Antibody Prevalence and Risk Factors Associated with Rickettsia spp. in a Pediatric Cohort: SFGR Remains Underdiagnosed and Underreported in El Salvador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Recruitment
2.2. Ethics Statement
2.3. Antibody Screening
2.4. Data Analysis
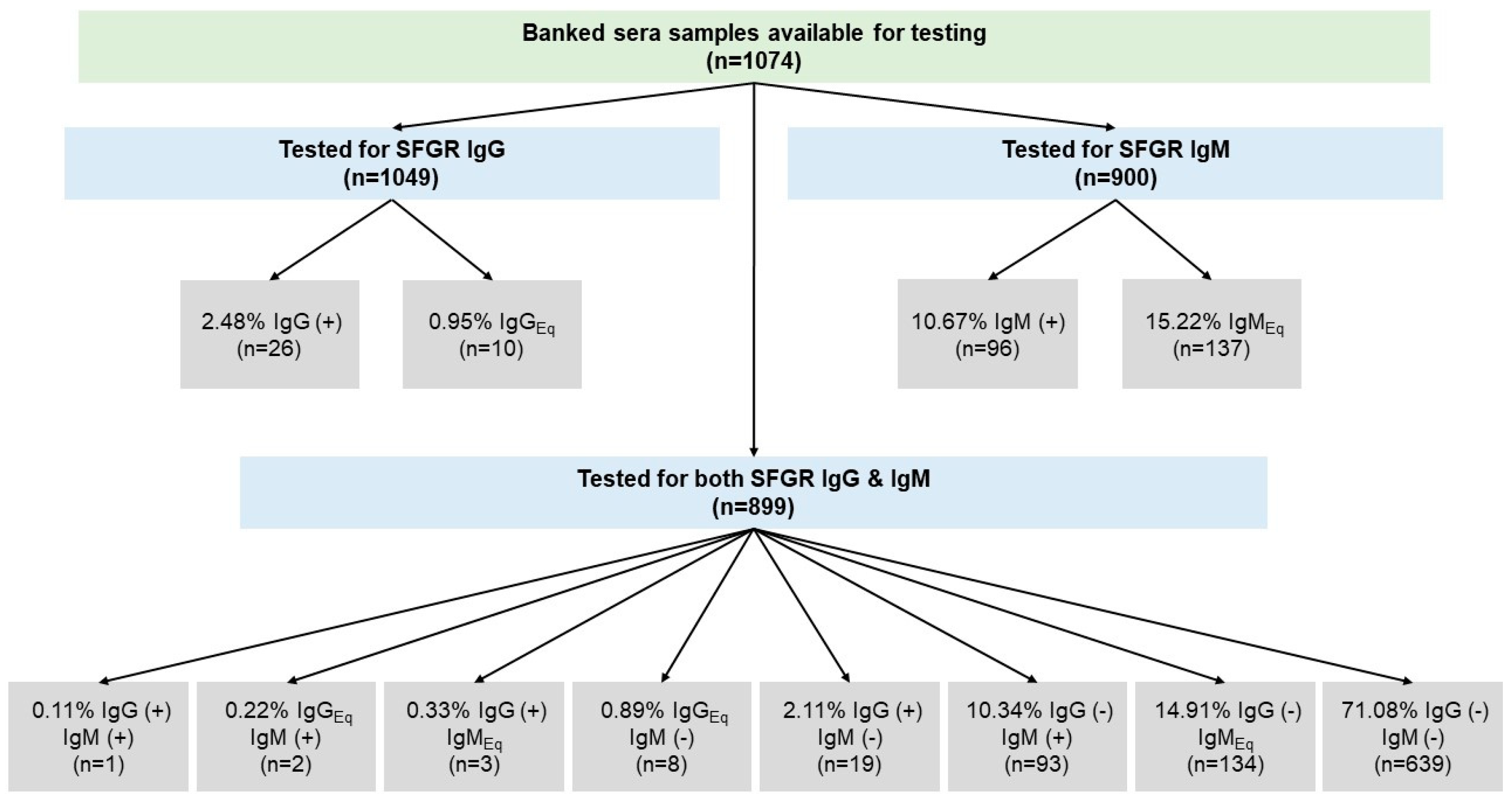
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanton, L.S. The rickettsioses: A practical update. Infect. Dis. Clin. 2019, 33, 213–229. [Google Scholar] [CrossRef]
- Mansueto, P.; Vitale, G.; Cascio, A.; Seidita, A.; Pepe, I.; Carroccio, A.; Di Rosa, S.; Rini, G.B.; Cillari, E.; Walker, D.H. New insight into immunity and immunopathology of Rickettsial diseases. Clin. Dev. Immunol. 2012, 2012, 967852. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.; Rymaszewska, A. Expansion of tick-borne rickettsioses in the world. Microorganisms 2020, 8, 1906. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, F.S.; Paddock, C.D.; Springer, Y.P.; Eisen, R.J.; Behravesh, C.B. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am. J. Trop. Med. Hyg. 2016, 94, 35. [Google Scholar] [CrossRef]
- Bishop, A.; Borski, J.; Wang, H.-H.; Donaldson, T.G.; Michalk, A.; Montgomery, A.; Heldman, S.; Mogg, M.; Derouen, Z.; Grant, W.E. Increasing Incidence of Spotted Fever Group Rickettsioses in the United States, 2010–2018. Vector-Borne Zoonotic Dis. 2022, 22, 491–497. [Google Scholar] [CrossRef]
- Charles, R.A.; Bermúdez, S.; Banović, P.; Alvarez, D.O.; Díaz-Sánchez, A.A.; Corona-González, B.; Etter, E.M.C.; Rodríguez González, I.; Ghafar, A.; Jabbar, A. Ticks and tick-borne diseases in Central America and the Caribbean: A one health Perspective. Pathogens 2021, 10, 1273. [Google Scholar] [CrossRef] [PubMed]
- Noden, B.H.; Roselli, M.A.; Loss, S.R. Effect of Urbanization on Presence, Abundance, and Coinfection of Bacteria and Protozoa in Ticks in the US Great Plains. J. Med. Entomol. 2022, 59, 957–968. [Google Scholar] [CrossRef]
- Ortiz, D.I.; Piche-Ovares, M.; Romero-Vega, L.M.; Wagman, J.; Troyo, A. The Impact of Deforestation, Urbanization, and Changing Land Use Patterns on the Ecology of Mosquito and Tick-Borne Diseases in Central America. Insects 2021, 13, 20. [Google Scholar] [CrossRef]
- Snellgrove, A.N.; Krapiunaya, I.; Scott, P.; Levin, M.L. Assessment of the pathogenicity of Rickettsia amblyommatis, Rickettsia bellii, and Rickettsia montanensis in a guinea pig model. Vector-Borne Zoonotic Dis. 2021, 21, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Farovitch, L.; Sippy, R.; Beltrán-Ayala, E.; Endy, T.P.; Stewart-Ibarra, A.M.; Leydet, B.F., Jr. Detection of Antibodies to Spotted Fever Group Rickettsiae and Arboviral Coinfections in Febrile Individuals in 2014–2015 in Southern Coastal Ecuador. Am. J. Trop. Med. Hyg. 2019, 101, 1087. [Google Scholar] [CrossRef]
- Bermúdez, C.S.E.; Troyo, A. A review of the genus Rickettsia in Central America. Res. Rep. Trop. Med. 2018, 9, 103. [Google Scholar] [PubMed] [Green Version]
- Brites-Neto, J.; Duarte, K.M.R.; Martins, T.F. Tick-borne infections in human and animal population worldwide. Vet. World 2015, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States: A practical guide for health care and public health professionals. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, S.V.d.; Guimarães, J.N.; Reckziegel, G.C.; Neves, B.M.d.C.; Araújo-Vilges, K.M.d.; Fonseca, L.X.; Pinna, F.V.; Pereira, S.V.C.; Caldas, E.P.d.; Gazeta, G.S. An update on the epidemiological situation of spotted fever in Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22. [Google Scholar] [CrossRef] [Green Version]
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.; Bjoersdorff, A.; Blanco, J.; Caruso, G.; Cinco, M.; Fournier, P.-E.; Francavilla, E. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132. [Google Scholar] [CrossRef]
- Kováčová, E.; Sixl, W.; Stünzner, D.; Ürvögyi, J.; Kazár, J. Serological examination of human and animal sera from six countries of three continents for the presence of rickettsial antibodies. Eur. J. Epidemiol. 1996, 12, 85–89. [Google Scholar] [CrossRef]
- WHO. Global surveillance of rickettsial diseases: Memorandum from a WHO meeting. Bull. WHO 1993, 71, 293–296. [Google Scholar]
- Chen, L.H.; Wilson, M.E. Tick-borne rickettsiosis in traveler returning from Honduras. Emerg. Infect. Dis. 2009, 15, 1321. [Google Scholar] [CrossRef]
- McCown, M.; Grzeszak, B. Zoonotic and infectious disease surveillance in Central America: Honduran feral cats positive for toxoplasma, trypanosoma, leishmania, rickettsia, and Lyme disease. J. Spec. Oper. Med. Peer Rev. J. SOF Med. Prof. 2010, 10, 41–43. [Google Scholar] [CrossRef]
- Chao, C.-C.; Zhang, Z.; Belinskaya, T.; Chen, H.-W.; Ching, W.-M. Leptospirosis and Rickettsial Diseases Sero-Conversion Surveillance Among US Military Personnel in Honduras. Mil. Med. 2021, 187, 802–807. [Google Scholar] [CrossRef]
- Novakova, M.; Literak, I.; Chevez, L.; Martins, T.F.; Ogrzewalska, M.; Labruna, M.B. Rickettsial infections in ticks from reptiles, birds and humans in Honduras. Ticks Tick-Borne Dis. 2015, 6, 737–742. [Google Scholar] [CrossRef]
- Reller, M.E.; Chikeka, I.; Miles, J.J.; Dumler, J.S.; Woods, C.W.; Mayorga, O.; Matute, A.J. First identification and description of rickettsioses and Q fever as causes of acute febrile illness in Nicaragua. PLoS Negl. Trop. Dis. 2016, 10, e0005185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springer, A.; Montenegro, V.M.; Schicht, S.; Wölfel, S.; Schaper, S.R.; Chitimia-Dobler, L.; Siebert, S.; Strube, C. Detection of Rickettsia monacensis and Rickettsia amblyommatis in ticks collected from dogs in Costa Rica and Nicaragua. Ticks Tick-Borne Dis. 2018, 9, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Fiorello, C.V.; Straub, M.H.; Schwartz, L.M.; Liu, J.; Campbell, A.; Kownacki, A.K.; Foley, J.E. Multiple-host pathogens in domestic hunting dogs in Nicaragua’s Bosawás Biosphere Reserve. Acta Trop. 2017, 167, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Eremeeva, M.E.; Berganza, E.; Suarez, G.; Gobern, L.; Dueger, E.; Castillo, L.; Reyes, L.; Wikswo, M.E.; Abramowicz, K.F.; Dasch, G.A. Investigation of an outbreak of rickettsial febrile illness in Guatemala, 2007. Int. J. Infect. Dis. 2013, 17, e304–e311. [Google Scholar] [CrossRef] [Green Version]
- Nolan, M.S.; Murray, K.O.; Mejia, R.; Hotez, P.J.; Villar Mondragon, M.J.; Rodriguez, S.; Palacios, J.R.; Murcia Contreras, W.E.; Lynn, M.K.; Torres, M.E. Elevated pediatric Chagas disease burden complicated by concomitant intestinal parasites and malnutrition in El Salvador. Trop. Med. Infect. Dis. 2021, 6, 72. [Google Scholar] [CrossRef]
- Clements, M.; Dumler, J.; Fiset, P.; Wisseman, C., Jr.; Snyder, M.; Levine, M. Serodiagnosis of Rocky Mountain spotted fever: Comparison of IgM and IgG enzyme-linked immunosorbent assays and indirect fluorescent antibody test. J. Infect. Dis. 1983, 148, 876–880. [Google Scholar] [CrossRef]
- Robinson, M.T.; Satjanadumrong, J.; Hughes, T.; Stenos, J.; Blacksell, S.D. Diagnosis of spotted fever group Rickettsia infections: The Asian perspective. Epidemiol. Infect. 2019, 147, e286. [Google Scholar] [CrossRef] [Green Version]
- Fournier, P.-E.; Jensenius, M.; Laferl, H.; Vene, S.; Raoult, D. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin. Vaccine Immunol. 2002, 9, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Lewin, M.R.; Bouyer, D.H.; Walker, D.H.; Musher, D.M. Rickettsia sibirica infection in members of scientific expeditions to northern Asia. Lancet 2003, 362, 1201–1202. [Google Scholar] [CrossRef]
- Salje, J.; Weitzel, T.; Newton, P.N.; Varghese, G.M.; Day, N. Rickettsial Infections: A Blind Spot in Our View of Neglected Tropical Diseases; Public Library of Science: San Francisco, CA USA, 2021. [Google Scholar]
- Alvarez, D.; Ochoa, E.; Nichols Heitman, K.; Binder, A.M.; Alvarez, G.; Armstrong, P.A. Epidemiology and clinical features of Rocky Mountain spotted fever from enhanced surveillance, Sonora, Mexico: 2015–2018. Am. J. Trop. Med. Hyg. 2020, 104, 190–197. [Google Scholar] [CrossRef]
- Álvarez-Hernández, G.; Roldán, J.F.G.; Milan, N.S.H.; Lash, R.R.; Behravesh, C.B.; Paddock, C.D. Rocky Mountain spotted fever in Mexico: Past, present, and future. Lancet Infect. Dis. 2017, 17, e189–e196. [Google Scholar] [CrossRef]
- Mora, J.D.-D.l.; Licona-Enríquez, J.D.; Leyva-Gastélum, M.; Mora, D.D.-D.l.; Rascón-Alcantar, A.; Álvarez-Hernández, G. A fatal case series of Rocky Mountain spotted fever in Sonora, México. Biomédica 2018, 38, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weil, E. Zur serologischen Diagnose des Flekfiebers. Wien Klin. Wochenschr. 1916, 29, 33–35. [Google Scholar]
- Plotz, H.; Wertman, K.; Bennett, B. Identification of rickettsial agents isolated in guinea pigs by means of specific complement fixation. Proc. Soc. Exp. Biol. Med. 1946, 61, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Hechemy, K.; Anacker, R.; Philip, R.; Kleeman, K.; MacCormack, J.; Sasowski, S.; Michaelson, E. Detection of Rocky Mountain spotted fever antibodies by a latex agglutination test. J. Clin. Microbiol. 1980, 12, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Halle, S.; Dasch, G.A.; Weiss, E. Sensitive enzyme-linked immunosorbent assay for detection of antibodies against typhus rickettsiae, Rickettsia prowazekii and Rickettsia typhi. J. Clin. Microbiol. 1977, 6, 101–110. [Google Scholar] [CrossRef]
- Horta, M.C.; Labruna, M.B.; Pinter, A.; Linardi, P.M.; Schumaker, T.T. Rickettsia infection in five areas of the state of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2007, 102, 793–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, M.G.; Ormsbee, R.A.; Johnson, K.M. Rickettsioses of central America. Am. J. Trop. Med. Hyg. 1971, 20, 941–949. [Google Scholar] [CrossRef]
- Wood, H.; Drebot, M.A.; Dewailly, E.; Dillon, L.; Dimitrova, K.; Forde, M.; Grolla, A.; Lee, E.; Loftis, A.; Makowski, K. Seroprevalence of seven zoonotic pathogens in pregnant women from the Caribbean. Am. J. Trop. Med. Hyg. 2014, 91, 642. [Google Scholar] [CrossRef]
- Forshey, B.M.; Stewart, A.; Morrison, A.C.; Gálvez, H.; Rocha, C.; Astete, H.; Eza, D.; Chen, H.-W.; Chao, C.-C.; Montgomery, J.M. Epidemiology of spotted fever group and typhus group rickettsial infection in the Amazon basin of Peru. Am. J. Trop. Med. Hyg. 2010, 82, 683. [Google Scholar] [CrossRef] [PubMed]
- Salmon-Mulanovich, G.; Simons, M.P.; Flores-Mendoza, C.; Loyola, S.; Silva, M.; Kasper, M.; Rázuri, H.R.; Canal, L.E.; Leguia, M.; Bausch, D.G. Seroprevalence and risk factors for Rickettsia and Leptospira infection in four ecologically distinct regions of Peru. Am. J. Trop. Med. Hyg. 2019, 100, 1391. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Acosta-Jamett, G.; Jiang, J.; Martínez-Valdebenito, C.; Farris, C.M.; Richards, A.L.; Abarca, K. Human seroepidemiology of Rickettsia and Orientia species in Chile–A cross-sectional study in five regions. Ticks Tick-Borne Dis. 2020, 11, 101503. [Google Scholar] [CrossRef]
- Montenegro, D.C.; Bitencourth, K.; De Oliveira, S.V.; Borsoi, A.P.; Cardoso, K.M.; Sousa, M.S.; Giordano-Dias, C.; Amorim, M.; Serra-Freire, N.M.; Gazêta, G.S. Spotted fever: Epidemiology and vector-rickettsia-host relationship in Rio de Janeiro state. Front. Microbiol. 2017, 8, 505. [Google Scholar] [CrossRef] [Green Version]
- The World Bank. Primary and Secondary Education, Pupils-El Salvador. 2022. Available online: https://data.worldbank.org/indicator/SE.PRM.ENRL?locations=SV (accessed on 27 July 2022).
- Pisharody, S.; Rubach, M.P.; Carugati, M.; Nicholson, W.L.; Perniciaro, J.L.; Biggs, H.M.; Maze, M.J.; Hertz, J.T.; Halliday, J.E.; Allan, K.J. Incidence Estimates of Acute Q Fever and Spotted Fever Group Rickettsioses, Kilimanjaro, Tanzania, from 2007 to 2008 and from 2012 to 2014. Am. J. Trop. Med. Hyg. 2022, 106, 494. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.T.; Ho, T.M.; Rohani, M.; Devi, S. Antibodies to Orientia tsutsugamushi, Rickettsia typhi and spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 280–284. [Google Scholar] [CrossRef]
- Omodior, O.; Kianersi, S.; Luetke, M. Prevalence of risk and protective factors for tick exposure and tick-borne disease among residents of Indiana. J. Public Health Manag. Pract. 2021, 27, E210–E219. [Google Scholar] [CrossRef]
- Iriani, D.U.; Matsukawa, T.; Tadjudin, M.K.; Itoh, H.; Yokoyama, K. Cross-sectional study on the effects of socioeconomic factors on lead exposure in children by gender in Serpong, Indonesia. Int. J. Environ. Res. Public Health 2012, 9, 4135–4149. [Google Scholar] [CrossRef] [Green Version]
- Younis, L.G.; Kroeger, A.; Joshi, A.B.; Das, M.L.; Omer, M.; Singh, V.K.; Gurung, C.K.; Banjara, M.R. Housing structure including the surrounding environment as a risk factor for visceral leishmaniasis transmission in Nepal. PLoS Negl. Trop. Dis. 2020, 14, e0008132. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, N.D.; Avoi, R. Prevalence and risk factors for positive lymphatic filariasis antibody in Sabah, Malaysia: A cross-sectional study. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 369–374. [Google Scholar] [CrossRef]
- Dzul-Rosado, K.R.; Reyes-Novelo, E.; Lugo-Caballero, C.; Cuxim-Koyoc, A.D.; Collí-Padrón, F.; Tello-Martín, R.; López-Ávila, K.B.; Palma-Chan, A.; Peniche-Lara, G.; Ruiz-Piña, H.A. Urban ecology of hosts and vectors of Rickettsia in a rickettsiosis-endemic city of the Yucatan peninsula, Mexico. Acta Trop. 2021, 216, 105832. [Google Scholar] [CrossRef]
- Prabhu, M.; Nicholson, W.L.; Roche, A.J.; Kersh, G.J.; Fitzpatrick, K.A.; Oliver, L.D.; Massung, R.F.; Morrissey, A.B.; Bartlett, J.A.; Onyango, J.J. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin. Infect. Dis. 2011, 53, e8–e15. [Google Scholar] [CrossRef]
- Omballa, V.O.; Musyoka, R.N.; Vittor, A.Y.; Wamburu, K.B.; Wachira, C.M.; Waiboci, L.W.; Abudo, M.U.; Juma, B.W.; Kim, A.A.; Montgomery, J.M. Serologic evidence of the geographic distribution of bacterial zoonotic agents in Kenya, 2007. Am. J. Trop. Med. Hyg. 2016, 94, 43. [Google Scholar] [CrossRef] [Green Version]
- Chaisiri, K.; Tanganuchitcharnchai, A.; Kritiyakan, A.; Thinphovong, C.; Tanita, M.; Morand, S.; Blacksell, S.D. Risk factors analysis for neglected human rickettsioses in rural communities in Nan province, Thailand: A community-based observational study along a landscape gradient. PLoS Negl. Trop. Dis. 2022, 16, e0010256. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.M.; Heitman, K.N.; Drexler, N.A. Diagnostic methods used to classify confirmed and probable cases of spotted fever rickettsioses—United States, 2010–2015. Morb. Mortal. Wkly. Rep. 2019, 68, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuiston, J.H.; Wiedeman, C.; Singleton, J.; Carpenter, L.R.; McElroy, K.; Mosites, E.; Chung, I.; Kato, C.; Morris, K.; Moncayo, A.C. Inadequacy of IgM antibody tests for diagnosis of Rocky Mountain spotted fever. Am. J. Trop. Med. Hyg. 2014, 91, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, K.; Wallménius, K.; Påhlson, C. Coinfection with Rickettsia helvetica and herpes simplex virus 2 in a young woman with meningoencephalitis. Case Rep. Infect. Dis. 2011, 2011, 469194. [Google Scholar] [PubMed] [Green Version]
- McQuiston, J.H.; Zemtsova, G.; Perniciaro, J.; Hutson, M.; Singleton, J.; Nicholson, W.L.; Levin, M.L. Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector-Borne Zoonotic Dis. 2012, 12, 1059–1061. [Google Scholar] [CrossRef] [Green Version]
- Ostfeld, R.S.; Lewis, D.N. Experimental studies of interactions between wild turkeys and black-legged ticks. J. Vector Ecol. 1999, 24, 182–186. [Google Scholar]
- Samish, M.; Ginsberg, H.; Glazer, I. Biological control of ticks. Parasitology 2004, 129, S389–S403. [Google Scholar] [CrossRef]
- Tappe, D.; Gross, Y.; Ngui, R.; Rauch, J.; Tay, S.T.; Lim, Y.A.L. High seroprevalence against typhus group and spotted fever group Rickettsiae in rural indigenous populations of peninsular Malaysia. Vector-Borne Zoonotic Dis. 2019, 19, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Devamani, C.S.; Schmidt, W.-P.; Ariyoshi, K.; Anitha, A.; Kalaimani, S.; Prakash, J.A. Risk factors for scrub typhus, murine typhus, and spotted fever seropositivity in urban areas, rural plains, and peri-forest hill villages in South India: A cross-sectional study. Am. J. Trop. Med. Hyg. 2020, 103, 238. [Google Scholar] [CrossRef] [PubMed]
- Piranda, E.M.; Faccini, J.L.H.; Pinter, A.; Saito, T.B.; Pacheco, R.C.; Hagiwara, M.K.; Labruna, M.B. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: Clinical and laboratory findings. Mem. Inst. Oswaldo Cruz 2008, 103, 696–701. [Google Scholar] [CrossRef]
- Schmidt, W.P.; Devamani, C.S.; Elangovan, D.; Alexander, N.; Rose, W.; Prakash, J.A. Clinical characteristics of and antibody response to spotted fever group rickettsial infections in South India: Case series and serological cohort study. Trop. Med. Int. Health 2021, 26, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Kharod, G.A.; Schaad, N.; Furukawa, N.W.; Vora, N.M.; Blaney, D.D.; Crump, J.A.; Clarke, K.R. Global knowledge gaps in acute febrile illness etiologic investigations: A scoping review. PLoS Negl. Trop. Dis. 2019, 13, e0007792. [Google Scholar] [CrossRef]


| ELISA IgG and/or IgM | ELISA IgG | ELISA IgM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive N (%) or Mean ± SD | Negative N (%) or Mean ± SD | Model 1 Odds Ratio (95% CI) ꜝ | Positive N (%) or Mean ± SD | Negative N (%) or Mean ± SD | Model 2 Odds Ratio (95% CI) | Positive N (%) or Mean ± SD | Negative N (%) or Mean ± SD | Model 3 Odds Ratio (95% CI) | |
| N = 121 | N = 887 | N = 26 | N = 971 | N = 95 | N = 662 | ||||
| Participant characteristics | |||||||||
| Male | 58 (47.9) | 478 (53.9) | 1.81 (1.01, 3.25) † | 17 (65.4) | 442 (45.5) | 5.33 (1.40, 20.25) † | 41 (43.2) | 328 (48.0) | |
| Febrile at time of enrollment | 32/117 (27.4) | 540 (64.2) | 0.36 (0.18, 0.71) ‡ | 7 (26.2) | 322/922 (34.9) | 25/91 (27.5) | 228/642 (35.5) | 0.38 (0.17, 0.82) † | |
| Enrolled in the wet season | 81 (66.9) | 503 (56.7) | 14/26 (53.8) | 561 (57.8) | 67 (70.5) | 380 (57.4) | 2.527 (1.25, 5.11) ‡ | ||
| Age (years) | 10.6 ± 4.6 | 8.5 ± 4.7 | 1.16 (1.08, 1.23) * | 12.0 ± 4.1 | 8.6 ± 4.7 | 1.18 (1.03, 1.35) † | 10.2 ± 4.7 | 8.3 ± 4.8 | 1.15 (1.08, 1.24) * |
| Household occupancy and composition | |||||||||
| N living in household | 5.2 ± 2.1 | 5.5 ± 3.0 | 5.0 ± 2.0 | 5.5 ± 3.0 | 5.3 ± 2.1 | 5.5 ± 3.1 | |||
| N kids in household | 2.5 ± 1.3 | 2.5 ± 1.4 | 2.4 ± 1.4 | 2.5 ± 1.4 | 2.6 ± 1.3 | 2.5 ± 1.3 | |||
| Mother’s last year of education | 5.1 ± 4.0 | 5.4 ± 4.1 | 1.07 (0.99, 1.15) | 4.2 ± 4.6 | 5.4 ± 4.1 | 5.5 ± 4.0 | 5.4 ± 4.0 | 1.11 (1.02, 1.21) † | |
| Father’s last year of education | 5.5 ± 4.2 | 6.0 ± 4.4 | 5.0 ± 4.5 | 5.9 ± 4.4 | 5.7 ± 4.1 | 6.1 ± 4.4 | |||
| N beds in home | 4.7 ± 1.9 | 4.8 ± 2.3 | 4.7 ± 1.9 | 4.8 ± 2.2 | 4.7 ± 1.9 | 4.8 ± 2.3 | |||
| Household poverty indicators | |||||||||
| Agriculture as primary household income source | 69/110 (62.7) | 513/816 (62.9) | 13/22 (59.1) | 564/894 (63.1) | 57/88 (64.8) | 389/607 (64.1) | |||
| Type of cooking fuel used | |||||||||
| Firewood | 14/119 (11.8) | 126/886 (14.2) | 1/25 (4.0) | 139/970 (14.3) | 13 (13.7) | 95/660 (14.4) | |||
| Gas | 6/119 (5.0) | 50/886 (5.6) | 1/25 (4.0) | 53/970 (5.5) | 5 (5.3) | 37/660 (5.6) | |||
| Both | 99/119 (83.2) | 710/886 (80.1) | 23/25 (92.0) | 778/970 (80.2) | 77 (81.0) | 248/660 (37.6) | |||
| Type of floor in bedroom | |||||||||
| Bare earth | 96/119 (80.7) | 695 (78.4) | 18/25 (72.0) | 765 (78.8) | 79 (83.2) | 514/661 (77.8) | |||
| Cement | 23/119 (19.3) | 192 (21.7) | 7/25 (28.0) | 206 (21.2) | 0.26 (0.08, 0.86) † | 16 (16.8) | 147/661 (22.2) | ||
| Type of wall material in bedroom | |||||||||
| Blocks | 21/119 (17.6) | 177/885 (20.0) | 2/25 (8.0) | 191/969 (19.7) | 19 (20.0) | 132/559 (20.0) | |||
| Adobe | 58/119 (48.7) | 357/885 (40.3) | 12/25 (48.0) | 401/969 (41.4) | 46 (48.4) | 279/559 (42.3) | |||
| Other | 40/119 (33.6) | 351/885 (39.7) | 11/25 (44.0) | 377/969 (38.9) | 30 (31.6) | 248/559 (37.6) | |||
| Family uses outside latrine for bathroom | 108/120 (90.0) | 823/886 (92.9) | 0.25 (0.11, 0.56) ‡ | 21/25 (84.0) | 901/970 (92.9) | 0.10 (0.03, 0.42) ‡ | 88 (92.6) | 618/660 (93.6) | |
| Potable water in the house | 112/120 (93.3) | 776 (87.5) | 24/25 (96.0) | 854 (88.0) | 89 (93.7) | 581/660 (87.9) | |||
| No electricity | 58/116 (50.0) | 308/876 (35.2) | 2.41 (1.31, 4.45) ‡ | 12/24 (50.0) | 351/957 (36.7) | 46/92 (50.0) | 233/651 (35.8) | ||
| House has fowl (chickens, turkeys, and/or ducks) | 53/113 (46.9) | 471/860 (54.8) | 0.49 (0.27, 0.87) † | 9/25 (36.0) | 511/937 (54.5) | 44/88 (50.0) | 336/636 (52.8) | ||
| House has cats | 17/113 (15.0) | 121/860 (14.1) | 2/25 (8.0) | 135/937 (14.4) | 15/88 (15.8) | 88/636 (13.3) | |||
| House has dogs | 60/113 (53.1) | 462/860 (53.7) | 11/25 (44.0) | 507/937 (54.1) | 50/88 (56.8) | 335/636 (52.7) | |||
| Proxy assessment of vector-borne disease exposure and knowledge: household Chagas disease vector exposure and parent or legal guardian Chagas disease knowledge | |||||||||
| Knows what a “chinche” is | 74/120 (61.7) | 610 (68.8) | 13/25 (52.0) | 663 (68.3) | 0.13 (0.03, 0.49) ‡ | 61 (64.2) | 449/661 (67.9) | ||
| Knows about Chagas disease | 17/120 (14.2) | 178 (20.1) | 6/25 (24.0) | 188 (19.4) | 11 (11.6) | 126/661 (19.1) | |||
| Has seen chinches inside house within the past year | 28/120 (23.3) | 283 (31.9) | 6/25 (24.0) | 302 (31.1) | 22 (23.2) | 218/661 (33.0) | |||
| Someone in household has been bitten by a chinche in the past year | 18/120 (15.0) | 161/884 (18.2) | 0.39 (0.16, 0.99) † | 3/25 (12.0) | 176/968 (18.2) | 15 (15.8) | 132/660 (20.0) | ||
| House has been fumigated in the past year | 7/120 (5.8) | 90/885 (10.2) | 2/25 (8.0) | 94/969 (9.7) | 5 (5.3) | 66/660 (10.0) | |||
| Family health concerns | |||||||||
| Someone in the household sought clinical care in the past year | 90/119 (16.0) | 599/867 (69.1) | 18/25 (72.0) | 665/950 (70.0) | 73/94 (77.7) | 455/646 (70.4) | |||
| Someone in the household sought clinical care for an infection in the past year | 59/118 (50.0) | 341/865 (39.4) | 2.17 (1.21, 3.91) ‡ | 12/25 (48.0) | 386/948 (40.7) | 48/93 (51.6) | 257/645 (39.8) | 1.90 (0.99, 3.62) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dye-Braumuller, K.C.; Aquino, M.S.R.; Zellars, K.; Waltz, H.; Meyer, M.; Gual-Gonzalez, L.; Self, S.C.W.; Kanyangarara, M.; Nolan, M.S. Antibody Prevalence and Risk Factors Associated with Rickettsia spp. in a Pediatric Cohort: SFGR Remains Underdiagnosed and Underreported in El Salvador. Pathogens 2022, 11, 1241. https://doi.org/10.3390/pathogens11111241
Dye-Braumuller KC, Aquino MSR, Zellars K, Waltz H, Meyer M, Gual-Gonzalez L, Self SCW, Kanyangarara M, Nolan MS. Antibody Prevalence and Risk Factors Associated with Rickettsia spp. in a Pediatric Cohort: SFGR Remains Underdiagnosed and Underreported in El Salvador. Pathogens. 2022; 11(11):1241. https://doi.org/10.3390/pathogens11111241
Chicago/Turabian StyleDye-Braumuller, Kyndall C., Marvin Stanley Rodríguez Aquino, Kia Zellars, Hanna Waltz, Madeleine Meyer, Lídia Gual-Gonzalez, Stella C. W. Self, Mufaro Kanyangarara, and Melissa S. Nolan. 2022. "Antibody Prevalence and Risk Factors Associated with Rickettsia spp. in a Pediatric Cohort: SFGR Remains Underdiagnosed and Underreported in El Salvador" Pathogens 11, no. 11: 1241. https://doi.org/10.3390/pathogens11111241
APA StyleDye-Braumuller, K. C., Aquino, M. S. R., Zellars, K., Waltz, H., Meyer, M., Gual-Gonzalez, L., Self, S. C. W., Kanyangarara, M., & Nolan, M. S. (2022). Antibody Prevalence and Risk Factors Associated with Rickettsia spp. in a Pediatric Cohort: SFGR Remains Underdiagnosed and Underreported in El Salvador. Pathogens, 11(11), 1241. https://doi.org/10.3390/pathogens11111241






