Human Neurocysticercosis: An Overview
Abstract
1. Introduction
2. Clinical Manifestations
2.1. Seizures/Epilepsy
2.2. Headaches
2.3. Other Manifestations
3. Diagnosis
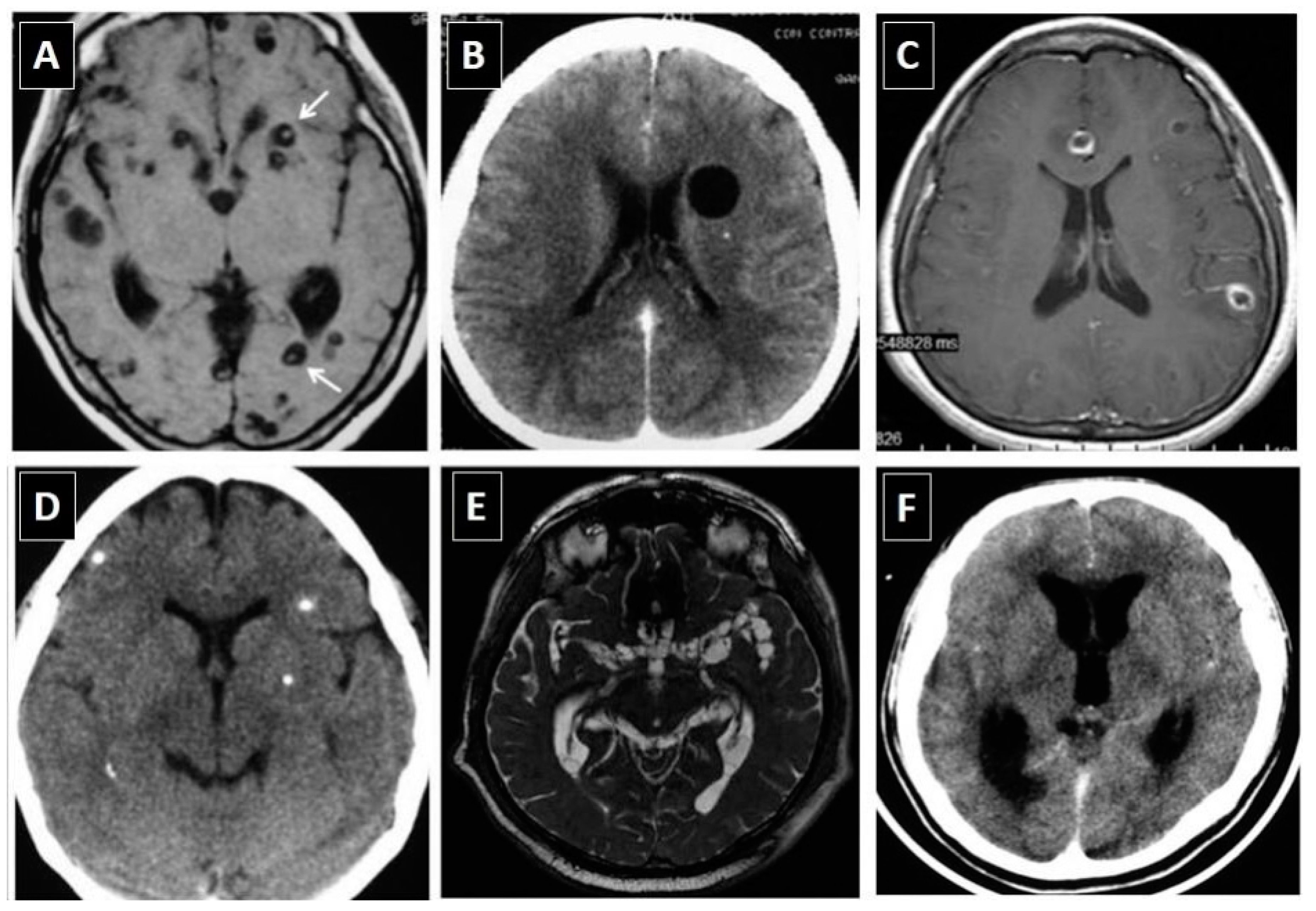
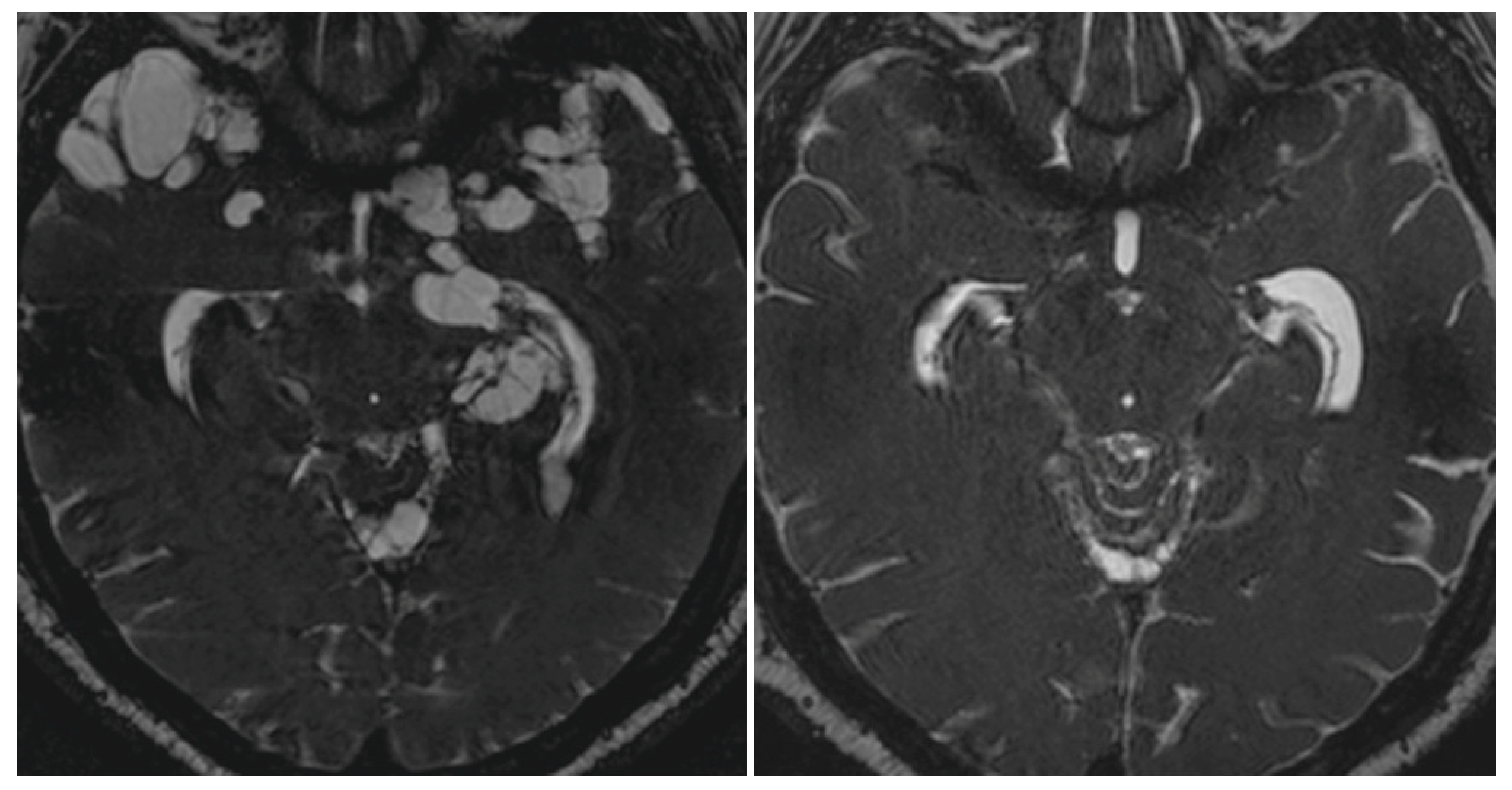
3.1. Neuroimaging Studies
3.2. Immune Diagnostic Tests
3.3. Unification of Diagnostic Criteria
| Diagnostic Criteria |
|---|
| Absolute Criteria: |
|
| Neuroimaging criteria: |
Major neuroimaging criteria:
|
Confirmative neuroimaging criteria:
|
Minor neuroimaging criteria:
|
| Clinical/exposure criteria: |
Major clinical/exposure:
|
Minor clinical/exposure:
|
| Degrees of diagnostic certainty |
Definitive Diagnosis:
|
Probable Diagnosis:
|
4. Treatment
5. Control Measures
6. Final Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coyle, C.M. Neurocysticercosis: An update. Curr. Infect. Dis. Rep. 2014, 16, 347. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.H. Parasitic infections of the nervous system. Continuum (Minneap Minn) 2021, 27, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.H.; Del Brutto, O.H. Fake news in neglected tropical diseases: The case of neurocysticercosis. PLoS Negl. Trop. Dis. 2020, 14, e0008208. [Google Scholar] [CrossRef]
- Hunter, E.; Cliff, M.; Armstrong, M.; Manji, H.; Jager, H.R.; Chiodini, P.; Brown, M. Active neurocysticercosis at the hospital for tropical diseases, London: A clinical case series. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H. Neurocysticercosis among international travelers to disease-endemic areas. J. Travel Med. 2012, 19, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Schantz, P.M.; Moore, A.C.; Muñoz, J.L.; Hartman, B.J.; Schaefer, J.A.; Aron, A.M.; Persaud, D.; Sarti, E.; Wilson, M.; Flisser, A. Neurocysticercosis in an Orthodox Jewish community in New York City. N. Engl. J. Med. 1992, 327, 692–695. [Google Scholar] [CrossRef]
- Garcia, H.H.; Nash, T.E.; Del Brutto, O.H. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014, 13, 1202–1215. [Google Scholar] [CrossRef]
- Moyano, L.M.; O’Neal, S.E.; Ayvar, V.; Gonzalvez, G.; Gamboa, R.; Vílchez, P.; Rodriguez, S.; Reistetter, J.; Tsang, V.C.W.; Gilman, R.H.; et al. High prevalence of asymptomatic neurocysticercosis in an endemic rural community in Perú. PloS Negl. Trop. Dis. 2016, 10, e0005130. [Google Scholar] [CrossRef]
- Fleury, A.; Gomez, T.; Alvarez, I.; Meza, D.; Huerta, M.; Chavarría, A.; Carrillo Mezo, R.A.; Lloyd, C.; Dessein, A.; Preux, P.M.; et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology 2003, 22, 139–145. [Google Scholar] [CrossRef]
- Carabin, H.; Ndimubanzi, P.C.; Budke, C.M.; Nguyen, H.; Qian, Y.; Cowan, L.D.; Stoner, J.A.; Rainwater, E.; Dickey, M. Clinical manifestations associated with neurocysticercosis: A systematic review. PLoS Negl. Trop. Dis. 2011, 5, e1152. [Google Scholar] [CrossRef]
- Nash, T.E.; Mahanty, S.; Loeb, J.A.; Theodore, W.H.; Friedman, A.; Sander, J.W.; Singh, G.; Cavalheiro, E.; Del Brutto, O.H.; Takayanagui, O.M.; et al. Neurocysticercosis: A natural human model of epileptogenesis. Epilepsia 2015, 56, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.E. Edema surrounding calcified intracranial cysticerci: Clinical manifestations, natural history, and treatment. Pathog. Glob. Health 2012, 106, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, P.; Singh, I.; Rani, A.; Kaushal, S.; Avasthi, G. Epidemiologic classification of seizures associated with neurocysticercosis: Observations from a sample of seizure disorders in neurologic care in India. Acta Neurol. Scand. 2006, 113, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Engel, J., Jr.; Eliashiv, D.S.; Garcia, H.H. Update on cysticercosis epileptogenesis: The role of the hippocampus. Curr. Neurol. Neurosci. Rep. 2016, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.A.; Bustos, J.A.; Clapham, P.; García, H.H.; Loeb, J.A.; Cysticercosis Working Group in Perú. Unique characteristics of epilepsy development in neurocysticercosis. Am. J. Trop. Med. Hyg. 2020, 103, 639–645. [Google Scholar] [CrossRef]
- Lachuriya, G.; Garg, R.K.; Jain, A.; Malhotra, H.S.; Singh, A.K.; Jain, B.; Kumar, N.; Verma, R.; Sharma, P.K. Toll-like receptor-4 polymorphisms and serum matrix metalloproteinase-9 in newly diagnosed patients with calcified neurocysticercosis and seizures. Medicine 2016, 95, e3288. [Google Scholar] [CrossRef]
- Prasad, A.; Prasad, K.N.; Gupta, R.K.; Pradhan, S. Increased expression of ICAM-1 among symptomatic neurocysticercosis. J. Neuroimmunol. 2009, 206, 118–120. [Google Scholar] [CrossRef]
- Verma, A.; Prasad, K.N.; Gupta, R.K.; Singh, A.K.; Nyati, K.K.; Rizwan, A.; Pandey, C.M.; Paliwal, V.K. Toll-like receptor 4 polymorphism and its association with symptomatic neurocysticercosis. J. Infect. Dis. 2010, 202, 1219–1225. [Google Scholar] [CrossRef]
- Herrick, J.A.; Maharathi, B.; Kim, J.S.; Abundis, G.G.; Garg, A.; Gonzales, I.; Saavedra, H.; Bustos, J.A.; Garcia, H.H.; Loeb, J.A. Inflammation is a key risk factor for persistent seizures in neurocysticercosis. Ann. Clin. Transl. Neurol. 2018, 5, 630–639. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Arroyo, G.; Del Brutto, V.J.; Zambrano, M.; García, H.H. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: A large-scale, computed tomography-based population study in rural Ecuador. Epilepsia 2017, 58, 1955–1961. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Mera, R.M.; Atahualpa Project Investigators. The importance of people compliance (social desirability bias) in the assessment of epilepsy prevalence in rural areas of developing countries. Results of the Atahualpa Project. Epilepsia 2016, 57, e221–e224. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Recalde, B.Y.; Mera, R.M. Incidence of adult-onset epilepsy and the contributory role of neurocysticercosis in a five-year, population-based, prospective study in rural Ecuador. Am. J. Trop. Med. Hyg. 2021, 106, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Garvey, B.T.; Moyano, L.M.; Ayvar, V.; Rodriguez, S.; Gilman, R.H.; Gonzalez, A.E.; Garcia, H.H.; O’Neal, S.E. Neurocysticercosis among people living near pigs heavily infected with cysticercosis in rural endemic Perú. Am. J. Trop. Med. Hyg. 2018, 98, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Prasad, A.; Gupta, R.K.; Nath, K.; Pradhan, S.; Tripathi, M.; Pandey, C.M. Neurocysticercosis in patients with active epilepsy from the pig farming community of Lucknow district, north India. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 144–150. [Google Scholar] [CrossRef]
- Amelot, A.; Faillot, T. Hydrocephalus and neurocysticercosis: Cases illustrative of three distinct mechanisms. J. Clin. Neurol. 2014, 10, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.E.; Cruz, I.; Preux, P.M.; Schantz, P.M.; Dumas, M. Headache and cysticercosis in Ecuador, South America. Headache 1995, 35, 93–97. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Del Brutto, V.J. Calcified neurocysticercosis among patients with primary headache. Cephalalgia 2012, 32, 250–254. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Robles, A.M.; Mera, R.M.; Costa, A.F.; Darsan, E.; Milla, L.; Montes, J.; Lama, J.; Del Brutto, V.; Zambrano, M.; et al. Calcified neurocysticercosis and headache in an endemic village: A case-control study nested to a population-based cohort. Am. J. Trop. Med. Hyg. 2018, 99, 729–734. [Google Scholar] [CrossRef]
- Nash, T.E.; Pretell, E.J.; Lescano, A.G.; Bustos, J.A.; Gilman, R.H.; Gonzalez, A.E.; Garcia, H.H.; Cysticercosis Working Group in Peru. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: A prospective cohort and nested case-control study. Lancet Neurol. 2008, 7, 1099–1105. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Robles, A.M.; Láinez, J.M. Migrainous headaches, calcified cysticercosis and breakthrough seizures. Cephalalgia Rep. 2022, 5, 25158163221076464. [Google Scholar] [CrossRef]
- Fleury, A.; Carrillo-Mezo, R.; Flisser, A.; Sciutto, E.; Corona, T. Subarachnoid basal neurocysticercosis: A focus on the most severe form of the disease. Expert Rev. Anti-Infect. Ther. 2011, 9, 123–133. [Google Scholar] [CrossRef]
- Carod Artal, F.J. Clinical management of infectious cerebral vasculitidis. Expert Rev. Neurother. 2016, 16, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Mera, R.M.; Zambrano, M.; Costa, A.F.; Román, G.C. The association between calcified neurocysticercosis and cognitive performance: A case-control study nested to a population-based cohort. Am. J. Trop. Med. Hyg. 2019, 100, 323–326. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Del Brutto, V.J. Intrasellar cysticercosis: A systematic review. Acta Neurol. Belg. 2013, 113, 225–327. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; García, H.H. Intramedullary cysticercosis of the spinal cord: A review of patients evaluated with MRI. J. Neurol. Sci. 2013, 331, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Panchal, B.; Pathengay, A. Relationship between scolex, shape of the cyst and timing of surgery in subretinal cysticercosis. BMJ Case Rep. 2020, 13, e236805. [Google Scholar] [CrossRef]
- Guzman, C.; Garcia, H.H. Cysticercosis Working Group in Peru. Current diagnostic criteria for neurocysticercosis. Res. Rep. Trop. Med. 2021, 12, 197–203. [Google Scholar] [CrossRef]
- Kimura-Hayama, E.T.; Higuera, J.A.; Corona-Cedillo, R.; Chávez-Macías, L.; Perochena, A.; Quiroz-Rojas, L.Y.; Rodríguez-Carbajal, J.; Criales, J.L. Neurocysticercosis: Radiologic-pathologic correlation. Radiographics 2010, 30, 1705–1719. [Google Scholar] [CrossRef]
- Pappala, B.C.S.; Indugula, J.P.; Shrivastava, A.K.; Jumar, S.; Talabhatula, S.K.; Kolli, R.S.; Sahu, P.S. Comparative evaluation of indigenous ELISAs for detection of anti-cysticercus IgG antibodies in serum from clinically and radiologically suspected cases of neurocysticercosis. Trop. Biomed. 2017, 4, 622–635. [Google Scholar]
- Carod, J.F.; Randrianarison, M.; Razafimahefa, J.; Ramahefarisoa, R.M.; Rakotondrazaka, M.; Debruyne, M. Evaluation of the performance of 5 commercialized enzyme immunoassays for the detection of Taenia solium antibodies and for the diagnosis of neurocysticercosis. Diagn. Microbiol. Infect. Dis. 2012, 72, 85–89. [Google Scholar] [CrossRef]
- García, H.H.; Castillo, Y.; Gonzales, I.; Bustos, J.A.; Saavedra, H.; Jacob, L.; Del Brutto, O.H.; Wilkins, P.; Gonzalez, A.E.; Gilman, R.H. Low sensitivity and frequent cross-reactions in commercially available antibody detection ELISA assays for Taenia solium cysticercosis. Trop. Med. Int. Health 2018, 23, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Wilkins, P.; Dorny, P. Immunological and molecular diagnosis of cysticercosis. Pathog. Glob. Health 2012, 106, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.M.; White, A.C., Jr. Update on the diagnosis and management of neurocysticercosis. Curr. Infect. Dis. Rep. 2016, 18, 44. [Google Scholar] [CrossRef]
- Garcia, H.H.; O’Neal, S.E.; Noh, J.; Handali, S.; Cysticercosis Working Group in Peru. Laboratory diagnosis of neurocysticercosis (Taenia solium). J. Clin. Microbiol. 2018, 56, e00424-18. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, G.; Bustos, J.A.; Lescano, A.G.; Gonzales, I.; Saavedra, H.; Pretell, E.J.; Castillo, Y.; Perez, E.; Dorny, P.; Gilman, R.H.; et al. Improved diagnosis of viable parenchymal neurocysticercosis by combining antibody bandings patterns on enzyme-linked immunoelectrotransfer blot (EITB) with antigen ELISA assay. J. Clin. Microbiol. 2022, 20, e0155021. [Google Scholar] [CrossRef]
- O’Connell, E.M.; Harrison, S.; Dahlstrom, E.; Nash, T.; Nutman, T.B. A novel, highly sensitive quantitative polymerase chain reaction assay for the diagnosis of subarachnoid and ventricular neurocysticercosis and for assessing responses to treatment. Clin. Infect. Dis. 2020, 70, 1875–1881. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Wadia, N.H.; Dumas, M.; Cruz, M.; Tsang, V.C.; Schantz, P.M. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J. Neurol. Sci. 1996, 142, 1–6. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Rajshekhar, V.; White, A.C., Jr.; Nash, T.E.; Takayanagui, O.M.; Schantz, P.M.; Evans, C.A.; Flisser, A.; Correa, D.; Botero, D.; et al. Proposed diagnostic criteria for neurocysticercosis. Neurology 2001, 57, 177–183. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Nash, T.E.; White, A.C., Jr.; Rajshekhar, V.; Wilkins, P.P.; Singh, G.; Vasquez, C.M.; Salgado, P.; Gilman, R.H.; Garcia, H.H. Revised diagnostic criteria for neurocysticercosis. J. Neurol. Sci. 2017, 371, 202–210. [Google Scholar] [CrossRef]
- Del Brutto, O.H. Twenty-five years of evolution of standard diagnostic criteria for neurocysticercosis. How have they impacted diagnosis and patient outcomes? Expert Rev. Neurother. 2020, 20, 147–155. [Google Scholar] [CrossRef]
- Garg, R.K. Diagnostic criteria for neurocysticercosis: Some modifications are needed for Indian patients. Neurol. India 2004, 52, 171–177. [Google Scholar] [PubMed]
- Gabriel, S.; Blocher, J.; Dorny, P.; Abatih, E.N.; Schmutzhard, E.; Ombay, M.; Mathias, B.; Winkler, A.S. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl. Trop. Dis. 2012, 6, e1851. [Google Scholar] [CrossRef] [PubMed]
- Carpio, A.; Fleury, A.; Romo, M.L.; Abraham, R.; Fandiño, J.; Durán, J.C.; Cárdenas, G.; Moncayo, J.; Rodrigues, C.L.; San-Juan, D.; et al. New diagnostic criteria for neurocysticercosis: Reliability and validity. Ann. Neurol. 2016, 80, 434–442. [Google Scholar] [CrossRef]
- Gilman, R.H. Infectious disease: Diagnostic criteria for neurocysticercosis–a difficult update. Nat. Rev. Neurol. 2016, 12, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.M. Neurocysticercosis: An individualized approach. Infect. Dis. Clin. N. Am. 2019, 33, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Takayanagui, O.M.; Marques de Haes, T. Update on the diagnosis and management of neurocysticercosis. Arq. Neuropsiquiatr. 2022, 80, 296–306. [Google Scholar] [CrossRef]
- Bustos, J.A.; Garcia, H.H.; Del Brutto, O.H. Antiepileptic drug therapy and recommendations for withdrawal in patients with seizures and epilepsy due to neurocysticercosis. Expert Rev. Neurother. 2016, 16, 1079–1085. [Google Scholar] [CrossRef]
- Nash, T.E.; Mahanty, S.; Garcia, H.H.; Cysticercosis Group in Peru. Corticosteroid use in neurocysticercosis. Expert Rev. Neurother. 2011, 11, 1175–1183. [Google Scholar] [CrossRef]
- Mitre, E.; Talaat, K.R.; Sperling, M.R.; Nash, T.E. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin. Infect. Dis. 2007, 44, 549–553. [Google Scholar] [CrossRef]
- Nash, T.E.; Ware, J.A.M.; Coyle, C.M.; Mahanty, S. Etanercept to control inflammation in the treatment of complicated neurocysticercosis. Am. J. Trop. Med. Hyg. 2019, 100, 609–616. [Google Scholar] [CrossRef]
- Barrie, U.; Badejo, O.; Aoun, S.G.; Adeyemo, E.; Moler, N.; Christian, Z.K.; Caruso, J.P.; El Ahmadieh, T.Y.; Ban, V.S.; MacAllister, W.C.; et al. Systematic review and meta-analysis of management strategies and outcomes in adult spinal neurocysticercosis. World Neurosurg. 2020, 138, 504–511e8. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto Filho, P.T.; Zanini, M.A.; Fleury, A. Hydrocephalus in neurocysticercosis: Challenges for clinical practice and basic research perspectives. World Neurosurg. 2019, 126, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.E.; Ware, J.A.M.; Mahanty, S. Intraventricular neurocysticercosis: Experience and long-term outcome from a tertiary referral center in the United States. Am. J. Trop. Med. Hyg. 2018, 98, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H. A personal account regarding the origin and evolution of controversies in the management of neurocysticercosis. Am. J. Trop. Med. Hyg. 2019, 100, 780–782. [Google Scholar] [CrossRef]
- Garcia, H.H.; Pretell, E.J.; Gilman, R.H.; Martinez, S.M.; Moulton, L.H.; Del Brutto, O.H.; Herrera, G.; Evans, C.A.W.; Gonzalez, A.E.; Cysticercosis Working Group in Peru. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N. Engl. J. Med. 2004, 350, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Roos, K.L.; Coffey, C.S.; Garcia, H.H. Meta-analysis: Cysticidal drugs for neurocysticercosis: Albendazole and praziquantel. Ann. Intern. Med. 2006, 145, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.A.; Wiebe, S.; Zunt, J.R.; Halperin, J.J.; Gronseth, G.; Roos, K.L. Evidence-based guideline: Treatment of parenchymal neurocysticercosis: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2013, 80, 1424–1429. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; García, H.H. The many facets of disseminated parenchymal brain cysticercosis: A differential diagnosis with important therapeutic implications. PloS Negl. Trop. Dis. 2021, 15, e0009883. [Google Scholar] [CrossRef]
- Nash, T.E.; O’Connell, E.M.; Hammoud, D.A.; Wetzler, L.; Ware, J.M.; Mahanty, S. Natural history of treated subarachnoid neurocysticercosis. Am. J. Trop. Med. Hyg. 2020, 102, 78–89. [Google Scholar] [CrossRef]
- White, A.C., Jr.; Coyle, C.M.; Rajshekhar, V.; Singh, G.; Hauser, W.A.; Mohanty, A.; Garcia, H.H.; Nash, T.E. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 2018, 66, 1159–1163. [Google Scholar] [CrossRef]
- Garcia, H.H.; Gonzales, I.; Lescano, A.G.; Bustos, J.A.; Zimic, M.; Escalante, D.; Saavedra, H.; Gavidia, M.; Rodriguez, L.; Najar, E.; et al. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: A double-blind, randomised controlled trial. Lancet Infect. Dis. 2014, 14, 687–695. [Google Scholar] [CrossRef]
- García, H.H.; Lescano, A.G.; Gonzales, I.; Bustos, J.A.; Pretell, E.J.; Horton, J.; Saavedra, H.; Gonzalez, A.E.; Gilman, R.H.; Cysticercosis Working Group in Peru. Cysticidal efficacy of combined treatment with praziquantel and albendazole for parenchymal brain cysticercosis. Clin. Infect. Dis. 2016, 62, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.H.; Del Brutto, O.H. Neurocysticercosis: Updated concepts about an old disease. Lancet Neurol. 2005, 4, 653–661. [Google Scholar] [CrossRef]
- Keilbach, N.M.; de Aluja, A.S.; Sarti-Gutierrez, E. A programme to control taeniasis-cysticercosis (T. solium): Experiences in a Mexican village. Acta Leiden 1989, 57, 181–189. [Google Scholar] [PubMed]
- Pawlowski, Z.S. Role of chemotherapy of taeniasis in prevention of neurocysticercosis. Parasitol. Int. 2006, 55, S105–S109. [Google Scholar] [CrossRef]
- García, H.H.; Gonzalez, A.E.; Gilman, R.H. Neurocysticercosis as an eradicable cause of epilepsy: A plan and actions are needed. JAMA Neurol. 2021, 78, 1045–1046. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.H.; Gonzalez, A.E.; Tsang, V.C.; O’Neal, S.E.; Llanos-Zavalaga, F.; Gonzalvez, G.; Romero, J.; Rodriguez, S.; Moyano, L.M.; Ayvar, V.; et al. Elimination of Taenia solium transmission in northern Perú. N. Engl. J. Med. 2016, 374, 2335–2344. [Google Scholar] [CrossRef]
- Lightowlers, M.W. Vaccines for the prevention of cysticercosis. Acta Trop. 2003, 87, 129–135. [Google Scholar] [CrossRef]
- Bonnet, G.; Pizzitutti, F.; Gonzales-Guftavson, A.A.; Gabriel, S.; Pan, W.K.; Garcia, H.H.; Bistos, J.A.; Vilcez, P.; O’Neal, S.E.; Cysticercosis Working Gropu in Peru. CystiHuman: A model of human neurocysticercosis. PloS Compu. Biol. 2022, 18, e1010118. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; O’Neal, S.E.; Dorny, P.; García, H.H. Spontaneously arrested transmission of cysticercosis in a highly endemic village with a very low migration rate. Am. J. Trop. Med. Hyg. 2018, 98, 776–778. [Google Scholar] [CrossRef]
- Bustos, J.A.; Garcia, H.H.; Del Brutto, O.H. Reliability of diagnostic criteria for neurocysticercosis for patients with ventricular cystic lesions of granulomas: A systematic review. Am. J. Trop. Med. Hyg. 2017, 97, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.E.; Del Brutto, O.H.; Butman, J.A.; Corona, T.; Delgado-Escueta, A.; Duron, R.M.; Evans, C.A.W.; Gilman, R.H.; Gonzalez, A.E.; Loeb, J.A.; et al. Calcific neurocysticercosis and epileptogenesis. Neurology 2004, 62, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Bianchin, M.M.; Rodrigues Velasco, T.; Wichert-Ana, L.; Dos Santos, A.C.; Sakamoto, A.C. Understanding the association of neurocysticercosis and mesial temporal lobe epilepsy and its impact on the surgical treatment of patients with drug-resistant epilepsy. Epilepsy Behav. 2017, 76, 168–177. [Google Scholar] [CrossRef] [PubMed]
| PARENCHYMAL NEUROCYSTICERCOSIS Vesicular cysts:
Single or multiple: No need for cysticidal drug therapy. AED for seizures. Corticosteroids for patients with recurrent seizures and perilesional edema surrounding calcifications. |
| EXTRAPARENCHYMAL NEUROCYSTICERCOSIS Small cysts over convexity of cerebral hemispheres:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Brutto, O.H. Human Neurocysticercosis: An Overview. Pathogens 2022, 11, 1212. https://doi.org/10.3390/pathogens11101212
Del Brutto OH. Human Neurocysticercosis: An Overview. Pathogens. 2022; 11(10):1212. https://doi.org/10.3390/pathogens11101212
Chicago/Turabian StyleDel Brutto, Oscar H. 2022. "Human Neurocysticercosis: An Overview" Pathogens 11, no. 10: 1212. https://doi.org/10.3390/pathogens11101212
APA StyleDel Brutto, O. H. (2022). Human Neurocysticercosis: An Overview. Pathogens, 11(10), 1212. https://doi.org/10.3390/pathogens11101212






