A Serodiagnostic IgM ELISA to Detect Acute Cytauxzoonosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Control Samples
2.2. Experimental Infection Samples
2.3. Clinical Samples (Groups 2 and 3)
2.4. Samples from Chronic C. felis Carriers (Group 4)
2.5. C88 Recombinant Protein
2.6. Microsphere Immunoassay (MIA)
2.7. C. felis IgM/IgG ELISA
2.8. Statistical Analysis
3. Results
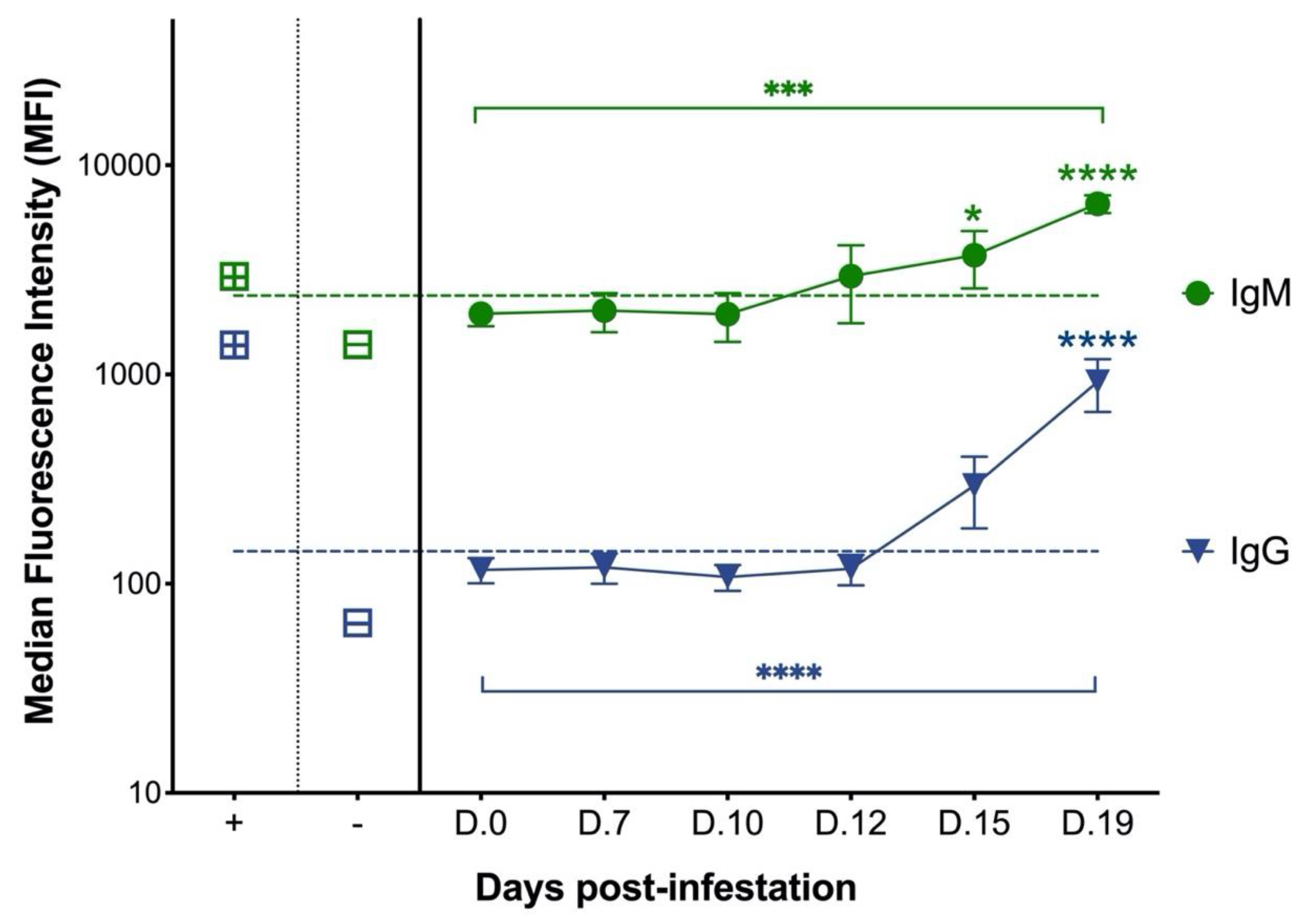
3.1. Cats with Experimental C. felis Infection Generate IgM and IgG Antibodies against C. felis C88 Recombinant Protein
3.2. Cats with Acute Cytauxzoonosis Have Significantly Higher Plasma Anti-C88 IgM and IgG Antibodies Compared to Cats in Other Groups
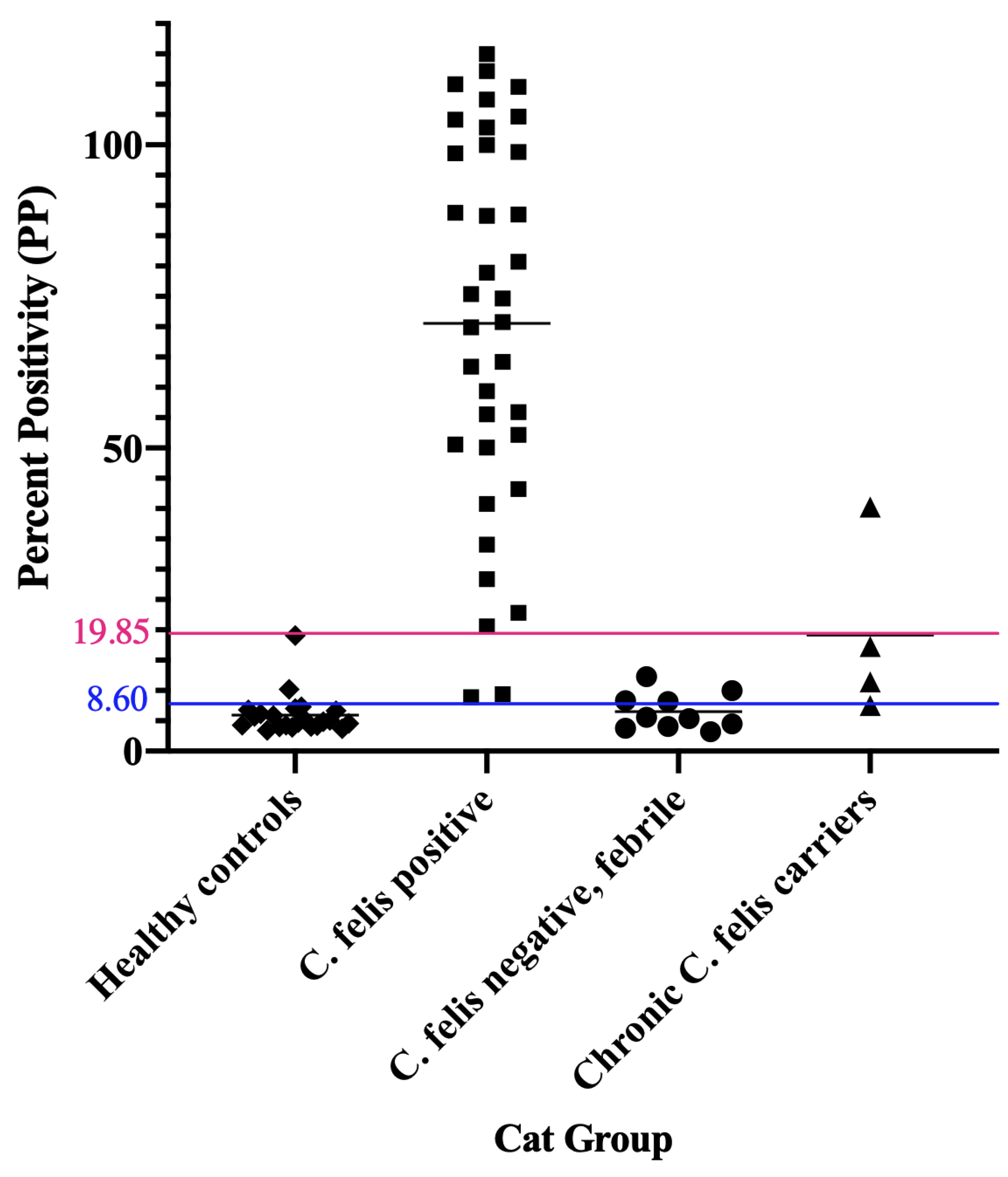
3.3. C. felis IgM ELISA Is Highly Specific and Can Successfully Detect Infection in Cats with Acute Cytauxzoonosis
3.4. Weak Positive IgM ELISA Results Can Be Evaluated in Combination with Other Diagnostics to Reach a Definitive Diagnosis
3.5. C. felis PCR Negative Cats Have Lower IgG than Cats with Acute Cytauxzoonosis
3.6. Asymptomatic Chronic C. felis Carriers Exhibit High IgG but Variable IgM Antibody Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reichard, M.V.; Meinkoth, J.H.; Edwards, A.C.; Snider, T.A.; Kocan, K.M.; Blouin, E.F.; Little, S.E. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet. Parasitol. 2009, 161, 110–115. [Google Scholar] [CrossRef]
- Reichard, M.V.; Edwards, A.C.; Meinkoth, J.H.; Snider, T.A.; Meinkoth, K.R.; Heinz, R.E.; Little, S.E. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J. Med. Entomol. 2010, 47, 890–896. [Google Scholar] [CrossRef]
- Nagamori, Y.; Slovak, J.E.; Reichard, M.V. Prevalence of Cytauxzoon felis infection in healthy free-roaming cats in north-central Oklahoma and central Iowa. JFMS Open Rep. 2016, 2, 2055116916655174. [Google Scholar] [CrossRef]
- Motzel, S.; Wagner, J. Treatment of experimentally induced cytauxzoonosis in cats with parvaquone and buparvaquone. Vet. Parasitol. 1990, 35, 131–138. [Google Scholar] [CrossRef]
- Greene, C.; Latimer, K.; Hopper, E.; Shoeffler, G.; Lower, K.; Cullens, F. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J. Am. Vet. Med. Assoc. 1999, 215, 497–500, 482. [Google Scholar]
- Cohn, L.; Birkenheuer, A.; Brunker, J.; Ratcliff, E.; Craig, A. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J. Vet. Intern. Med. 2011, 25, 55–60. [Google Scholar] [CrossRef]
- Sherrill, M.K.; Cohn, L.A. Cytauxzoonosis: Diagnosis and treatment of an emerging disease. J. Feline Med. Surg. 2015, 17, 940–948. [Google Scholar] [CrossRef]
- Lloret, A.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Horzinek, M.C.; Hosie, M.J.; Lutz, H. Cytauxzoonosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2015, 17, 637–641. [Google Scholar] [CrossRef]
- Reichard, M.V.; Thomas, J.E.; Arther, R.G.; Hostetler, J.A.; Raetzel, K.L.; Meinkoth, J.H.; Little, S.E. Efficacy of an Imidacloprid 10%/Flumethrin 4.5% Collar (Seresto®, Bayer) for Preventing the Transmission of Cytauxzoon felis to Domestic Cats by Amblyomma americanum. Parasitol. Res. 2013, 112, 11–20. [Google Scholar] [CrossRef]
- Tizard, I.R. Veterinary Immunology-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Schreeg, M.E.; Marr, H.S.; Tarigo, J.L.; Sherrill, M.K.; Outi, H.K.; Scholl, E.H.; Bird, D.M.; Vigil, A.; Hung, C.; Nakajima, R.; et al. Identification of Cytauxzoon felis antigens via protein microarray and assessment of expression library immunization against cytauxzoonosis. Clin. Proteom. 2018, 15, 44. [Google Scholar] [CrossRef]
- Kao, Y.F.; Peake, B.; Madden, R.; Cowan, S.R.; Scimeca, R.C.; Thomas, J.E.; Reichard, M.V.; Ramachandran, A.; Miller, C.A. A probe-based droplet digital polymerase chain reaction assay for early detection of feline acute cytauxzoonosis. J. Vet. Parasitol. 2021, 292, 109413. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Emanuelli, M.; Fink, E.; Musselman, E.; Mackie, R.; Troyer, R.; Elder, J.; VandeWoude, S. FIV vaccine with receptor epitopes results in neutralizing antibodies but does not confer resistance to challenge. NPJ Vaccines 2018, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.A.; Carver, S.; Troyer, R.M.; Elder, J.H.; VandeWoude, S. Domestic cat microsphere immunoassays: Detection of antibodies during feline immunodeficiency virus infection. J. Immunol. Methods 2013, 396, 74–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Classen, D.C.; Morningstar, J.M.; Shanley, J.D. Detection of antibody to murine cytomegalovirus by enzyme-linked immunosorbent and indirect immunofluorescence assays. J. Clin. Microbiol. 1987, 25, 600–604. [Google Scholar] [CrossRef]
- Dreitz, M.; Dow, S.; Fiscus, S.; Hoover, E. Development of monoclonal antibodies and capture immunoassays for feline immunodeficiency virus. Am. J. Vet. Res. 1995, 56, 764–768. [Google Scholar]
- Wright, P.F.; Nilsson, E.; Van, R.; Lelenta, M.; Jeggo, M.H. Standardisation and validation of enzyme-linked immunosorbent assay techniques for the detection of antibody in infectious disease diagnosis. Rev. Sci. Tech. 1993, 12, 435–450. [Google Scholar] [CrossRef]
- Hartfield, J.N.; Grooters, A.M.; Waite, K.J. Development and evaluation of an ELISA for the quantitation of anti-Lagenidium giganteum forma caninum antibodies in dogs. J. Vet. Intern. Med. 2014, 28, 1479–1484. [Google Scholar] [CrossRef]
- Reichard, M.V.; Sanders, T.L.; Weerarathne, P.; Meinkoth, J.H.; Miller, C.A.; Scimeca, R.C.; Almazán, C. Cytauxzoonosis in North America. Pathogens 2021, 10, 1170. [Google Scholar] [CrossRef]
- Kier-Schroeder, A.B. The Etiology and Pathogenesis of Feline Cytauxzoonosis; University of Missouri: Columbia, MI, USA, 1979. [Google Scholar]
- Murray, K.O.; Garcia, M.N.; Yan, C.; Gorchakov, R. Persistence of detectable immunoglobulin M antibodies up to 8 years after infection with West Nile virus. Am. J. Trop. Med. Hyg. 2013, 89, 996–1000. [Google Scholar] [CrossRef]
- Chien, Y.W.; Liu, Z.H.; Tseng, F.C.; Ho, T.C.; Guo, H.R.; Ko, N.Y.; Ko, W.C.; Perng, G.C. Prolonged persistence of IgM against dengue virus detected by commonly used commercial assays. BMC Infect. Dis. 2018, 18, 156. [Google Scholar] [CrossRef]
- Patgaonkar, M.; Herbert, F.; Powale, K.; Gandhe, P.; Gogtay, N.; Thatte, U.; Pied, S.; Sharma, S.; Pathak, S. Vivax infection alters peripheral B-cell profile and induces persistent serum IgM. Parasite Immunol. 2018, 40, e12580. [Google Scholar] [CrossRef] [PubMed]




| PP | Specificity/Sensitivity | Interpretation | Comments |
|---|---|---|---|
| >19.85 | 100%/94.44% | Strong Positive | Cytauxzoonosis is confirmed. |
| 8.60–19.85 | 87.88%/100% | Weak Positive | Possible cytauxzoonosis if clinical history is suggestive. Additional diagnostics are recommended (i.e., Blood smear evaluation and/or PCR). Empirical therapy should be initiated while awaiting other diagnostic results. |
| <8.60 | N/A | Negative | Cytauxzoonosis is unlikely. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, Y.-F.; Spainhour, R.; Cowan, S.R.; Nafe, L.; Birkenheuer, A.; Reichard, M.V.; Miller, C.A. A Serodiagnostic IgM ELISA to Detect Acute Cytauxzoonosis. Pathogens 2022, 11, 1183. https://doi.org/10.3390/pathogens11101183
Kao Y-F, Spainhour R, Cowan SR, Nafe L, Birkenheuer A, Reichard MV, Miller CA. A Serodiagnostic IgM ELISA to Detect Acute Cytauxzoonosis. Pathogens. 2022; 11(10):1183. https://doi.org/10.3390/pathogens11101183
Chicago/Turabian StyleKao, Yun-Fan, Rebecca Spainhour, Shannon R. Cowan, Laura Nafe, Adam Birkenheuer, Mason V. Reichard, and Craig A. Miller. 2022. "A Serodiagnostic IgM ELISA to Detect Acute Cytauxzoonosis" Pathogens 11, no. 10: 1183. https://doi.org/10.3390/pathogens11101183
APA StyleKao, Y.-F., Spainhour, R., Cowan, S. R., Nafe, L., Birkenheuer, A., Reichard, M. V., & Miller, C. A. (2022). A Serodiagnostic IgM ELISA to Detect Acute Cytauxzoonosis. Pathogens, 11(10), 1183. https://doi.org/10.3390/pathogens11101183







