosky, G.R.; Larcombe, A.N.; Turner, D.J.; Hantos, Z.; Sly, P.D. Acute Influenza A infection induces bronchial hyper-responsiveness in mice. Respir. Physiol. Neurobiol. 2008, 162, 190–196. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Siefker, D.T.; Shrestha, B.; Saravia, J.; Cormier, S.A. Building a better neonatal mouse model to understand infant respiratory syncytial virus disease. Respir. Res. 2015, 16, 91. [Google Scholar] [CrossRef]
- Limkar, A.R.; Percopo, C.M.; Redes, J.L.; Druey, K.M.; Rosenberg, H.F. Persistent Airway Hyperresponsiveness Following Recovery from Infection with Pneumonia Virus of Mice. Viruses 2021, 13, 728. [Google Scholar] [CrossRef]
- Li, L.F.; Liao, S.K.; Ko, Y.S.; Lee, C.H.; Quinn, D.A. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: A prospective, controlled animal experiment. Crit. Care 2007, 11, R25. [Google Scholar] [CrossRef]
- Sinclair, S.E.; Altemeier, W.A.; Matute-Bello, G.; Chi, E.Y. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit. Care Med. 2004, 32, 2496–2501. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.F.; Schouten, L.R.A.; Wagenaar, G.T.M.; Juffermans, N.P.; Roelofs, J.J.T.H.; Schultz, M.J.; de Jonge, E.; van Westerloo, D.J. Hyperoxia provokes a time- and dose-dependent inflammatory response in mechanically ventilated mice, irrespective of tidal volumes. Intensive Care Med. Exp. 2017, 5, 27. [Google Scholar] [CrossRef]
- Cui, T.X.; Maheshwer, B.; Hong, J.Y.; Goldsmith, A.M.; Bentley, J.K.; Popova, A.P. Hyperoxic Exposure of Immature Mice Increases the Inflammatory Response to Subsequent Rhinovirus Infection: Association with Danger Signals. J. Immunol. 2016, 196, 4692–4705. [Google Scholar] [CrossRef]
- Black, K.R.; Suki, B.; Madwed, J.B.; Jackson, A.C. Airway resistance and tissue elastance from input or transfer impedance in bronchoconstricted monkeys. J. Appl. Physiol. 2001, 90, 571–578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaczka, D.W.; Ingenito, E.P.; Israel, E.; Lutchen, K.R. Airway and lung tissue mechanics in asthma. Effects of albuterol. Am. J. Respir. Crit Care Med. 1999, 159, 169–178. [Google Scholar] [CrossRef] [PubMed]
- LaPrad, A.S.; Lutchen, K.R. Respiratory impedance measurements for assessment of lung mechanics: Focus on asthma. Respir. Physiol. Neurobiol. 2008, 163, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lutchen, K.R.; Jensen, A.; Atileh, H.; Kaczka, D.W.; Israel, E.; Suki, B.; Ingenito, E.P. Airway constriction pattern is a central component of asthma severity: The role of deep inspirations. Am. J. Respir. Crit. Care Med. 2001, 164, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef]
- Karayama, M.; Inui, N.; Mori, K.; Kono, M.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Hashimoto, D.; Enomoto, N.; Fujisawa, T.; et al. Respiratory impedance is correlated with airway narrowing in asthma using three-dimensional computed tomography. Clin. Exp. Allergy 2018, 48, 278–287. [Google Scholar] [CrossRef]
- Anh, D.B.; Faisca, P.; Desmecht, D.J. Differential resistance/susceptibility patterns to pneumovirus infection among inbred mouse strains. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L426–L435. [Google Scholar] [CrossRef]
- Whitehead, G.S.; Burch, L.H.; Berman, K.G.; Piantadosi, C.A.; Schwartz, D.A. Genetic basis of murine responses to hyperoxia-induced lung injury. Immunogenetics 2006, 58, 793–804. [Google Scholar] [CrossRef]
| NOX-RPMI | HYX-RPMI | NOX-PVM | HYX-PVM | ANOVA p Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||||||||||
| V (lung) [cm3] | 0.376 ± 0.036 | 0.368 ± 0.023 | 0.337 ± 0.031 | 0.349 ± 0.015 | 0.1432 | ||||||||||
| CE | CV | CE2/CV2 | 0.043 | 0.096 | 0.200 | 0.028 | 0.062 | 0.200 | 0.041 | 0.091 | 0.200 | 0.019 | 0.043 | 0.200 | |
| Vv (par/lung) [%] | 87.310 ± 1.301 | 86.930 ± 1.159 | 86.870 ± 0.871 | 87.870 ± 0.861 | 0.4457 | ||||||||||
| CE | CV | CE2/CV2 | 0.007 | 0.015 | 0.200 | 0.006 | 0.013 | 0.200 | 0.004 | 0.010 | 0.200 | 0.004 | 0.010 | 0.200 | |
| N (alv, lung) ×106 | 5.830 ± 0.764 | 5.433 ± 0.333 | 5.272 ± 0.306 | 5.234 ± 0.263 | 0.2011 | ||||||||||
| CE | CV | CE2/CV2 | 0.059 | 0.131 | 0.200 | 0.027 | 0.061 | 0.200 | 0.026 | 0.058 | 0.200 | 0.022 | 0.050 | 0.200 | |
| Nv (alv/par) ×106 [cm−3] | 17.730 ± 0.681 | 16.970 ± 0.391 (p = 0.0168 vs. NOX-PVM) | 18.030 ± 0.561 | 17.090 ± 0.169 (p = 0.0361 vs. NOX-PVM) | 0.0095 * | ||||||||||
| CE | CV | CE2/CV2 | 0.017 | 0.038 | 0.200 | 0.010 | 0.023 | 0.200 | 0.014 | 0.031 | 0.200 | 0.004 | 0.010 | 0.200 | |
| S (alv epi, lung) [cm2] | 269.900 ± 29.430 | 264.000 ± 13.540 | 239.400 ± 18.770 | 250.900 ± 10.710 | 0.1010 | ||||||||||
| CE | CV | CE2/CV2 | 0.049 | 0.109 | 0.200 | 0.023 | 0.051 | 0.200 | 0.035 | 0.078 | 0.200 | 0.019 | 0.043 | 0.200 | |
| Sv (alv epi/par) [cm−1] | 822.100 ± 12.730 | 824.900 ± 14.710 | 817.600 ± 17.050 | 819.400 ± 13.410 | 0.8672 | ||||||||||
| CE | CV | CE2/CV2 | 0.007 | 0.015 | 0.200 | 0.008 | 0.018 | 0.200 | 0.009 | 0.021 | 0.200 | 0.007 | 0.016 | 0.200 | |
| τ (sep) [µm] | 6.948 ± 0.437 | 7.442 ± 0.312 | 7.403 ± 0.320 | 7.363 ± 0.105 | 0.0859 | ||||||||||
| CE | CV | CE2/CV2 | 0.028 | 0.063 | 0.200 | 0.019 | 0.042 | 0.200 | 0.019 | 0.043 | 0.200 | 0.006 | 0.014 | 0.200 | |
| MLI [µm] | 34.770 ± 0.998 | 33.620 ± 1.226 | 34.130 ± 1.438 | 34.100 ± 0.737 | 0.4764 | ||||||||||
| CE | CV | CE2/CV2 | 0.013 | 0.029 | 0.200 | 0.016 | 0.036 | 0.200 | 0.019 | 0.042 | 0.200 | 0.010 | 0.022 | 0.200 | |
| NOX-RPMI | HYX-RPMI | NOX-PVM | HYX-PVM | ANOVA p Value | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Structural airway properties: | |||||
| Epithelial thickness (µm) | 8.160 ± 0.688 | 8.000 ± 0.860 | 8.980 ± 1.375 | 8.340 ± 0.737 | 0.4130 |
| Normalized to base perimeter (µm.µm−1) | 0.019 ± 0.002 | 0.019 ± 0.002 | 0.020 ± 0.003 | 0.018 ± 0.003 | 0.7297 |
| Inner airway / Outer airway (%) | 80.3 ± 1.0 | 80.3 ± 1.7 | 78.8 ± 1.5 | 80.4 ± 2.0 | 0.3745 |
| Area of collagen: | |||||
| Normalized to base perimeter (px.µm−1) | 37 ± 21 | 81 ± 38 | 59 ± 33 | 56 ± 24 | 0.1943 |
| Normalized to airway area (%) | 1.0 ± 0.5 | 1.7 ± 1.0 | 2.4 ± 1.2 | 1.5 ± 0.5 | 0.1408 |
| αSMA positive area: | |||||
| Normalized to base perimeter (px.µm−1) | 62 ± 13 | 54 ± 22 | 86 ± 27 | 93 ± 30 | 0.0557 |
| Normalized to airway area (%) | 1.7 ± 0.5 | 1.4 ± 0.6 | 2.2 ± 0.6 | 2.2 ± 0.7 | 0.1336 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lilien, T.A.; Gunjak, M.; Myti, D.; Casado, F.; van Woensel, J.B.M.; Morty, R.E.; Bem, R.A. Long-Term Pulmonary Dysfunction by Hyperoxia Exposure during Severe Viral Lower Respiratory Tract Infection in Mice. Pathogens 2022, 11, 1334. https://doi.org/10.3390/pathogens11111334
Lilien TA, Gunjak M, Myti D, Casado F, van Woensel JBM, Morty RE, Bem RA. Long-Term Pulmonary Dysfunction by Hyperoxia Exposure during Severe Viral Lower Respiratory Tract Infection in Mice. Pathogens. 2022; 11(11):1334. https://doi.org/10.3390/pathogens11111334
Chicago/Turabian StyleLilien, Thijs A., Miša Gunjak, Despoina Myti, Francisco Casado, Job B. M. van Woensel, Rory E. Morty, and Reinout A. Bem. 2022. "Long-Term Pulmonary Dysfunction by Hyperoxia Exposure during Severe Viral Lower Respiratory Tract Infection in Mice" Pathogens 11, no. 11: 1334. https://doi.org/10.3390/pathogens11111334
APA StyleLilien, T. A., Gunjak, M., Myti, D., Casado, F., van Woensel, J. B. M., Morty, R. E., & Bem, R. A. (2022). Long-Term Pulmonary Dysfunction by Hyperoxia Exposure during Severe Viral Lower Respiratory Tract Infection in Mice. Pathogens, 11(11), 1334. https://doi.org/10.3390/pathogens11111334








