Parasitemia and Differential Tissue Tropism in Mice Infected with Trypanosoma cruzi Isolates Obtained from Meccus phyllosoma in the State of Oaxaca, Mexico
Abstract
1. Introduction
2. Material and Methods
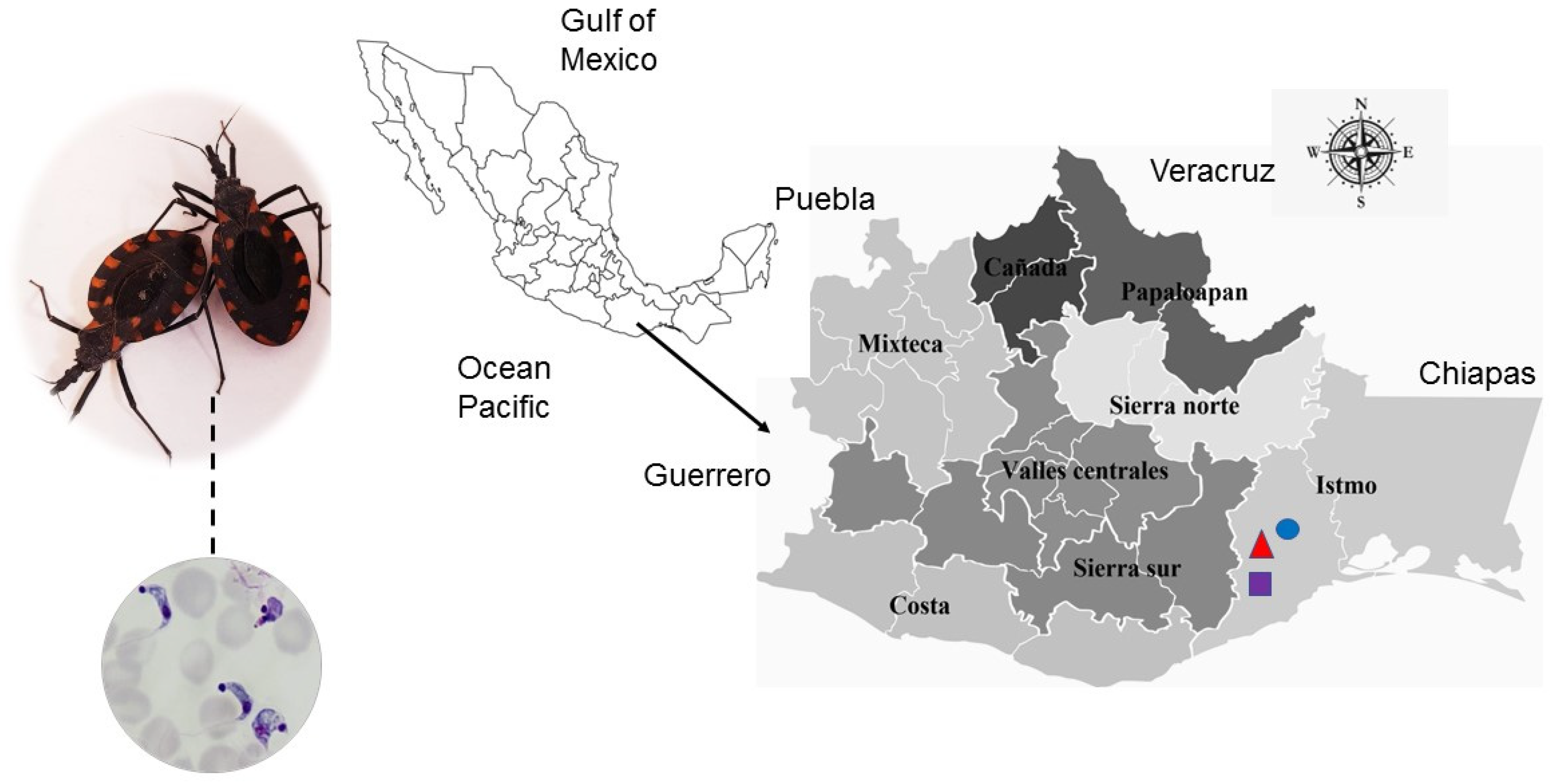
2.1. Origin of Isolates
2.2. Parasitemia
2.3. Survival
2.4. Organ Harvesting
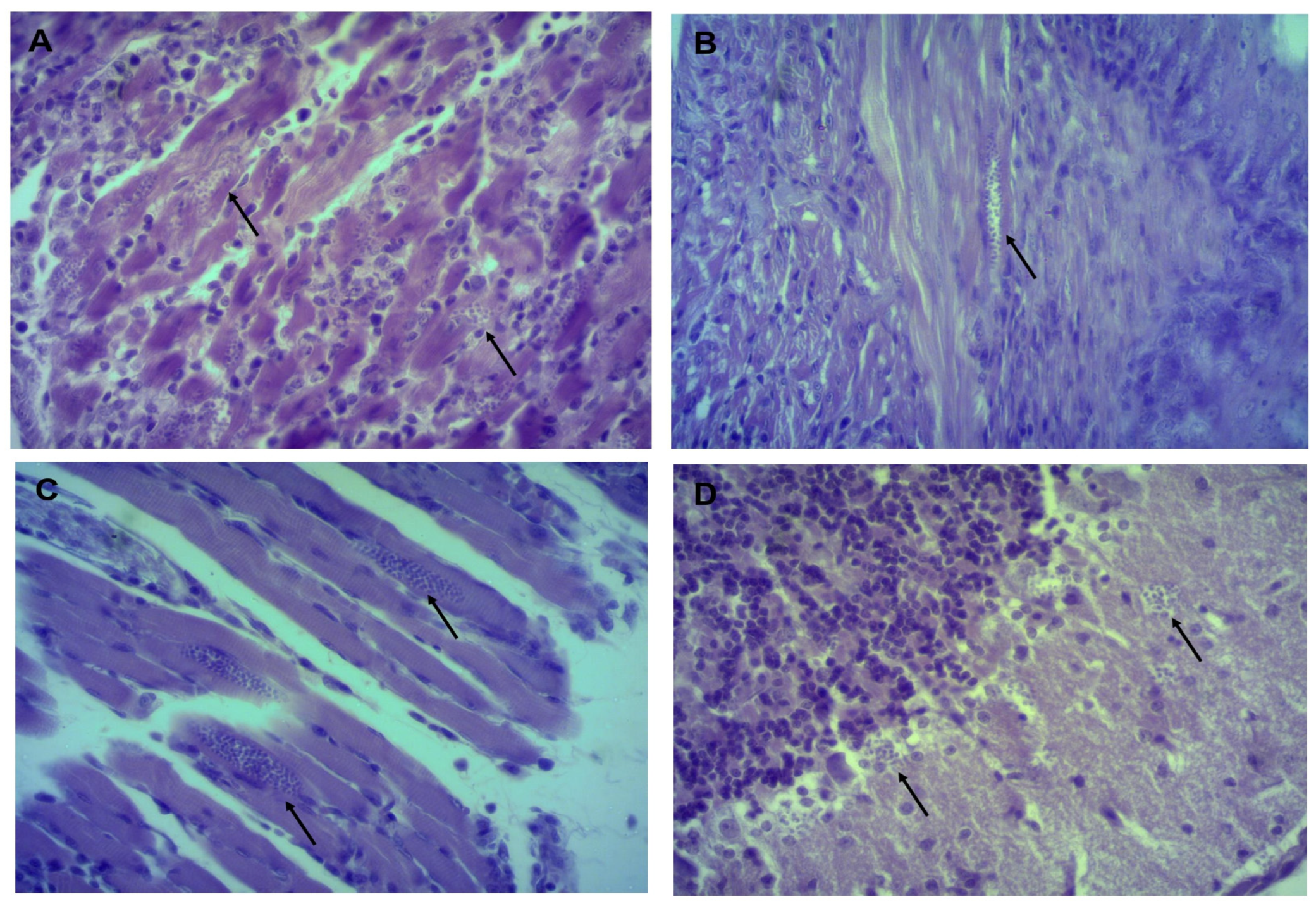
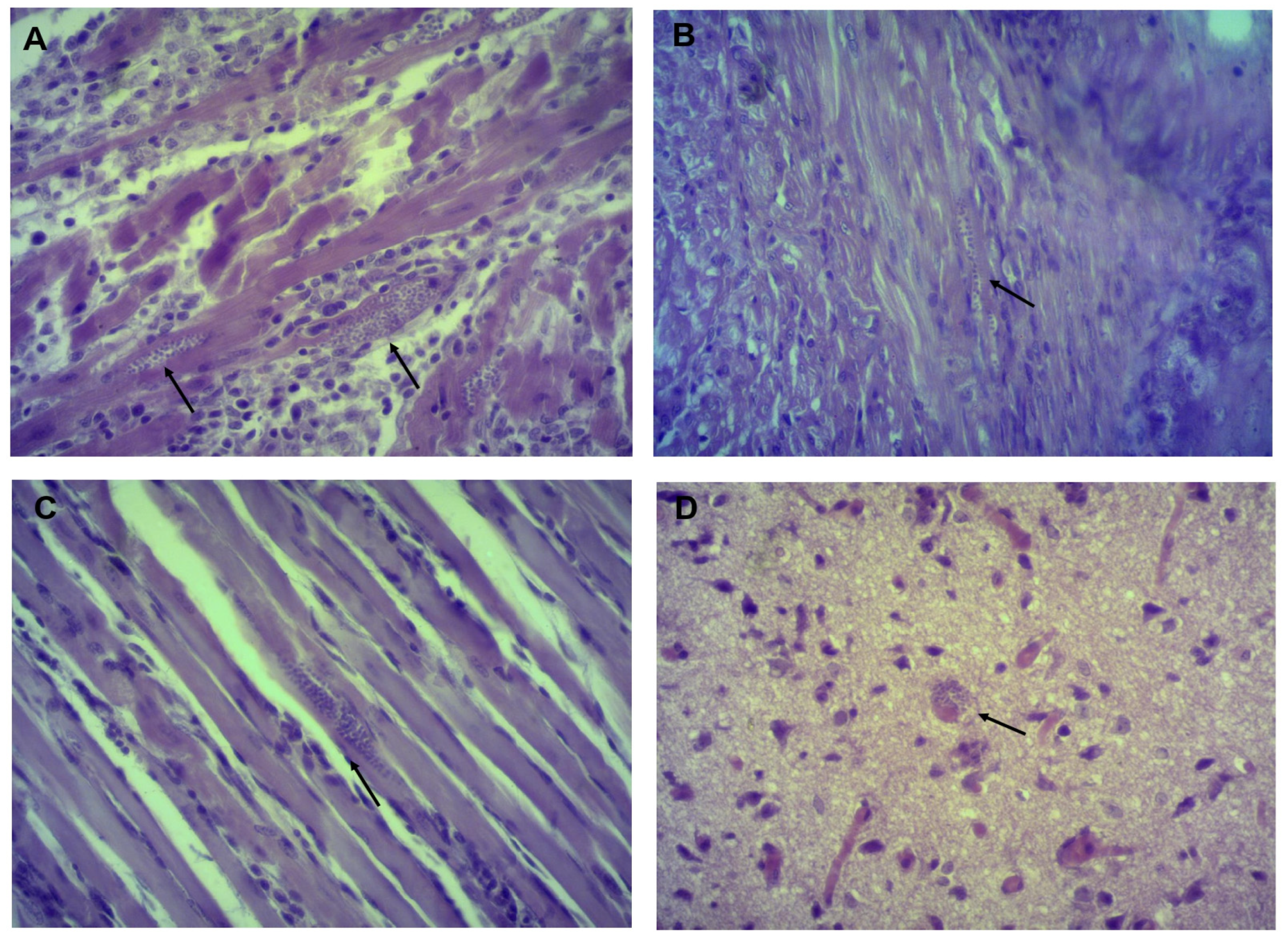
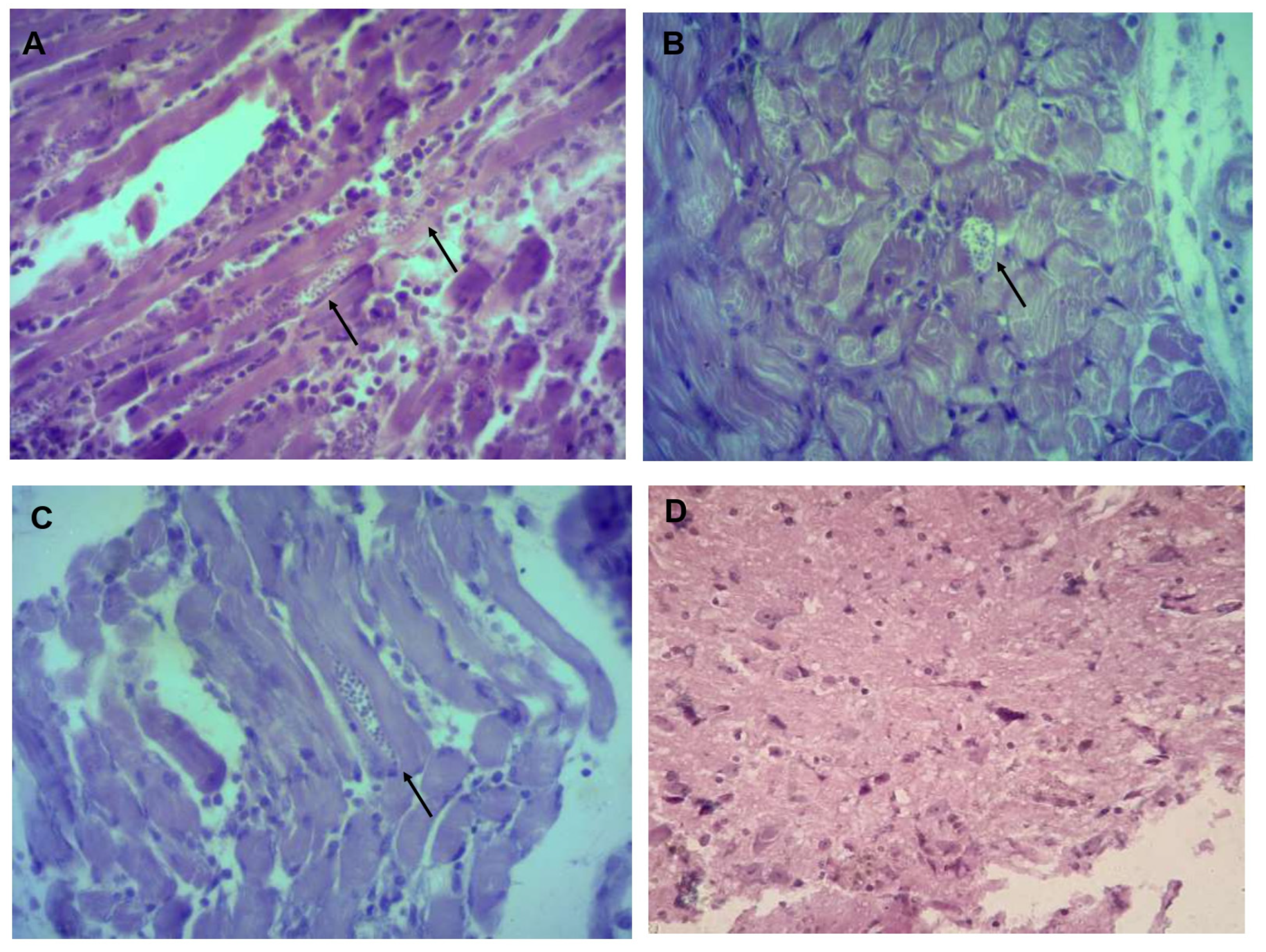
2.5. Histopathological Studies and Amastigote Nest Count
2.6. DNA Extraction
2.7. Identifying Discrete Typing Units by PCR
2.8. RNA Extraction and cDNA Synthesis
2.9. qPCR
2.10. Statistical Analysis
3. Results
3.1. Parasitemia Elicited by Isolates Obtained from Meccus phyllosoma
3.2. Survival in T. cruzi-Infected Mice
3.3. Identifying and Counting Amastigotes in Organs
3.4. Phylogenetic Identification of T. cruzi
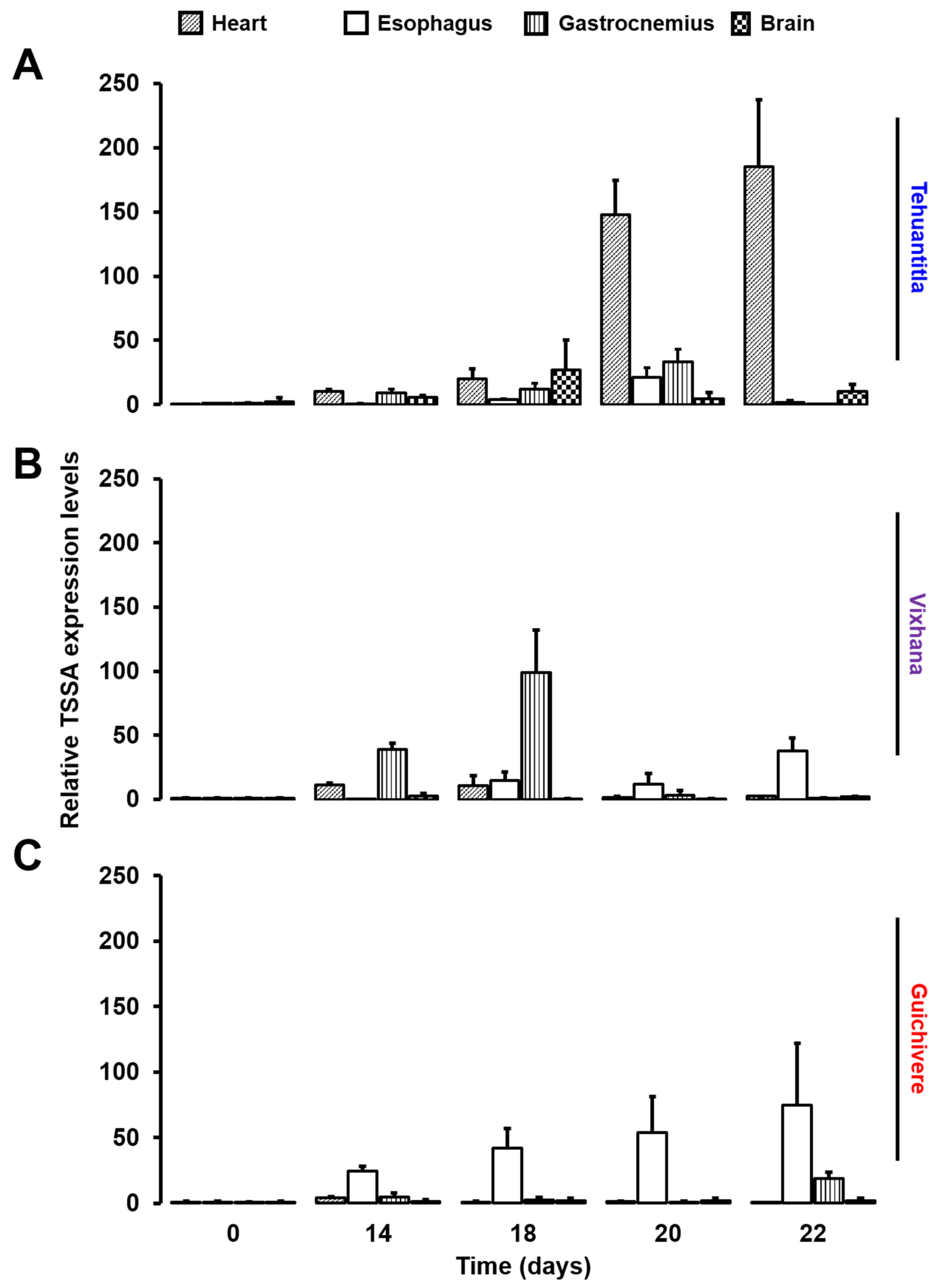
3.5. Expression of the T. cruzi Tssa Gene Reveals a Differential Tissue Tropism in the Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]
- Arnal, A.; Waleckx, E.; Rico-Chávez, O.; Herrera, C.; Dumonteil, E. Estimating the current burden of Chagas disease in Mexico: A systematic review and meta-analysis of epidemiological surveys from 2006 to 2017. PLoS Negl. Trop. Dis. 2019, 13, e0006859. [Google Scholar] [CrossRef]
- Carabarin-Lima, A.; González-Vázquez, M.C.; Rodríguez-Morales, O.; Baylón-Pacheco, L.; Rosales-Encina, J.L.; Reyes-López, P.A.; Arce-Fonseca, M. Chagas disease (American trypanosomiasis) in Mexico: An update. Acta Trop. 2013, 127, 126–135. [Google Scholar] [CrossRef]
- Secretaría de Salud (SSA). Manual de Procedimientos para la Enfermedad de Chagas en México, 1st ed.; Secretaría de Salud: Mexico City, Mexico, 2019; pp. 1–10.
- 5 Salazar-Schettino, P.M.; Tay, J.; Bucio, M.I.; de Haro, I.; Anzures, M.E.; Flores-Ayala, S. Primer caso de megaesófago con serología positiva a Trypanosoma cruzi. Salud Públ. Méx. 1984, 26, 452–455. [Google Scholar]
- Martínez-Ibarra, J.A.; Alejandre-Aguilar, R.; Torres-Morales, A.; Trujillo-García, J.C.; Nogueda-Torres, B.; Trujillo-Contreras, F. Biology of three species of the Meccus phyllosomus complex (Hemiptera: Reduviidae: Triatominae) fed on blood of hens and rabbits. Mem. Inst. Oswaldo Cruz 2006, 101, 787–794. [Google Scholar] [CrossRef]
- Carcavallo, R.U.; Jurberg, J.; Lent, H.; Noireau, F.; Galvao, C. Phylogeny of the Triatominae (Hemiptera, Reduviidae), proposals for taxonomic arrangements. Entom. Vect. 2000, 7, 1–99. [Google Scholar]
- Hypsa, V.; Tietz, D.; Zrzavy, J.; Rego, R.O.; Galvão, C.; Jurberg, J. Phylogeny and biogeography of Triatominae (Hemiptera, Reduviidae): Molecular evidence of a New World origin of the Asiatic clade. Mol. Phylog. Evol. 2012, 23, 447–457. [Google Scholar] [CrossRef]
- Cesaretto, N.R.; de Oliveira, J.; Ravazi, A.; Fernandez-Madeira, F.; dos Reis, Y.V.; Bortolozo de Oliveira, A.B.; Vicente, R.D.; Cesaretto-Cristal, D.; Galvao, C.; de Azeredo-Oliveira, M.T.V.; et al. Trends in taxonomy of Triatomini (Hemiptera, Reduviidae, Triatominae): Reproductive compatibility reinforces the synonymization of Meccus Stål, 1859 with Triatoma Laporte, 1832. Parasit. Vectors 2021, 14, 340. [Google Scholar] [CrossRef]
- Vidal-Acosta, V.; Ibáñez-Bernal, S.; Martínez-Campos, C. Infección natural de chinches Triatominae con Trypanosoma cruzi asociadas a la vivienda humana en México. Salud Públ. Méx. 2000, 42, 496–503. [Google Scholar] [CrossRef][Green Version]
- Salazar-Schettino, P.M.; Rojas-Wastavino, G.E.; Cabrera-Bravo, M.; Bucio-Torres, M.I.; Martínez-Ibarra, J.A.; Monroy-Escobar, M.C.; Rodas-Retana, A.; Guevara-Gómez, Y.; Vences-Blanco, M.O.; Ruiz-Hernández, A.L.; et al. A revision of thirteen species of Triatominae (Hemiptera: Reduviidae) vectors of Chagas disease in Mexico. J. Selva Andina Res. Soc. 2010, 1, 57–81. [Google Scholar] [CrossRef]
- Villalobos, G.; Martínez-Hernández, F.; de la Torre, P.; Laclette, J.P.; Espinoza, B. Entomological indices, feeding sources, and molecular identification of Triatoma phyllosoma (Hemiptera: Reduviidae) one of the main vectors of Chagas disease in the Istmo de Tehuantepec, Oaxaca, Mexico. Am. J. Trop. Med. Hyg. 2011, 85, 490–497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Díaz, L.; Covarrubias, K.; Licón, Á.; Astorga, M.; Moreno, Y.; Martínez, J.A. Parámetros biológicos de Meccus phyllosomus phyllosomus (Burmeister) 1835, de Triatoma recurva (Stål) 1868 (Hemiptera, Reduviidae) y de sus híbridos de laboratorio. Biomédica 2017, 37, 77–82. [Google Scholar] [CrossRef]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52. [Google Scholar] [CrossRef]
- De Fuentes-Vicente, J.A.; Vidal-López, D.G.; Flores-Villegas, A.L.; Moreno-Rodríguez, A.; De Alba-Alvarado, M.C.; Salazar-Schettino, P.M.; Rodríguez-López, M.H.; Gutiérrez-Cabrera, A.E. Trypanosoma cruzi: A review of biological and methodological factors in Mexican strains. Acta Trop. 2019, 195, 51–57. [Google Scholar] [CrossRef]
- De Fuentes-Vicente, J.A.; Cabrera-Bravo, M.; Enríquez-Vara, J.N.; Bucio-Torres, M.I.; Gutiérrez-Cabrera, A.E.; Vidal-López, D.G.; Martínez-Ibarra, J.A.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. Relationships between altitude, triatomine (Triatoma dimidiata) immune response and virulence of Trypanosoma cruzi, the causal agent of Chagas’ disease. Med. Vet. Entomol. 2017, 31, 63–71. [Google Scholar] [CrossRef]
- Salazar-Schettino, P.M.; de Haro, I.; Tay, J.; Bucio, M.I.; Robert, L. Estudio de la virulencia de cepas de Trypanosoma cruzi en el ratón blanco. Rev. Mex. Patol. Clin. 1987, 34, 105–109. [Google Scholar]
- Cámara, M.L.M.; Cánepa, G.E.; Lantos, A.B.; Balouz, V.; Yu, H.; Chen, X.; Campetella, O.; Mucci, J.; Buscaglia, C.A. The Trypomastigote Small Surface Antigen (TSSA) regulates Trypanosoma cruzi infectivity and differentiation. PLoS Negl. Trop. Dis 2017, 11, e0005856. [Google Scholar] [CrossRef] [PubMed]
- Favila-Ruiz, G.; Jiménez-Cortés, J.G.; Córdoba-Aguilar, A.; Salazar-Schettino, P.M.; Gutiérrez-Cabrera, A.E.; Pérez-Torres, A.; De Fuentes-Vicente, J.A.; Vences-Blanco, M.O.; Bucio-Torres, M.I.; Flores-Villegas, A.L.; et al. Effects of Trypanosoma cruzi on the phenoloxidase and prophenoloxidase activity in the vector Meccus pallidipennis (Hemiptera: Reduviidae). Parasit. Vectors 2018, 11, 434. [Google Scholar] [CrossRef]
- Salazar-Schettino, P.M.; Tay, J.; Navarrete, F.; Ramos, S. Comportamiento en el ratón de una cepa mexicana de Trypanosoma cruzi de peculiar virulencia. Rev. Inv. Salud Públ. 1975, 35, 37–45. [Google Scholar]
- Suárez, N.; Cabrera, R.; Cartagena, L.; Ayaqui, R. Características biológicas de una cepa de Trypanosoma cruzi en un modelo murino y análisis de supervivencia. Rev. Perú. Med. Exp. Salud Pública 2009, 26, 187–192. [Google Scholar]
- Risso, M.G.; Garbarino, G.B.; Mocetti, E.; Campetella, O.; González-Cappa, S.M.; Buscaglia, C.A.; Leguizamon, M.S. Differential expression of a virulence factor, the trans-sialidase, by the main Trypanosoma cruzi phylogenetic lineages. J. Infect. Dis. 2004, 189, 2250–2259. [Google Scholar] [CrossRef]
- Di Noia, J.M.; Buscaglia, C.A.; De Marchi, C.R.; Almeida, I.C.; Frasch, A.C. A Trypanosoma cruzi small surface molecule provides the first immunological evidence that Chagas’ disease is due to a single parasite lineage. J. Exp. Med. 2002, 195, 401–413. [Google Scholar] [CrossRef]
- Aguilar-Díaz, J.H. Caracterización Biológica e Inmunoquímica de Cuatro Aislados de Trypanosoma cruzi. Bachelor’s Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2004. [Google Scholar]
- Mendoza-Rodríguez, M. Caracterización Biológica y Bioquímica de Cuatro Aislados de Trypanosoma cruzi. Bachelor’s Thesis, Facultad de Química, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2015. [Google Scholar]
- Gómez-Hernández, C.; Rezende-Oliveira, K.; Nogueira-Nascentes, G.A.; Rocha-Batista, L.; Borges-Kappel, H.; Martínez-Ibarra, J.A.; Trujillo-Contreras, F.; Lages-Silva, E.; Ramírez, L.E. Molecular characterization of Trypanosoma cruzi Mexican strains and their behavior in the mouse experimental model. Rev. Soc. Bras. Med. Trop. 2011, 44, 684–690. [Google Scholar] [CrossRef]
- Ramsey, J.M.; Ordoñez, R.; Cruz-Celis, A.; Alvear, A.L.; Chavez, V.; Lopez, R.; Pintor, J.R.; Gama, F.; Carrillo, S. Distribution of domestic triatominae and stratification of Chagas Disease transmission in Oaxaca, Mexico. Med. Vet. Entomol. 2000, 14, 19–30. [Google Scholar] [CrossRef]
- Tay, J.; Gutiérrez-Quiroz, M.; Salazar-Schettino, P.M.; Castillo, M.; Ortega-G, M. Estudios sobre seis cepas mexicanas de Trypanosoma cruzi. Rev. Inv. Salud Pública 1973, 33, 67–76. [Google Scholar]
- De Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E.; Flores-Villegas, A.L.; Lowenberger, C.; Benelli, G.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Peña-Callejas, G.; González, J.; Jiménez-Cortés, J.G.; de Fuentes-Vicente, J.A.; Salazar-Schettino, P.M.; Bucio-Torres, M.I.; Cabrera-Bravo, M.; Flores-Villegas, A.L. Enfermedad de Chagas: Biología y Transmisión de Trypanosoma cruzi. TIP Rev. Esp. Cienc. Quím. Biol. 2022, 25, 1–19. [Google Scholar] [CrossRef]
- Buscaglia, C.A.; Di Noia, J.M. Trypanosoma cruzi clonal diversity and the epidemiology of Chagas’ disease. Microbes Infect. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Espinoza, B.; Rico, T.; Sosa, S.; Oaxaca, E.; Vizcaino-Castillo, A.; Caballero, M.L.; Martínez, I. Mexican Trypanosoma cruzi TCI strains with different degrees of virulence induce diverse humoral and cellular immune responses in a murine experimental infection model. J. Biomed. Biotechnol. 2010, 1, 890672. [Google Scholar] [CrossRef]
- Espinoza, B.; Solorzano-Domínguez, N.; Vizcaino-Castillo, A.; Martínez, I.; Elias-López, A.L.; Rodríguez-Martínez, J.A. Gastrointestinal infection with Mexican TcI Trypanosoma cruzi strains: Different degrees of colonization and diverse immune responses. Int. J. Biol. Sci. 2011, 7, 1357–1370. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Bartholomeu, D.C. Trypanosoma cruzi genetic diversity: Impact on transmission cycles and Chagas disease. Mem. Inst. Oswaldo Cruz 2022, 117, e210193. [Google Scholar] [CrossRef]
- Bosseno, M.F.; Barnabé, C.; Magallón Gastélum, E.; Lozano-Kasten, F.; Ramsey, J.; Espinoza, B.; Brenière, S.F. Predominance of Trypanosoma cruzi lineage I in Mexico. J. Clin. Microbiol. 2002, 40, 627–632. [Google Scholar] [CrossRef]
- Ramos-Ligonio, A.; Torres-Montero, J.; López-Monteon, A.; Dumonteil, E. Extensive diversity of Trypanosoma cruzi discrete typing units circulating in Triatoma dimidiata from central Veracruz, Mexico. Infect. Genet. Evol. 2012, 12, 1341–1343. [Google Scholar] [CrossRef]
- Cánepa, G.E.; Degese, M.S.; Budu, A.; Garcia, C.R.; Buscaglia, C.A. Involvement of TSSA (trypomastigote small surface antigen) in Trypanosoma cruzi invasion of mammalian cells. Biochem. J. 2012, 444, 211–218. [Google Scholar] [CrossRef]
- Balouz, V.; Melli, L.J.; Volcovich, R.; Moscatelli, G.; Moroni, S.; González, N.; Ballering, G.; Bisio, M.; Ciocchini, A.E.; Buscaglia, C.A.; et al. The Trypomastigote Small Surface Antigen from Trypanosoma cruzi Improves Treatment Evaluation and Diagnosis in Pediatric Chagas Disease. J. Clin. Microbiol. 2017, 55, 3444–3453. [Google Scholar] [CrossRef] [PubMed]




| Nomenclature | Triatomine Species and Sex | Geographic Area |
|---|---|---|
| Tehuantitla (ITRI/MX/2017/TEHUANTITLA) | M. phyllosoma Female | Tehuantitla, Tehuantepec municipality, Oaxaca 50 masl Latitude 16°19′06″ N Longitude 95°14′52″ W |
| Vixhana (ITRI/MX/2017/VIXHANA) | M. phyllosoma Female | Vixhana, Tehuantepec municipality, Oaxaca 43 masl Latitude 16°19′06″ N Longitude 95°14′52″ W |
| Guichivere (ITRI/MX/2017/GUICHIVERE) | M. phyllosoma Male | Guichivere, Tehuantepec municipality, Oaxaca 33 masl Latitude 16°19′06″ N Longitude 95°14′52″ W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Villegas, A.L.; Jiménez-Cortés, J.G.; González, J.; Moreno-Rodríguez, A.; Pérez-Cabeza de Vaca, R.; Segal-Kischinevzky, C.; Bucio-Torres, M.I.; De Fuentes-Vicente, J.A.; Nava-Lazaro, E.; Salazar-Schettino, P.M.; et al. Parasitemia and Differential Tissue Tropism in Mice Infected with Trypanosoma cruzi Isolates Obtained from Meccus phyllosoma in the State of Oaxaca, Mexico. Pathogens 2022, 11, 1141. https://doi.org/10.3390/pathogens11101141
Flores-Villegas AL, Jiménez-Cortés JG, González J, Moreno-Rodríguez A, Pérez-Cabeza de Vaca R, Segal-Kischinevzky C, Bucio-Torres MI, De Fuentes-Vicente JA, Nava-Lazaro E, Salazar-Schettino PM, et al. Parasitemia and Differential Tissue Tropism in Mice Infected with Trypanosoma cruzi Isolates Obtained from Meccus phyllosoma in the State of Oaxaca, Mexico. Pathogens. 2022; 11(10):1141. https://doi.org/10.3390/pathogens11101141
Chicago/Turabian StyleFlores-Villegas, Any Laura, Jesús Guillermo Jiménez-Cortés, James González, Adriana Moreno-Rodríguez, Rebeca Pérez-Cabeza de Vaca, Claudia Segal-Kischinevzky, Martha I. Bucio-Torres, José A. De Fuentes-Vicente, Elisabeth Nava-Lazaro, Paz María Salazar-Schettino, and et al. 2022. "Parasitemia and Differential Tissue Tropism in Mice Infected with Trypanosoma cruzi Isolates Obtained from Meccus phyllosoma in the State of Oaxaca, Mexico" Pathogens 11, no. 10: 1141. https://doi.org/10.3390/pathogens11101141
APA StyleFlores-Villegas, A. L., Jiménez-Cortés, J. G., González, J., Moreno-Rodríguez, A., Pérez-Cabeza de Vaca, R., Segal-Kischinevzky, C., Bucio-Torres, M. I., De Fuentes-Vicente, J. A., Nava-Lazaro, E., Salazar-Schettino, P. M., & Cabrera Bravo, M. (2022). Parasitemia and Differential Tissue Tropism in Mice Infected with Trypanosoma cruzi Isolates Obtained from Meccus phyllosoma in the State of Oaxaca, Mexico. Pathogens, 11(10), 1141. https://doi.org/10.3390/pathogens11101141






