The Presence of Dirofilaria immitis in Domestic Dogs on San Cristobal Island, Galapagos
Abstract
:1. Introduction
2. Materials and Methods
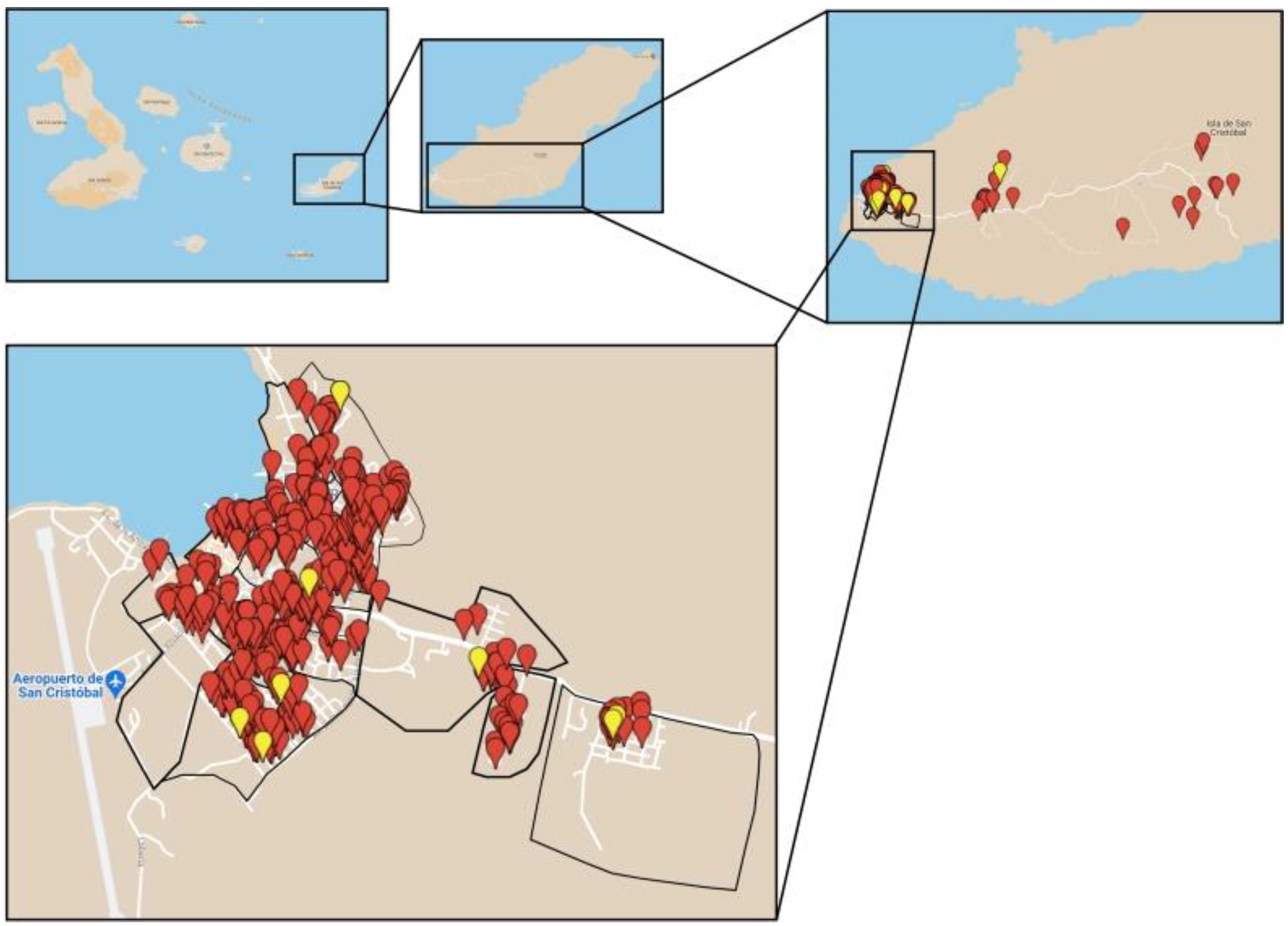
2.1. Sample Collection
2.2. Blood Examination
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Ethics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwin, C. On the Origin of Species; Routledge: London, UK, 1859. [Google Scholar]
- Cittadino, E. Nature as the Laboratory: Darwinian Plant Ecology in the German Empire; Cambridge University Press: Cambridge, UK, 1990; pp. 1880–1900. [Google Scholar]
- Edgar, G.J.; Banks, S.; Bensted-Smith, R.; Calvopiña, M.; Chiriboga, A.; Garske, L.E.; Henderson, S.; Miller, K.A.; Salazar, S. Conservation of threatened species in the Galapagos Marine Reserve through identification and protection of marine key biodiversity areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 955–968. [Google Scholar] [CrossRef]
- Barrow, M. Nature’s Ghosts: Confronting Extinction from the Age of Jefferson to the Age of Ecology; University of Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- Swash, A.; Still, R. Birds, Mammals, and Reptiles of the Galapagos Islands; Bloomsbury Publishing PLC: London, UK, 2005. [Google Scholar]
- Jiménez-Uzcátegui, G.; Milstead, B.; Márquez, C.; Zabala, J.; Buitrón, P.; Llerena, A.; Salazar, S.; Fessl, B. Galapagos vertebrates: Endangered status and conservation actions. Galapagos Rep. 2007, 2006, 104–110. [Google Scholar]
- Harvell, C.D.; Kim, K.; Burkholder, J.M.; Colwell, R.R.; Epstein, P.R.; Grimes, D.J.; Hofmann, E.E.; Lipp, E.K.; Osterhaus, A.D.M.E.; Overstreet, R.M.; et al. Emerging Marine Diseases--Climate Links and Anthropogenic Factors. Science 1999, 285, 1505–1510. [Google Scholar] [CrossRef]
- Alava, J.J.; Salazar, S. Status and Conservation of Otariids in Ecuador and the Galápagos Islands. In Sea Lions of the World; AK-SG-06-01; Alaska Sea Grant College Program: Fairbanks, AK, USA, 2006; pp. 495–519. [Google Scholar]
- Páez-Rosas, D.; Guevara, N. Management strategies and conservation status in populations of Galapagos sea lion (Zalophus wollebaeki). In Tropical Pinnipeds, Bio-Ecology, Threats and Conservation; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Páez-Rosas, D.; Torres, J.; Espinoza, E.; Marchetti, A.; Seim, H.; Riofrío-Lazo, M. Declines and recovery in endangered Galapagos pinnipeds during the El Niño event. Sci. Rep. 2021, 11, 8785. [Google Scholar] [CrossRef] [PubMed]
- Trillmich, F. Zalophus wollebaeki. The IUCN Red List of Threatened Species 2015. e.T41668A45230540. Available online: https://doi.org/10.2305/IUCN.UK.2015-2.RLTS.T41668A45230540.en (accessed on 25 August 2022).
- Denkinger, J.; Gordillo, L.; Montero-Serra, I.; Murillo, J.C.; Guevara, N.; Hirschfeld, M.; Fietz, K.; Rubianes, F.; Dan, M. Urban life of Galapagos sea lions (Zalophus wollebaeki) on San Cristobal Island, Ecuador: Colony trends and threats. J. Sea Res. 2015, 105, 10–14. [Google Scholar] [CrossRef]
- Denkinger, J.; Guevara, N.; Ayala, S.; Murillo, J.C.; Hirschfeld, M.; Montero-Serra, I.; Fietz, K.; Goldstein, T.; Ackermann, M.; Barragán, V.; et al. Pup Mortality and Evidence for Pathogen Exposure in Galapagos Sea Lions (Zalophus wollebaeki) on San Cristobal Island, Galapagos, Ecuador. J. Wildl. Dis. 2017, 53, 491–498. [Google Scholar] [CrossRef]
- Riofrío-Lazo, M.; Arreguín-Sánchez, F.; Páez-Rosas, D. Population Abundance of the Endangered Galapagos Sea Lion Zalophus wollebaeki in the Southeastern Galapagos Archipelago. PLoS ONE 2017, 12, e0168829. [Google Scholar] [CrossRef]
- Benelli, G.; Mehlhorn, H. Mosquito-Borne Diseases; Parasitology Research Monographs; Springer: Cham, Switzerland, 2018; Volume 10. [Google Scholar]
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.B.; Dahl, C.; Kaiser, A. Mosquitoes: Identification, Ecology and Control; Springer Nature: Singapore, 2020. [Google Scholar]
- Sarzosa, M.S.; Duignan, P.; DeRango, E.J.; Field, C.; Ríos, C.; Sanchez, S.; Espinoza, E.; Loyola, A.; Rueda, D.; Páez-Rosas, D. Occurrence of Mycoplasmas in Galapagos Sea Lions (Zalophus wollebaeki) and their Association with Other Respiratory Pathogens. J. Wildl. Dis. 2021, 57, 623–627. [Google Scholar] [CrossRef]
- Walden, H.D.; Grijalva, C.J.; Páez-Rosas, D.; Hernandez, J.A. Intestinal Parasites in Galapagos Sea Lions (Zalophus wollebaeki) Sivertsen, 1953 on San Cristóbal Island, Galapagos, Ecuador. J. Parasitol. 2018, 104, 718–721. [Google Scholar] [CrossRef]
- Burek, K.A.; Gulland, F.M.D.; Sheffield, G.; Beckmen, K.B.; Keyes, E.; Spraker, T.R.; Smith, A.W.; Skilling, D.E.; Evermann, J.F.; Stott, J.L.; et al. Infectious disease and the decline of Steller sea lions (Eumetopias jubatus) in Alaska, USA: Insights from serologic data. J. Wildl. Dis. 2005, 41, 512–524. [Google Scholar] [CrossRef]
- Dubey, J.; Zarnke, R.; Thomas, N.; Wong, S.; Bonn, W.; Briggs, M.; Davis, J.; Ewing, R.; Mense, M.; Kwok, O.; et al. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 2003, 116, 275–296. [Google Scholar] [CrossRef]
- Conrad, P.; Miller, M.; Kreuder, C.; James, E.; Mazet, J.; Dabritz, H.; Jessup, D.; Gulland, F.; Grigg, M. Transmission of Toxoplasma: Clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. Int. J. Parasitol. 2005, 35, 1155–1168. [Google Scholar] [CrossRef]
- Levy, J.; Crawford, P.; Lappin, M.; Dubovi, E.; Levy, M.; Alleman, R.; Tucker, S.; Clifford, E. Infectious Diseases of Dogs and Cats on Isabela Island, Galapagos. J. Vet. Intern. Med. 2008, 22, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, E.; Scorza, A.; Clifford, E.; Olea-Popelka, F.; Lappin, M. Intestinal parasites of dogs on the Galapagos Islands. Vet. Parasitol. 2010, 169, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Rosenberg, D.E.; Yirui, H. Prevalence of vector-borne diseases in a sample of client-owned dogs on Santa Cruz in the Galápagos Islands: A pilot study. Vet. Parasitol. Reg. Stud. Rep. 2016, 6, 28–30. [Google Scholar] [CrossRef]
- Diaz, N.M.; Mendez, G.S.; Grijalva, C.J.; Walden, H.S.; Cruz, M.; Aragon, E.; Hernandez, J.A. Dog overpopulation and burden of exposure to canine distemper virus and other pathogens on Santa Cruz Island, Galapagos. Prev. Vet. Med. 2016, 123, 128–137. [Google Scholar] [CrossRef]
- Jimenez, I.A.; Mariño, P.A.V.; Stapleton, G.S.; Prieto, J.B.; Bowman, D.D. Canine vector-borne disease in domestic dogs on Isla Santa Cruz, Galápagos. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100373. [Google Scholar] [CrossRef]
- Barnett, B.D. Dogs of the Galapagos Islands: Evolution, Ecology, Impact and Control (Feral, Galapagos, Dogs). Ph.D. Thesis, University of California, Davis, CA, USA, 1985. [Google Scholar]
- Hendrix, C.M.; Brunner, C.J.; Bellamy, L.K. Natural transmission of Dirofilaria immitis by Aedes aegypti. J. Am. Mosq. Control Assoc. 1986, 2, 48–51. [Google Scholar] [PubMed]
- Bataille, A.; Cunningham, A.A.; Cedeño, V.; Cruz, M.; Eastwood, G.; Fonseca, D.M.; Causton, C.E.; Azuero, R.; Loayza, J.; Martinez, J.D.C.; et al. Evidence for regular ongoing introductions of mosquito disease vectors into the Galápagos Islands. Proc. R. Soc. B Boil. Sci. 2009, 276, 3769–3775. [Google Scholar] [CrossRef]
- Bataille, A.; Cunningham, A.A.; Cedeño, V.; Patiño, L.; Constantinou, A.; Kramer, L.D.; Goodman, S.J. Natural colonization and adaptation of a mosquito species in Galápagos and its implications for disease threats to endemic wildlife. Proc. Natl. Acad. Sci. USA 2009, 106, 10230–10235. [Google Scholar] [CrossRef]
- Tiawsirisup, S.; Nithiuthai, S. Vector competence of Aedes aegypti (L.) and Culex quinquefasciatus (Say) for Dirofilaria immitis (Leidy). Southeast Asian J. Trop. Med. Public Health 2006, 37, 110. [Google Scholar] [PubMed]
- Vezzani, D.; Eiras, D.F.; Wisnivesky, C. Dirofilariasis in Argentina: Historical review and first report of Dirofilaria immitis in a natural mosquito population. Vet. Parasitol. 2006, 136, 259–273. [Google Scholar] [CrossRef] [PubMed]
- King, J.E. Seals of the World; British Museum of Natural History: London, UK, 1964. [Google Scholar]
- Alho, A.M.; Marcelino, I.; Colella, V.; Flanagan, C.; Silva, N.; Correia, J.J.; Latrofa, M.S.; Otranto, D.; de Carvalho, L.M. Dirofilaria immitis in pinnipeds and a new host record. Parasites Vectors 2017, 10, 142. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, J.H.; Kwon, Y.B.; Park, S.H. A harbor seal infection with Dirofilaria. J. Vet. Clin. 2002, 19, 92–94. [Google Scholar]
- Forrester, D.J.; Jackson, R.F.; Miller, J.F.; Townsend, B.C. Heartworms in captive California sea lions. J. Am. Vet. 1973, 163, 568–570. [Google Scholar]
- White, G.L. Dirofilaria immitis and Heartworm Disease in the California Sea Lion. J. Zoo Anim. Med. 1975, 6, 23–24. [Google Scholar] [CrossRef]
- Sato, T.; Higuchi, T.; Shibuya, H.; Ohba, S.; Nogami, S.; Shirai, W.; Watanabe, H.; Honda, S. Lingual squamous cell carcinoma in a California sea lion (Zalophus californianus). J. Zoo Wildl. Med. 2002, 33, 367–370. [Google Scholar] [CrossRef]
- Farriols, M.; Arellano-Carbajal, F.; Elorriaga-Verplancken, F.R.; Adame-Fernández, K.; Garrido, E.; Álvarez-Martínez, R.C.; Bárcenas, R.T.; Flores-Morán, A.E.; Acevedo-Whitehouse, K. Filarial infections in California sea lions vary spatially within the Gulf of California, Mexico. Parasitol. Res. 2020, 119, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, C.J.; Walden, H.S.; Crawford, P.C.; Levy, J.K.; Pine, W.E.; Hernandez, J.A. Estimating the dog population, responsible pet ownership, and intestinal parasitism in dogs in Quito, Ecuador. Res. Sq. 2021, 1–15. [Google Scholar] [CrossRef]
- Vergara, S.P.P. Galápagos, Hábitats Humanos. Ph.D. Thesis, Pontificia Universidad Católica de Chile, Santiago, Chile, 2020. [Google Scholar]
- Working in Epidemiology. 2006. Available online: http://www.winepi.net/f101.php (accessed on 21 October 2022).
- Patel, J.R.; Devi, S.; Varshney, J.P.; Jadhav, K.M. Epizootiological observations on canine microfilaremia in Gujarat state, India. Vet. World 2018, 11, 1564–1568. [Google Scholar] [CrossRef]
- Saari, S.; Näreaho, A.; Nikander, S. Chapter 5-Nematoda (roundworms). In Canine Parasites and Parasitic Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 83–149. [Google Scholar] [CrossRef]
- Knott, J.; Earle, K. A method for making microfilarial surveys on day blood. Trans. R. Soc. Trop. Med. Hyg. 1939, 33, 191–196. [Google Scholar] [CrossRef]
- Newton, W.L.; Wright, W.H. The Occurrence of a Dog Filariid Other than Dirofilaria immitis in the United States. J. Parasitol. Res. 1956, 42, 246. [Google Scholar] [CrossRef]
- Georgi, J.R.; Georgi, M.E.; Theodorides, V.J. Parasitology for Veterinarians, 5th ed.; W.B. Saunders: Philadelphia, PA, USA, 1990; 412p. [Google Scholar]
- Magnis, J.; Lorentz, S.; Guardone, L.; Grimm, F.; Magi, M.; Naucke, T.J.; Deplazes, P. Morphometric analyses of canine blood microfilariae isolated by the Knott’s test enables Dirofilaria immitis and D. repens species-specific and Acanthocheilonema (syn. Dipetalonema) genus-specific diagnosis. Parasites Vectors 2013, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Di Cesare, A.; Conboy, G. Canine and feline cardiopulmonary parasitic nematodes in Europe: Emerging and underestimated. Parasites Vectors 2010, 3, 62. [Google Scholar] [CrossRef]
- Liotta, J.L.; Sandhu, G.K.; Rishniw, M.; Bowman, D.D. Differentiation of the microfilariae of Dirofilaria immitis and Dirofilaria repens in stained blood films. J. Parasitol. 2013, 99, 421–425. [Google Scholar] [CrossRef]
- Montarsi, F.; Ciocchetta, S.; Devine, G.; Ravagnan, S.; Mutinelli, F.; Di Regalbono, A.F.; Otranto, D.; Capelli, G. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasites Vectors 2015, 8, 177. [Google Scholar] [CrossRef]
- Younes, L.; Barré-Cardi, H.; Bedjaoui, S.; Ayhan, N.; Varloud, M.; Mediannikov, O.; Otranto, D.; Davoust, B. Dirofilaria immitis and Dirofilaria repens in mosquitoes from Corsica Island, France. Parasites Vectors 2021, 14, 427. [Google Scholar] [CrossRef]
- Meriem-Hind, B.M.; Mohamed, M. Prevalence of canine Dirofilaria immitis infection in the city of Algiers, Algeria. Afr. J. Agric. Res. 2009, 4, 1097–1100. [Google Scholar] [CrossRef]
- Ledesma, N.; Harrington, L. Fine-scale temperature fluctuation and modulation of Dirofilaria immitis larval development in Aedes aegypti. Vet. Parasitol. 2015, 209, 93–100. [Google Scholar] [CrossRef]
- Torres, M.L.; Mena, C.F. Understanding Invasive Species in the Galapagos Islands; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Montero-Serra, I.; Páez-Rosas, D.; Murillo, J.; Vegas-Vilarrúbia, T.; Fietz, K.; Denkinger, J. Environment-driven changes in terrestrial habitat use and distribution of the Galapagos sea lion. Endanger. Species Res. 2014, 24, 9–19. [Google Scholar] [CrossRef]
- Šebesta, O.; Gelbič, I.; Peško, J. Daily and seasonal variation in the activity of potential vector mosquitoes. Open Life Sci. 2011, 6, 422–430. [Google Scholar] [CrossRef]
- Little, S.; Braff, J.; Place, J.; Buch, J.; Dewage, B.G.; Knupp, A.; Beall, M. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013–2019. Parasites Vectors 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.; Carretón, E.; Corbera, J.A.; Juste, M.; Mellado, I.; Morchón, R.; Simón, F. Current prevalence of Dirofilaria immitis in dogs, cats and humans from the island of Gran Canaria, Spain. Vet. Parasitol. 2011, 176, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Morales, M.; Juste, M.C.; Banares, A.; Simon, F.; Genchi, C. Seroprevalence of canine heartworm disease (Dirofilaria immitis) in Tenerife island: An epidemiological update. Parasitol. Res. 2006, 100, 103–105. [Google Scholar] [CrossRef]
- Yaman, M.; Guzel, M.; Koltas, I.; Demirkazik, M.; Aktas, H. Prevalence of Dirofilaria immitis in dogs from Hatay province, Turkey. J. Helminthol. 2009, 83, 255–260. [Google Scholar] [CrossRef]
- Bowman, D.D.; Atkins, C.E. Heartworm Biology, Treatment, and Control. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 1127–1158. [Google Scholar] [CrossRef]
- Nguyen, C.; Koh, W.L.; Casteriano, A.; Beijerink, N.; Godfrey, C.; Brown, G.; Emery, D.; Šlapeta, J. Mosquito-borne heartworm Dirofilaria immitis in dogs from Australia. Parasites Vectors 2016, 9, 535. [Google Scholar] [CrossRef]
- Wang, L.-C. Canine filarial infections in north Taiwan. Acta Trop. 1997, 68, 115–120. [Google Scholar] [CrossRef]
- Panarese, R.; Iatta, R.; Mendoza-Roldan, J.A.; Szlosek, D.; Braff, J.; Liu, J.; Beugnet, F.; Dantas-Torres, F.; Beall, M.J.; Otranto, D. Comparison of Diagnostic Tools for the Detection of Dirofilaria immitis Infection in Dogs. Pathogens 2020, 9, 499. [Google Scholar] [CrossRef]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and Animal Dirofilariasis: The Emergence of a Zoonotic Mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef]
- Ash, L.R. Helminth Parasites of Dogs and Cats in Hawaii. J. Parasitol. Res. 1962, 48, 63–65. [Google Scholar] [CrossRef]
- Traversa, D.; Aste, G.; Milillo, P.; Capelli, G.; Pampurini, F.; Tunesi, C.; Santori, D.; Paoletti, B.; Boari, A. Autochthonous foci of canine and feline infections by Dirofilaria immitis and Dirofilaria repens in central Italy. Vet. Parasitol. 2010, 169, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Tasker, S.; Hartmann, K.; Belák, S.; Addie, D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Hofmann-Lehmann, R.; Hosie, M.; et al. Dirofilarioses in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2020, 22, 442–451. [Google Scholar] [CrossRef] [PubMed]

| Pathogen | Isabela | Santa Cruz | San Cristobal | Floreana |
|---|---|---|---|---|
| Canine distemper virus | + | + | − | − |
| Canine parvovirus | + | + | − | − |
| Canine adenovirus | + | + | − | − |
| Canine parainfluenza virus | + | + | − | − |
| Ehrlichia spp. | + | + | − | − |
| Anaplasma spp. | + | + | − | − |
| Bartonella spp. | + | − | − | − |
| Mycoplasma haemocanis | + | − | − | − |
| Giardia spp. | − | + | + | − |
| Leishmania donovani | + | − | − | − |
| Sarcocystis canis | + | + | − | − |
| Cystoisospora spp. | + | + | − | − |
| Cryptosporidium spp. | − | + | − | − |
| Toxocara spp. | + | + | + | − |
| Ancylostoma spp. | + | + | + | − |
| Dirofilaria immitis | + | + | − | + |
| Area | Estimated Human Population 1 | Estimated Number of Dogs 2 | Min. Sample Size | No. of Collected Samples |
|---|---|---|---|---|
| San Cristobal | 7330 | 1222 | 269 | 587 |
| No. of Samples | Positive (%) | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Total | 587 | 10 (1.7%) | 0.7–2.8% | ||
| Sex | |||||
| Males | 311 | 5 (1.6%) | 0.8856 | 0.2–3.0% | 1 |
| Females | 276 | 5 (1.8%) | 0.2–3.4% | ||
| Age | |||||
| <1 year | 74 | 0 | NA | 0% | 0.001 * |
| 1–4 years | 236 | 0 | 0% | ||
| 4–10 years | 208 | 10 (4.8%) | 1.9–7.8% | ||
| >10 years | 43 | 0 | 0% | ||
| unknown | 26 | 0 | 0% | ||
| Environment | |||||
| Urban | 532 | 8 (1.5%) | 0.4046 | 0.5–2.6% | 0.5377 |
| Rural | 55 | 2 (3.6%) | 0–8.6% | ||
| Housing | |||||
| Outdoor | 258 | 8 (3.1%) | NA | 1–5.2% | 0.0571 |
| Indoor | 109 | 0 | 0% | ||
| Outdoor and indoor | 220 | 2 (0.9%) | 0–2.2% | ||
| Neighborhood (Barrio) | No. of Dogs | Positive | Prevalence (%) | 95% CI | p-Value |
|---|---|---|---|---|---|
| Albatros | 30 | 0 | 0 | 0 | 0.2136 |
| Algarrobos | 42 | 0 | 0 | 0 | |
| Cactus | 49 | 1 | 2% | 0–6.0% | |
| Central | 18 | 0 | 0 | 0 | |
| Divino Niño | 24 | 1 | 4.1% | 0–12.1% | |
| Manatial and Isla Sur | 21 | 1 | 4.7% | 0–13.8% | |
| Estacion Terrena | 89 | 3 | 3.3% | 0–7.2% | |
| Fragatas | 48 | 0 | 0 | 0 | |
| Frío | 8 | 0 | 0 | 0 | |
| El Manzanillo | 23 | 0 | 0 | 0 | |
| Las Palmeras | 23 | 2 | 8.7% | 0–20.2% | |
| Peñas Altas | 76 | 0 | 0 | 0 | |
| Peñas Bajas | 35 | 0 | 0 | 0 | |
| Playa Mann | 23 | 0 | 0 | 0 | |
| San Francisco | 23 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culda, C.A.; Dionnet, R.; Barbu, A.C.; Cârstolovean, A.S.; Dan, T.; Grijalva, J.; Espin, P.; Vinueza, R.L.; Cruz, M.; Páez-Rosas, D.; et al. The Presence of Dirofilaria immitis in Domestic Dogs on San Cristobal Island, Galapagos. Pathogens 2022, 11, 1287. https://doi.org/10.3390/pathogens11111287
Culda CA, Dionnet R, Barbu AC, Cârstolovean AS, Dan T, Grijalva J, Espin P, Vinueza RL, Cruz M, Páez-Rosas D, et al. The Presence of Dirofilaria immitis in Domestic Dogs on San Cristobal Island, Galapagos. Pathogens. 2022; 11(11):1287. https://doi.org/10.3390/pathogens11111287
Chicago/Turabian StyleCulda, Carla Andreea, Romane Dionnet, Andra Celia Barbu, Andrada Silvia Cârstolovean, Teodora Dan, Jaime Grijalva, Priscilla Espin, Rommel Lenin Vinueza, Marylin Cruz, Diego Páez-Rosas, and et al. 2022. "The Presence of Dirofilaria immitis in Domestic Dogs on San Cristobal Island, Galapagos" Pathogens 11, no. 11: 1287. https://doi.org/10.3390/pathogens11111287
APA StyleCulda, C. A., Dionnet, R., Barbu, A. C., Cârstolovean, A. S., Dan, T., Grijalva, J., Espin, P., Vinueza, R. L., Cruz, M., Páez-Rosas, D., Renato, L., & Mihalca, A. D. (2022). The Presence of Dirofilaria immitis in Domestic Dogs on San Cristobal Island, Galapagos. Pathogens, 11(11), 1287. https://doi.org/10.3390/pathogens11111287










