Detection of Anti-Neospora caninum IgG in Blood Serum and Colostrum Samples in Naturally Infected Sheep and in Their Newborn Offspring
Abstract
1. Introduction
2. Materials and Methods
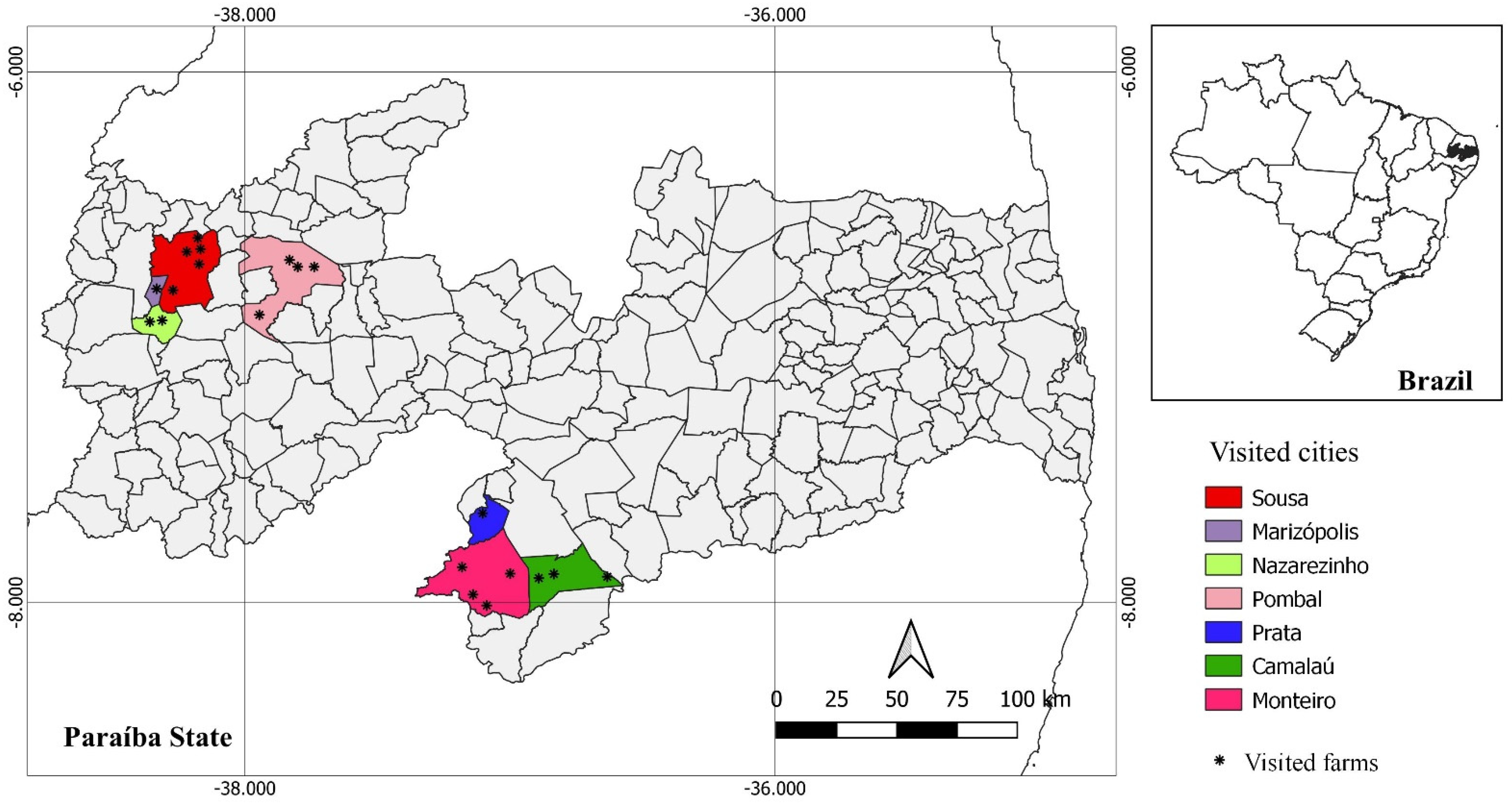
2.1. Experimental Design
2.2. Blood and Colostrum Sampling
2.3. Serological and Colostral Tests
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, K.A.G.; Sousa, R.S.; Varaschin, M.S.; Becker, A.P.B.B.; Monteiro, A.L.G.; Koch, M.O.; Costa, R.C.; Laskoski, R.M.; Galindo, C.M.; Cristo, T.G.; et al. Transplacental transmission of Neospora caninum to lambs in successive pregnancies of naturally infected sheep in Southern Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100537. [Google Scholar] [CrossRef]
- Gutiérrez-Exposito, D.; González-Warleta, M.; Espinosa, J.; Vallejo-García, R.; Castro Hermida, J.A.; Calvo, C.; Ferreras, M.C.; Pérez, V.; Benavides, J.; Mezo, M. Maternal immune response in the placenta of sheep during recrudescence of natural congenital infection of Neospora caninum. Vet. Parasitol. 2020, 285, 109204. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Neosporosis, Toxoplasmosis, and Sarcocystosis in Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 205–222. [Google Scholar] [CrossRef]
- Feitosa, T.F.; Costa, F.T.R.; Bezerra, R.A.; Alvares, F.B.V.; Ferreira, L.C.; Mota, R.A.; Gennari, S.M.; Pena, H.F.J.; Azevedo, S.S.; Vilela, V.L.R. Vertical transmission and kinetic of antibodies anti-Neospora caninum in naturally infected lambs in the semiarid region of Brazil. Rev. Bras. Parasitol. Vet. 2021, 30, e010621. [Google Scholar] [CrossRef]
- Tizard, I.R. Imunologia Veterinária, 10th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2019; 556p. [Google Scholar]
- Arranz-Solís, D.; Benavides, J.; Regidor-Cerrillo, J.; Fuertes, M.; Ferre, I.; Ferreras, M.C.; Collantes-Fernández, E.; Hemphill, A.; Pérez, V.; Ortega-Mora, L. Influence of the gestational stage on the clinical course, lesional development and parasite distribution in experimental ovine neosporosis. Vet. Res. 2015, 46, 1–13. [Google Scholar] [CrossRef]
- Ribeiro, E.L.A.; González-García, E. Indigenous sheep breeds in Brazil: Potential role for contributing to the sustainability of production systems. Trop. Anim. Health Prod. 2016, 48, 1305–1313. [Google Scholar] [CrossRef]
- Amouei, A.; Sharif, M.; Sarvi, S.; Nejad, R.B.; Aghayan, S.A.; Hashemi-Soteh, M.B.; Mizani, A.; Hosseini, S.A.; Gholami, S.; Sadeghi, A.; et al. Aetiology of livestock fetal mortality in Mazandaran province, Iran. PeerJ. 2019, 18, e5920. [Google Scholar] [CrossRef]
- Gonzalez-Warleta, M.; Castro-Hermida, J.A.; Calvo, C.; Perez, V.; Gutierrez-Exposito, D.; Regidor-Cerrillo, J.; Ortega-Mora, L.M.; Mezo, M. Endogenous transplacental transmission of Neospora caninum during successive pregnancies across three generations of naturally infected sheep. Vet. Res. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Howe, L.; West, D.M.; Collett, M.G.; Tattersfield, G.; Pattison, R.S.; Pomroy, W.E.; Kenyon, P.R.; Morris, S.T.; Williamson, N.B. The role of Neospora caninum in three cases of unexplained ewe abortions in the southern North Island of New Zealand. Small Rumin. Res. 2008, 75, 115–122. [Google Scholar] [CrossRef]
- Moreno, B.; Collantes-Fernández, E.; Villa, A.; Navarro, A.; Regidor-Cerrillo, J.; Ortega-Mora, L.M. Occurrence of Neospora caninum and Toxoplasma gondii infections in ovine and caprine abortions. Vet. Parasitol. 2012, 187, 312–318. [Google Scholar] [CrossRef]
- Masala, G.; Porcu, R.; Daga, C.; Denti, S.; Canu, G.; Patta, C.; Tola, S. Detection of pathogens in ovine and caprine abortion samples from Sardinia, Italy, by PCR. J. Vet. Diagn. Investig. 2007, 19, 96–98. [Google Scholar] [CrossRef]
- Figliuolo, L.P.C.; Kasai, N.; Ragozo, A.M.A.; Paula, V.S.O.; Dias, R.A.; Souza, S.L.P.; Gennari, S.M. Prevalence of anti-Toxoplasma gondii and anti-Neospora caninum antibodies in ovine from São Paulo State, Brazil. Vet. Parasitol. 2004, 123, 161–166. [Google Scholar] [CrossRef]
- Camillo, G.; Cezar, A.S.; Antonello, A.M.; Sangioni, L.A.; Flores, E.F.; Pereira, G.R.; Gonçalves, P.B.D.; Vogel, F.S.F. Detecção de anticorpos anti-Neospora caninum em amostras individuais e coletivas de leite de bovinos pela reação de imunofluorescência indireta. Pesq. Vet. Bras. 2011, 31, 482–486. [Google Scholar] [CrossRef]
- Dubey, J.P.; Beattie, C.P. Toxoplasmosis of Animals and Human, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; 313p. [Google Scholar]
- Camargo, M.E. Introdução às técnicas de imunofuorescência. Rev. Brasil. Patol. Clín. 1974, 10, 143–171. [Google Scholar]
- Correia, E.L.B.; Feitosa, T.F.; Santos, F.A.; Azevedo, S.S.; Pena, H.F.J.; Gennari, S.M.; Mota, R.A.; Alves, C.J. Prevalence and risk factors for Toxoplasma gondii in sheep in the state of ParaÍba. Northeastern Brazil. Rev. Bras. Parasitol. Vet. 2015, 24, 383–386. [Google Scholar] [CrossRef][Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Ooi, H.K.; Huang, C.C.; Yang, C.H.; Lee, S.H. Serological survey and first finding of Neospora caninum in Taiwan, and the detection of its antibodies in various body fluids of cattle. Vet. Parasitol. 2000, 90, 47–55. [Google Scholar] [CrossRef]
- Meirelles, A.C.F.; Locatelli-Dittrich, R.; Castilhos, B.; Busch, A.P.B. Concordância na detecção de anticorpos anti-Toxoplasma gondii e anti-Neospora caninum no sangue e no leite bovino pela reação de imunofluorescência indireta. Cienc. Rural. 2014, 44, 2204–2209. [Google Scholar] [CrossRef][Green Version]
- Alves, A.C.; Alves, N.G.; Ascari, I.J.; Junqueira, F.B.; Coutinho, A.S.; Lima, R.R.; Pérez, J.R.; Paula, S.O.; Furusho-Garcia, I.F.; Abreu, L.R. Colostrum composition of Santa Inês sheep and passive transfer of immunity to lambs. Int. J. Dairy Sci. 2015, 98, 3706–3716. [Google Scholar] [CrossRef]
- Tamponi, C.; Varcasia, A.; Pipia, A.P.; Zidda, A.; Panzalis, R.; Dore, F.; Dessì, G.; Sanna, G.; Falis, F.; Bjorkman, C.; et al. ISCOM ELISA in milk as screening for Neospora caninum in dairy sheep. Large Anim. Rev. 2015, 21, 213–216. [Google Scholar]
- Wapenaar, W.; Barkema, H.W.; VanLeeuwen, J.A.; McClure, J.T.; O’Handley, R.M.; Kwok, O.C.H.; Thulliez, P.; Dubey, J.P.; Jenkins, M.C. Comparison of serological methods for the diagnosis of Neospora caninum infection in cattle. Vet. Parasitol. 2007, 143, 166–173. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Funada, M.R.; Soares, R.M.; Gennari, S.M. Perfil sorológico dos anticorpos colostrais para Neospora caninum em bezerros livres da infecção. Braz. J. Vet. Res. Anim. Sci. 2008, 45, 379–384. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Ferre, I.; Re, M.; Regidor-Cerrillo, J.; Blanco-Murcia, J.; Ferrer, L.M.; Navarro, T.; Díaz, M.P.; Gonzáles-Huecas, M.; Tabanera, E.; et al. Influence of dose and route of administration on the outcome of infection with the virulent Neospora caninum isolate Nc-Spain7 in pregnant sheep at mid-gestation. Vet. Res. 2018, 42, 1–15. [Google Scholar] [CrossRef]
- Munhoz, A.D.; Jacintho, A.P.P.; Machado, R.Z. Bovine abortion associated with Neospora caninum: Diagnosis and epidemiological aspects of a dairy cattle herd in the Northeast Region of São Paulo State, Brazil. Braz. J. Vet. Pathol. 2011, 4, 112–116. [Google Scholar]

| ID of Ewes and Lambs | Postpartum Days | Serum Titration of Ewes | Titration of Colostrum | Serum Titration of Lambs |
|---|---|---|---|---|
| 2 | 4 | 200 | 200 | 100 |
| 3 | 3 | 3200 | 1600 | 100 |
| 5 | 3 | 200 | 100 | 50 |
| 6-L1 | 4 | 200 | 100 | 100 |
| 6-L2 | 50 | |||
| 9 | 2 | 200 | - | - |
| 10 | 5 | 50 | - | - |
| 11 | 2 | 200 | - | - |
| 12 | 3 | 50 | - | - |
| 18 | 4 | 50 | - | - |
| 21 | 2 | 50 | - | - |
| 27-L1 | 4 | 200 | 100 | 50 |
| 27-L2 | 50 | |||
| 28 | 2 | 50 | - | - |
| 29 | 2 | 50 | - | - |
| 31 | 3 | 100 | 100 | 100 |
| 34 | 3 | 6400 | 6400 | 100 |
| 35 | 4 | 200 | 200 | 200 |
| 37 | 2 | 50 | - | - |
| 38 | 2 | 100 | 50 | 50 |
| 39 | 4 | 50 | - | - |
| 42 | 4 | 50 | 50 | 50 |
| 61 | 2 | 100 | 50 | 50 |
| 63 | 2 | 200 | - | - |
| 64 | 2 | 400 | 400 | 400 |
| 71 | 3 | 50 | 50 | 50 |
| 81 | 4 | 50 | - | - |
| 82 | 3 | 50 | - | - |
| 83 | 2 | 50 | - | - |
| 101 | 5 | 50 | - | - |
| 103 | 5 | 100 | 100 | 100 |
| 107 | 4 | 50 | - | - |
| 108 | 3 | 50 | - | - |
| 112 | 3 | 50 | 50 | 50 |
| 113 | 3 | 50 | 50 | 50 |
| 115 | 4 | 100 | 50 | 50 |
| 123 | 4 | 50 | - | - |
| 125 | 4 | 50 | - | - |
| 126 | 3 | 50 | - | - |
| 127 | 3 | 50 | - | - |
| 128 | 2 | 50 | - | - |
| 134 | 5 | 50 | - | - |
| 135-L1 | 5 | 100 | 100 | 100 |
| 135-L2 | 100 | |||
| 140 | 5 | 50 | - | - |
| 146 | 5 | 50 | 50 | 50 |
| 147 | 4 | 100 | 50 | 50 |
| 148 | 2 | 50 | 50 | 50 |
| Titer of the Ewes | Positive Ewes/Analyzed Ewes (%) | Positive Colostrum/Positive Ewes (%) | Kappa |
|---|---|---|---|
| 1:50 | 27/162 (16.7%) | 6/27 (22.2%) | 0.323 |
| 1:100 | 7/162 (4.3%) | 7/7 (100%) | 1.000 |
| 1:200 | 8/162 (4.9%) | 5/8 (62.5%) | 0.760 |
| 1:400 | 1/162 (0.6%) | 1/1 (100%) | 1.000 |
| 1:3200 | 1/162 (0.6%) | 1/1 (100%) | 1.000 |
| 1:6400 | 1/162 (0.6%) | 1/1 (100%) | 1.000 |
| Total | 45/162 (27.7%) | 21 | 0.558 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, R.A.; Lima, B.A.; Alvares, F.B.V.; Rossi, G.A.M.; Braga, F.R.; de Melo, R.P.B.; Mota, R.A.; Vilela, V.L.R.; Feitosa, T.F. Detection of Anti-Neospora caninum IgG in Blood Serum and Colostrum Samples in Naturally Infected Sheep and in Their Newborn Offspring. Pathogens 2022, 11, 1263. https://doi.org/10.3390/pathogens11111263
Bezerra RA, Lima BA, Alvares FBV, Rossi GAM, Braga FR, de Melo RPB, Mota RA, Vilela VLR, Feitosa TF. Detection of Anti-Neospora caninum IgG in Blood Serum and Colostrum Samples in Naturally Infected Sheep and in Their Newborn Offspring. Pathogens. 2022; 11(11):1263. https://doi.org/10.3390/pathogens11111263
Chicago/Turabian StyleBezerra, Roberto Alves, Brendo Andrade Lima, Felipe Boniedj Ventura Alvares, Gabriel Augusto Marques Rossi, Fabio Ribeiro Braga, Renata Pimentel Bandeira de Melo, Rinaldo Aparecido Mota, Vinícius Longo Ribeiro Vilela, and Thais Ferreira Feitosa. 2022. "Detection of Anti-Neospora caninum IgG in Blood Serum and Colostrum Samples in Naturally Infected Sheep and in Their Newborn Offspring" Pathogens 11, no. 11: 1263. https://doi.org/10.3390/pathogens11111263
APA StyleBezerra, R. A., Lima, B. A., Alvares, F. B. V., Rossi, G. A. M., Braga, F. R., de Melo, R. P. B., Mota, R. A., Vilela, V. L. R., & Feitosa, T. F. (2022). Detection of Anti-Neospora caninum IgG in Blood Serum and Colostrum Samples in Naturally Infected Sheep and in Their Newborn Offspring. Pathogens, 11(11), 1263. https://doi.org/10.3390/pathogens11111263











