A Regulatory SRNA Rli43 Is Involved in the Modulation of Biofilm Formation and Virulence in Listeria monocytogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Primers, Plasmids, Strains, and Culture Conditions
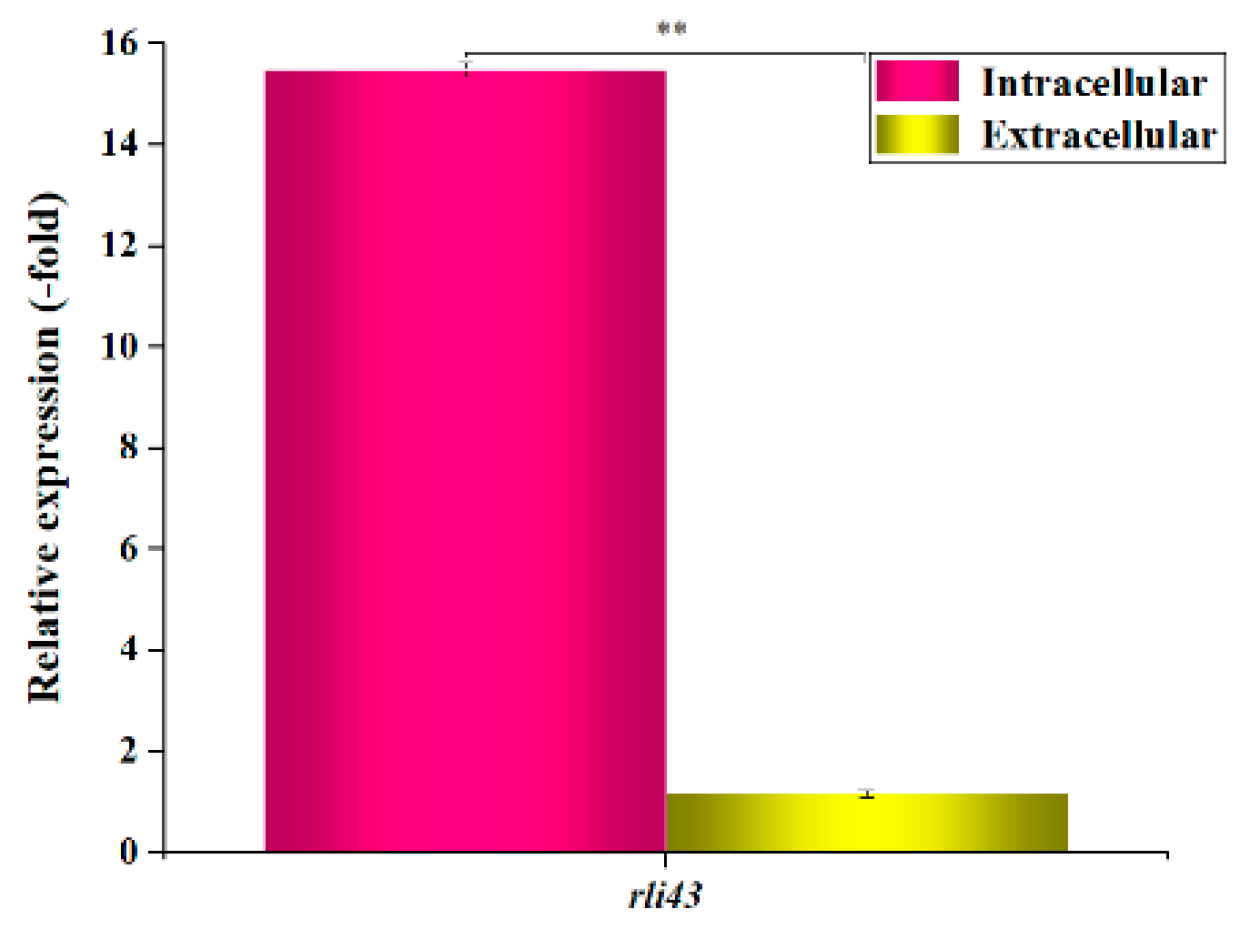
2.2. Intracellular and Extracellular Expression Profiles of Rli43 Gene
2.3. Generation of Rli43 Gene Deletion and Complementation Strains
2.4. Effects of Rli43 Gene Deletion on Responses to Environmental Stresses
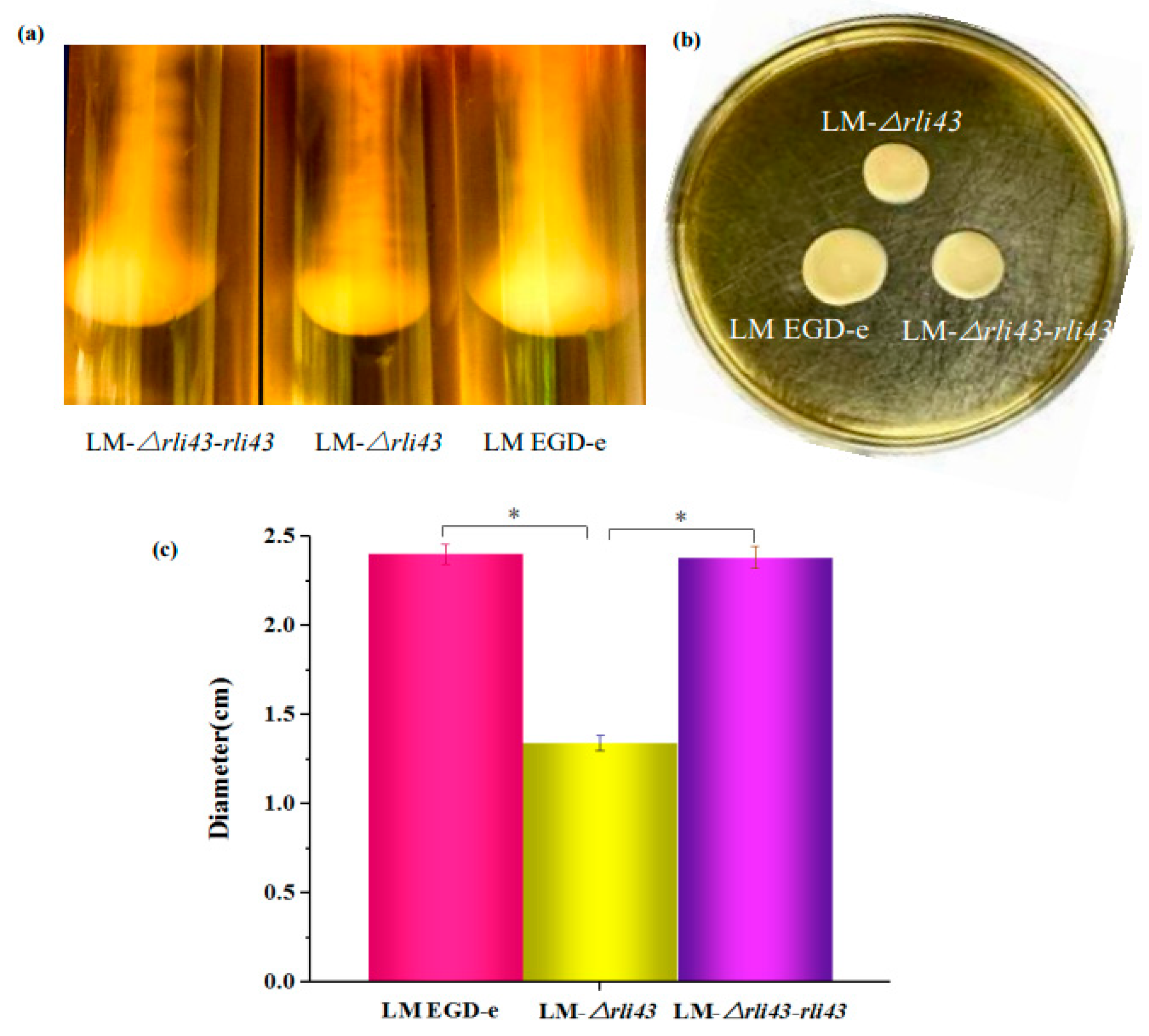
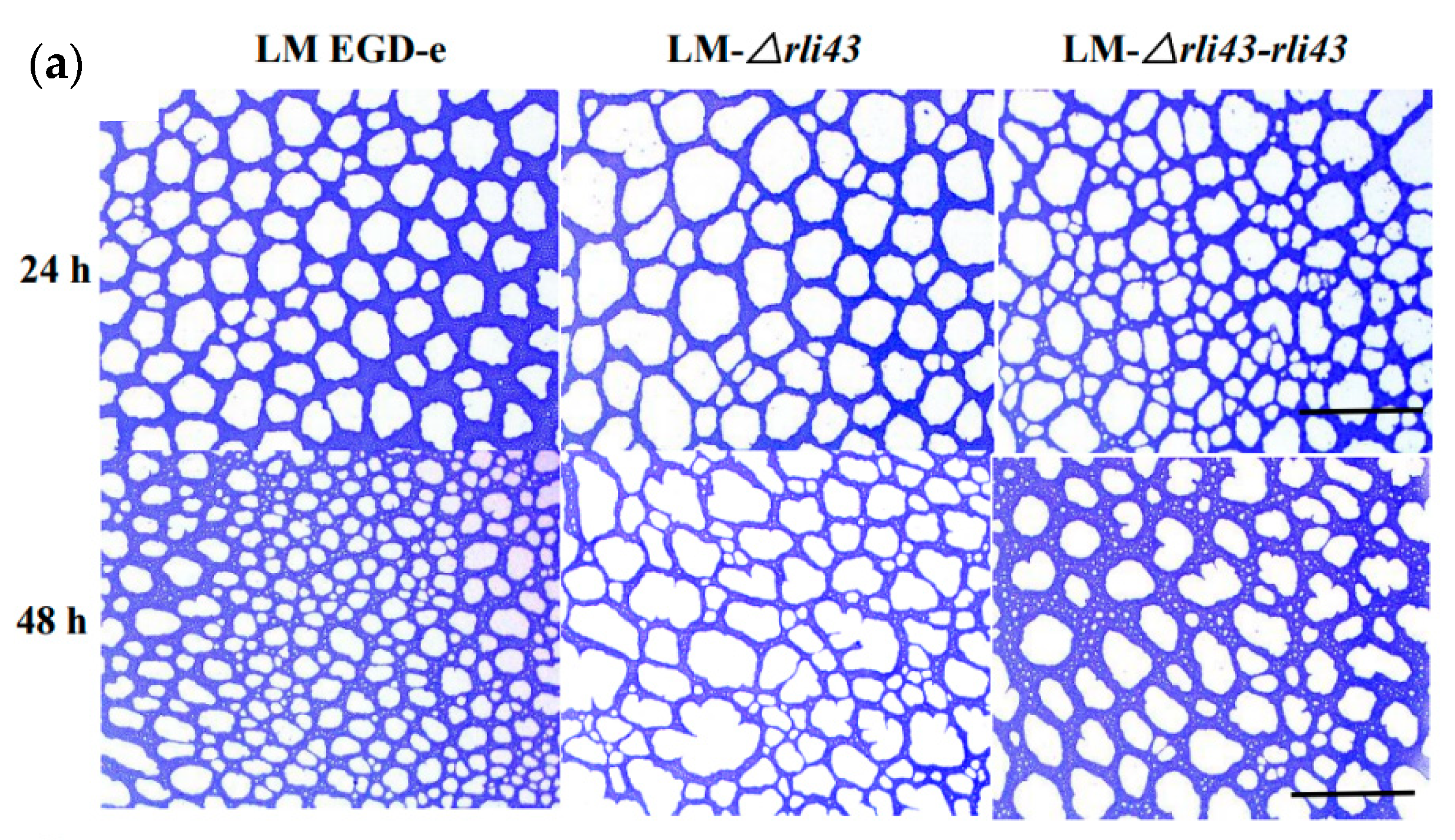
2.5. Impacts of Rli43 Gene Deletion on Motility and Biofilm Formation
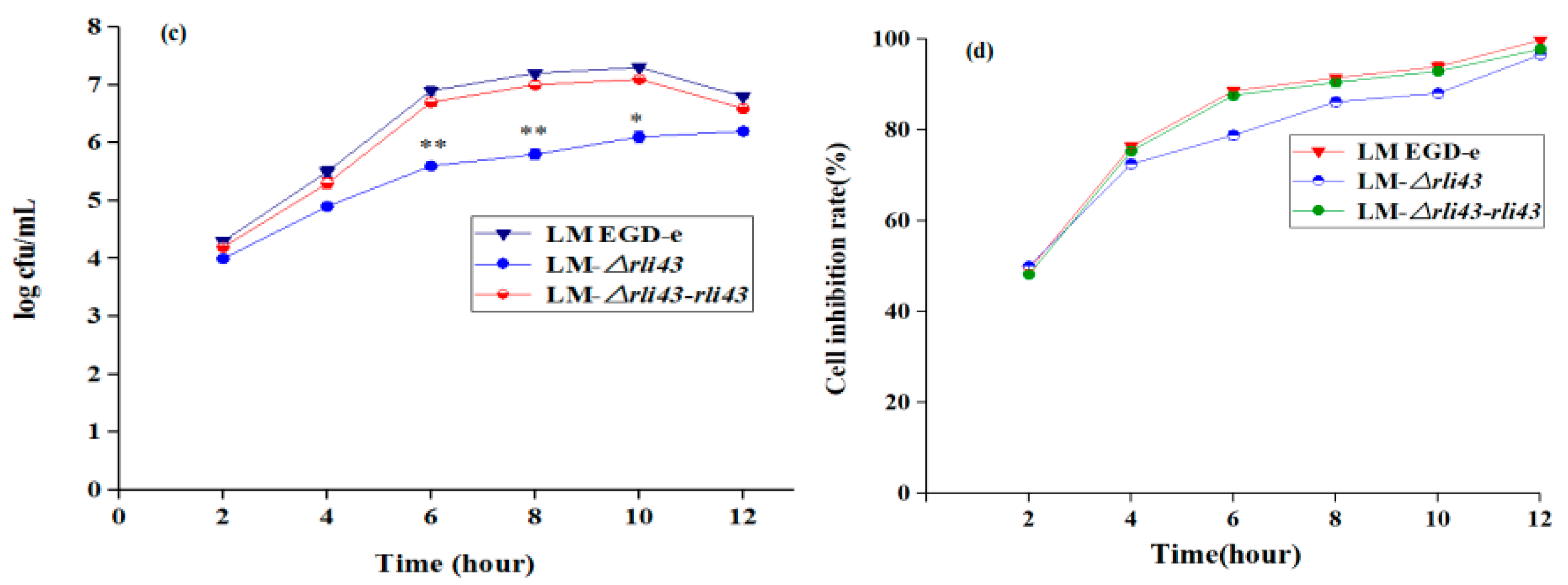
2.6. Cell Infection Experiments
2.7. Effects of Rli43 Gene Deletion on Virulence of L. monocytogenes
2.8. Determination of the Transcription Levels of Motility, Biofilm, and Virulence-Related Genes
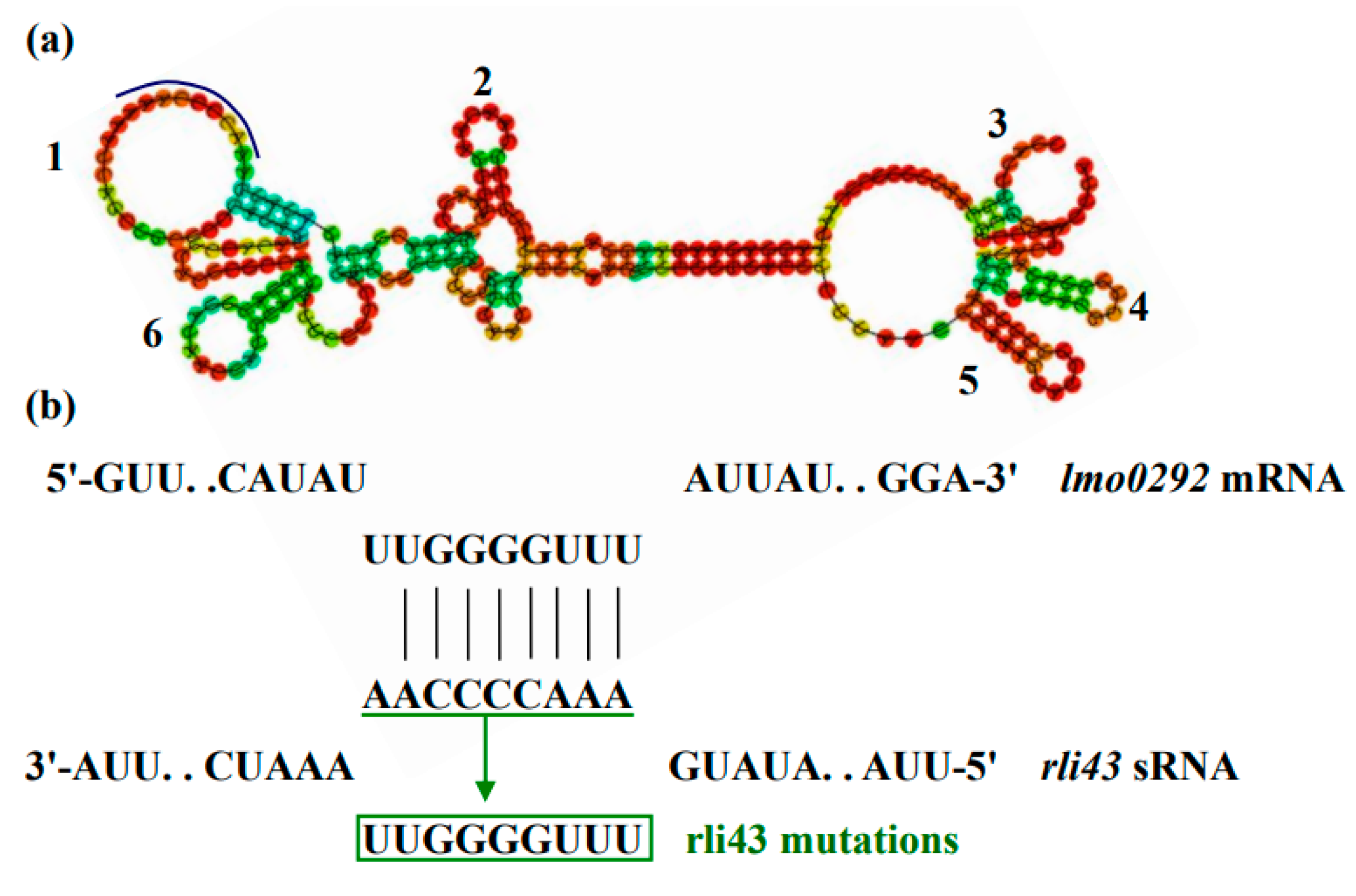
2.9. Analysis of Molecular Characteristics and Target Gene of SRNA Rli43
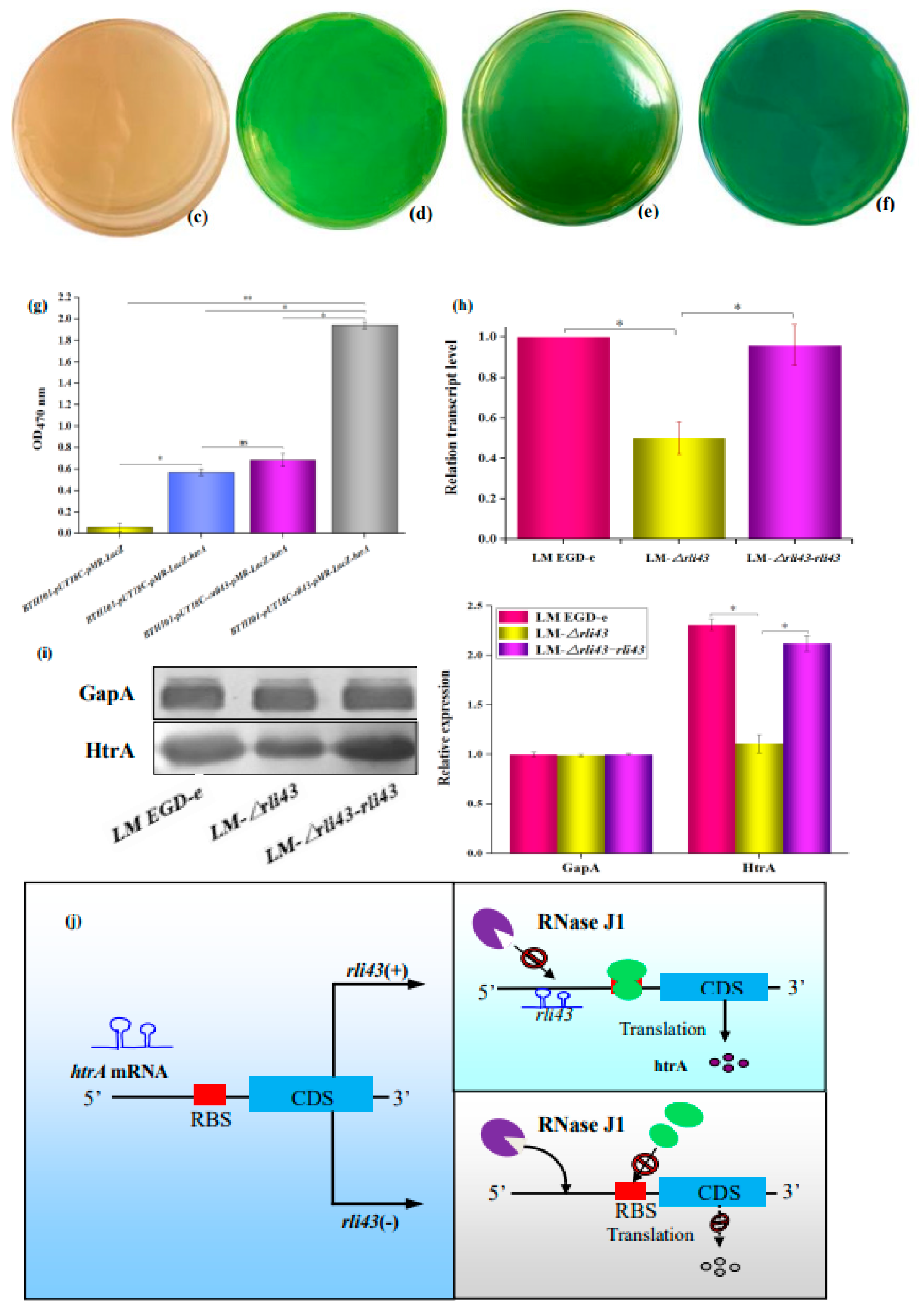
2.10. Verification of Interaction between Rli43 and Target Gene Htra Using Dual Plasmid Reporter System
2.11. Determination of the Expression Level of Target Gene Regulated by Rli43
2.12. Statistical Analysis of Data
3. Results
3.1. Expression Profiles of SRNA Rli43 Gene under Intra- and Extracellular Conditions
3.2. Deletion of Rli43 Gene Reduced Adaptation to Environmental Stress of L. monocytogenes
3.3. Deletion of Rli43 Gene Weakened L. monocytogenes Motility and Biofilm Formation
3.4. Deficiency of Rli43 Gene Impaired Adhesion, Invasion, and Intracellular Survival in Macrophage
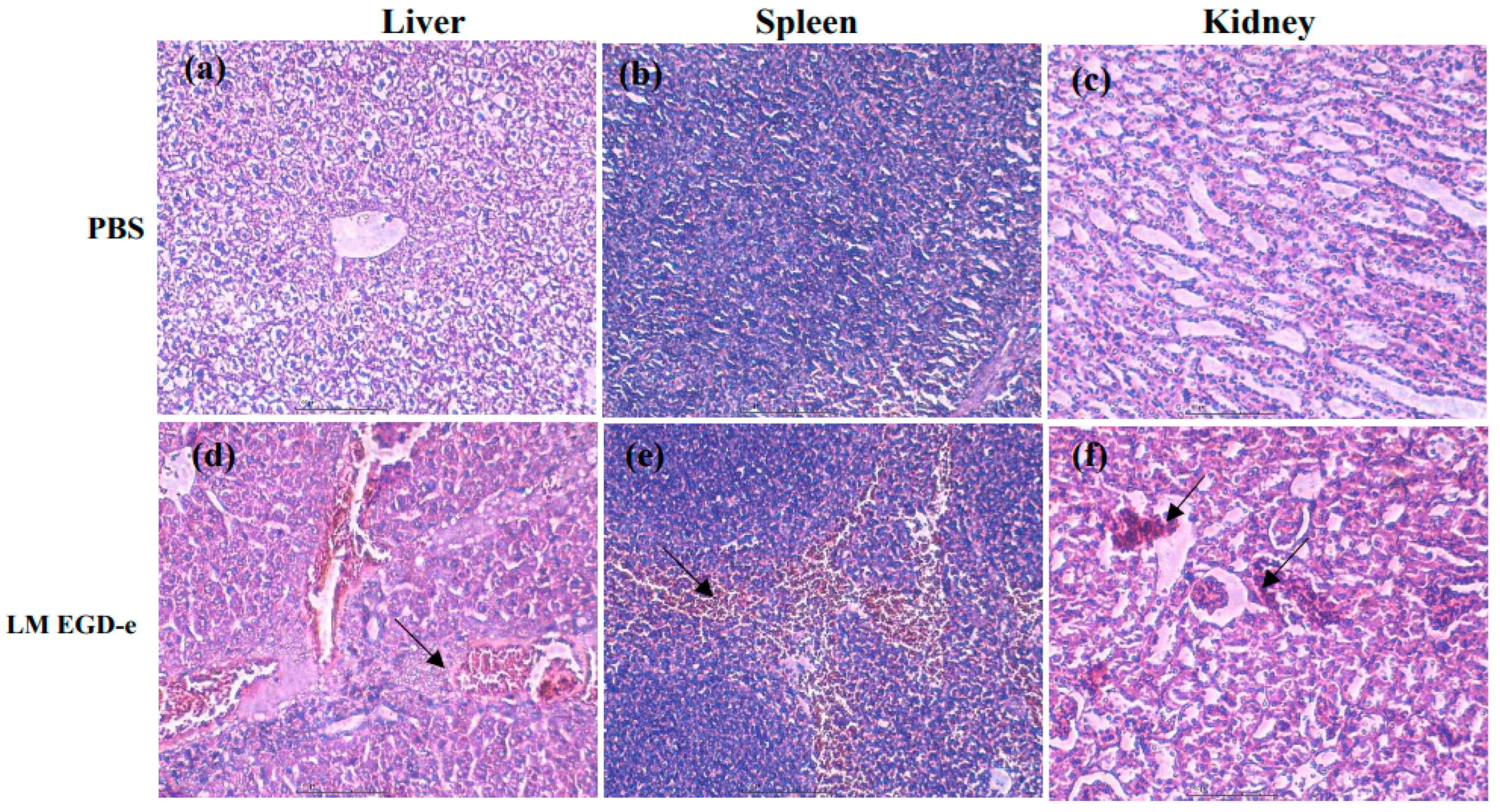
3.5. Deficiency of Rli43 Gene Weakened the Virulence of L. monocytogenes
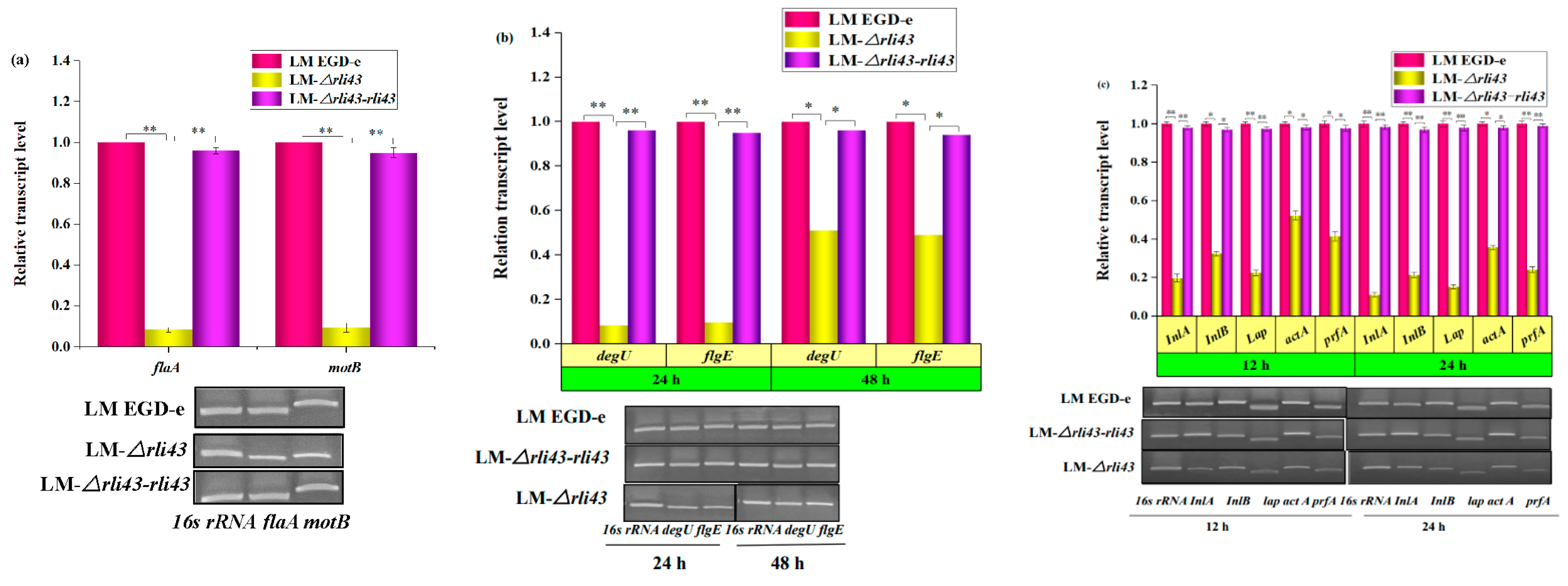
3.6. Determination of the Transcription Levels of Motility-, Biofilm-, and Virulence-Related Genes
3.7. Rli43 Can Modulate the Expression Level of Htra Gene via Targeting Its MRNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort. J. Vet. Res. 2020, 87, e1–e20. [Google Scholar] [CrossRef]
- Schonberg, A.; Gerigk, K. Listeria in effluents from the food-processing industry. Rev. Sci. Tech. 1991, 10, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.D.; Okoh, A.I. Preharvest Transmission Routes of Fresh Produce Associated Bacterial Pathogens with Outbreak Potentials: A Review. Int. J. Environ. Res. Public Health 2019, 16, 4407. [Google Scholar] [CrossRef] [PubMed]
- Oliver, H.F.; Orsi, R.H.; Ponnala, L.; Keich, U.; Wang, W.; Sun, Q.; Cartinhour, S.W.; Filiatrault, M.J.; Wiedmann, M.; Boor, K.J. Deep RNA sequencing of L. monocytogenes reveals overlapping and extensive stationary phase and sigma B-dependent transcriptomes, including multiple highly transcribed noncoding RNAs. BMC Genom. 2009, 10, 641. [Google Scholar] [CrossRef]
- Ortega, A.D.; Quereda, J.J.; Pucciarelli, M.G.; Garcia-del, P.F. Non-coding RNA regulation in pathogenic bacteria located inside eukaryotic cells. Front. Cell. Infect. Microbiol. 2014, 4, 162. [Google Scholar] [CrossRef]
- Nitzan, M.; Rehani, R.; Margalit, H. Integration of Bacterial Small RNAs in Regulatory Networks. Annu. Rev. Biophys. 2017, 46, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Mraheil, M.A.; Billion, A.; Mohamed, W.; Mukherjee, K.; Kuenne, C.; Pischimarov, J.; Krawitz, C.; Retey, J.; Hartsch, T.; Chakraborty, T.; et al. The intracellular sRNA transcriptome of Listeria monocytogenes during growth in macrophages. Nucleic Acids Res. 2011, 39, 4235–4248. [Google Scholar] [CrossRef]
- Toledo-Arana, A.; Dussurget, O.; Nikitas, G.; Sesto, N.; Guet-Revillet, H.; Balestrino, D.; Loh, E.; Gripenland, J.; Tiensuu, T.; Vaitkevicius, K.; et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature 2009, 459, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Izar, B.; Mraheil, M.A.; Hain, T. Identification and Role of Regulatory Non-Coding RNAs in Listeria monocytogenes. Int. J. Mol. Sci. 2011, 12, 5070–5079. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.L.; Meng, Q.L.; Qiao, J.; Xie, K.; Chen, C.; Liu, T.L.; Hu, Z.X.; Ma, Y.; Cai, X.P.; Chen, C.F. The Regulatory Roles of ncRNA Rli60 in Adaptability of Listeria monocytogenes to Environmental Stress and Biofilm Formation. Curr. Microbiol. 2016, 73, 77–83. [Google Scholar] [CrossRef]
- Wehner, S.; Mannala, G.K.; Qing, X.; Madhugiri, R.; Chakraborty, T.; Mraheil, M.A.; Hain, T.; Marz, M. Detection of very long antisense transcripts by whole transcriptome RNA-Seq analysis of Listeria monocytogenes by semiconductor sequencing technology. PLoS ONE 2014, 9, e108639. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdelhamed, H.; Karsi, A.; Lawrence, M.L. Efficient Gene Deletion Method for Listeria monocytogenes. Methods Mol. Biol. 2019, 2016, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Somwatcharajit, R.; Tiantad, I.; Panbangred, W. Coexpression of the silent cry2Ab27 together with cry1 genes in Bacillus thuringiensis subsp. aizawai SP41 leads to formation of amorphous crystal toxin and enhanced toxicity against Helicoverpa armigera. J. Invertebr. Pathol. 2014, 116, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Peng, X.; Liu, Y.; Wu, T.; Wang, X.; De, Y.; Han, T.; Yuan, L.; Ding, J.; Wang, C.; et al. BASI74, a Virulence-Related sRNA in Brucella abortus. Front. Microbiol. 2018, 9, 2173. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Chen, H.; Yu, S.; Qiu, X.; Song, C.; Chen, D.; Zhang, S.; Zhang, F.; He, S.; Shen, X.; et al. A SOE-PCR method of introducing multiple mutations into Mycoplasma gallisepticum neuraminidase. J. Microbiol. Methods 2013, 94, 117–120. [Google Scholar] [CrossRef]
- Taylor, A.J.; Stasiewicz, M.J. Persistent and sporadic Listeria monocytogenes strains do not differ when growing at 37 degrees C, in planktonic state, under different food associated stresses or energy sources. BMC Microbiol. 2019, 19, 257. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Ferretti, G.; Bacchetti, T.; Giuliani, A.; Pugnaloni, A.; Rippo, M.R.; Facinelli, B. Attenuation of Listeria monocytogenes Virulence by Cannabis sativa L. Essential Oil. Front. Cell. Infect. Microbiol. 2018, 8, 293. [Google Scholar] [CrossRef]
- Barzelighi, H.M.; Esfahani, B.N.; Bakhshi, B.; Daraei, B.; Moghim, S.; Fazeli, H. Influence of Heterologously Expressed azurin from Pseudomonas aeruginosa on the Adhesion and Invasion of Pathogenic Bacteria to the Caco-2 Cell Line. Probiotics Antimicrob. Proteins 2020, 12, 697–704. [Google Scholar] [CrossRef]
- Wang, X.; Ma, W.; Yin, J.; Chen, M.; Jin, H. Retraction Note to: HHIP gene overexpression inhibits the growth, migration and invasion of human liver cancer cells. J. BUON 2021, 26, 1693. [Google Scholar]
- Peng, Y.L.; Meng, Q.L.; Qiao, J.; Xie, K.; Chen, C.; Liu, T.L.; Hu, Z.X.; Ma, Y.; Cai, X.P.; Chen, C.F. The roles of noncoding RNA Rli60 in regulating the virulence of Listeria monocytogenes. J. Microbiol. Immunol. Infect. 2016, 49, 502–508. [Google Scholar] [CrossRef]
- Kun, X.; Qingling, M.; Qiao, J.; Yelong, P.; Tianli, L.; Cheng, C.; Yu, M.; Zhengxiang, H.; Xuepeng, C.; Chuangfu, C. Impact of rli87 gene deletion on response of Listeria monocytogenes to environmental stress. FEMS Microbiol. Lett. 2014, 359, 50–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cohen-Berneron, J.; Steinberg, D.; Featherstone, J.D.; Feuerstein, O. Sustained effects of blue light on Streptococcus mutans in regrown biofilm. Lasers Med. Sci. 2016, 31, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.; Scheller, C.; Krebs, F.; Watzig, H.; Oltmann-Norden, I. A comparative study of CE-SDS, SDS-PAGE, and Simple Western: Influences of sample preparation on molecular weight determination of proteins. Electrophoresis 2021, 42, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, H.A.; Raghavan, R. Origin, Evolution, and Loss of Bacterial Small RNAs. Microbiol. Spectr. 2018, 6, 10. [Google Scholar] [CrossRef]
- Lewis, C.; Skovierova, H.; Rowley, G.; Rezuchova, B.; Homerova, D.; Stevenson, A.; Spencer, J.; Farn, J.; Kormanec, J.; Roberts, M. Salmonella enterica Serovar Typhimurium HtrA: Regulation of expression and role of the chaperone and protease activities during infection. Microbiology 2009, 155, 873–881. [Google Scholar] [CrossRef]
- Ibrahim, Y.M.; Kerr, A.R.; McCluskey, J.; Mitchell, T.J. Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect. Immun. 2004, 72, 3584–3591. [Google Scholar] [CrossRef]
- Foucaud-Scheunemann, C.; Poquet, I. HtrA is a key factor in the response to specific stress conditions in Lactococcus lactis. FEMS Microbiol. Lett. 2003, 224, 53–59. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, T.; Li, Y.; Bai, X.; Yan, X.; Gao, Y.; Shi, Q.; Zhu, G. Contribution of the serine protease HtrA in Escherichia coli to infection in foxes. Microb. Pathog. 2019, 135, 103570. [Google Scholar] [CrossRef]
- Zarzecka, U.; Modrak-Wojcik, A.; Figaj, D.; Apanowicz, M.; Lesner, A.; Bzowska, A.; Lipinska, B.; Zawilak-Pawlik, A.; Backert, S.; Skorko-Glonek, J. Properties of the HtrA Protease From Bacterium Helicobacter pylori Whose Activity Is Indispensable for Growth Under Stress Conditions. Front. Microbiol. 2019, 10, 961. [Google Scholar] [CrossRef]
- Wilson, R.L.; Brown, L.L.; Kirkwood-Watts, D.; Warren, T.K.; Lund, S.A.; King, D.S.; Jones, K.F.; Hruby, D.E. Listeria monocytogenes 10403S HtrA is necessary for resistance to cellular stress and virulence. Infect. Immun. 2006, 74, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Wonderling, L.D.; Wilkinson, B.J.; Bayles, D.O. The htrA (degP) gene of Listeria monocytogenes 10403S is essential for optimal growth under stress conditions. Appl. Environ. Microbiol. 2004, 70, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.Y.; Liu, C.; Xiao, Q.T.; Sun, S.; Zou, Q.M.; Li, H.B. HtrA family proteases of bacterial pathogens: Pros and cons for their therapeutic use. Clin. Microbiol. Infect. 2021, 27, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Qin, Z.; Chang, Y.; Liu, J.; Malkowski, M.G.; Shipa, S.; Li, L.; Qiu, W.; Zhang, J.R.; Li, C. Analysis of a flagellar filament cap mutant reveals that HtrA serine protease degrades unfolded flagellin protein in the periplasm of Borrelia burgdorferi. Mol. Microbiol. 2019, 111, 1652–1670. [Google Scholar] [CrossRef]
- Hoy, B.; Brandstetter, H.; Wessler, S. The stability and activity of recombinant Helicobacter pylori HtrA under stress conditions. J. Basic Microbiol. 2013, 53, 402–409. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lemos, J.A.; Burne, R.A. Role of HtrA in growth and competence of Streptococcus mutans UA159. J. Bacteriol. 2005, 187, 3028–3038. [Google Scholar] [CrossRef]
- Chao, Y.; Bergenfelz, C.; Sun, R.; Han, X.; Achour, A.; Hakansson, A.P. The serine protease HtrA plays a key role in heat-induced dispersal of pneumococcal biofilms. Sci. Rep. 2020, 10, 22455. [Google Scholar] [CrossRef]
- Purdy, G.E.; Hong, M.; Payne, S.M. Shigella flexneri DegP facilitates IcsA surface expression and is required for efficient intercellular spread. Infect. Immun. 2002, 70, 6355–6364. [Google Scholar] [CrossRef]
- Elzer, P.H.; Phillips, R.W.; Robertson, G.T.; Roop, R.N. The HtrA stress response protease contributes to resistance of Brucella abortus to killing by murine phagocytes. Infect. Immun. 1996, 64, 4838–4841. [Google Scholar] [CrossRef]
- Li, S.R.; Dorrell, N.; Everest, P.H.; Dougan, G.; Wren, B.W. Construction and characterization of a Yersinia enterocolitica O:8 high-temperature requirement (htrA) isogenic mutant. Infect. Immun. 1996, 64, 2088–2094. [Google Scholar] [CrossRef]
- Boehm, M.; Simson, D.; Escher, U.; Schmidt, A.M.; Bereswill, S.; Tegtmeyer, N.; Backert, S.; Heimesaat, M.M. Function of Serine Protease HtrA in the Lifecycle of the Foodborne Pathogen Campylobacter jejuni. Eur. J. Microbiol. Immunol. 2018, 8, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Wen, Y.; Lau, G.W.; Huang, X.; Wu, R.; Yan, Q.; Huang, Y.; Zhao, Q.; Ma, X.; et al. HtrA Is Important for Stress Resistance and Virulence in Haemophilus parasuis. Infect. Immun. 2016, 84, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Cortes, G.; de Astorza, B.; Benedi, V.J.; Alberti, S. Role of the htrA gene in Klebsiella pneumoniae virulence. Infect. Immun. 2002, 70, 4772–4776. [Google Scholar] [CrossRef] [PubMed]
- Simson, D.; Boehm, M.; Backert, S. HtrA-dependent adherence and invasion of Campylobacter jejuni in human vs. avian cells. Lett. Appl. Microbiol. 2020, 70, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Pallen, M.J.; Wren, B.W. The HtrA family of serine proteases. Mol. Microbiol. 1997, 26, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.K.; Freitag, N.E. Secretion Chaperones PrsA2 and HtrA Are Required for Listeria monocytogenes Replication following Intracellular Induction of Virulence Factor Secretion. Infect. Immun. 2016, 84, 3034–3046. [Google Scholar] [CrossRef]
- Stack, H.M.; Sleator, R.D.; Bowers, M.; Hill, C.; Gahan, C.G. Role for HtrA in stress induction and virulence potential in Listeria monocytogenes. Appl. Environ. Microbiol. 2005, 71, 4241–4247. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Srivastava, S. Small RNA-mediated regulation in bacteria: A growing palette of diverse mechanisms. Gene 2018, 656, 60–72. [Google Scholar] [CrossRef] [PubMed]





| Primer Names | Primer Sequences (5′→3′) | |
|---|---|---|
| rli43 F | TTATTTGCAATTTTTCTCAATAAGTA | RT-PCR/qRT-PCR |
| rli43 R | ACCATCCCGCGCCATTTA | |
| R1 | CGGGGTACCCACAAAAGAGGATTTTCATATACTTCC | SOE-PCR |
| R2 | GCAGAAAGTTGGGTATGACTATAGAAAAAAAGCCTTGTCA | |
| R3 | TGACAAGGCTTTTTTTCTATAGTCATACCCAACTTTCTGC | |
| R4 | AAAACTGCAGCCTACGTGGTAAAGCGGCTC | |
| P1 | CCCAAGCTTTTATTTGCAATTTTTCTCAATAAGT | Complementation strains |
| P2 | GGAATTCTAAATGGCGCGGGATGGT | |
| 16S rRNA F | GAGCTAATCCCATAAAACTATTCTCA | qRT-PCR |
| 16S rRNA R | ACCTTGTTACGACTTCACCCC | |
| flaA F | AACAAGCAACTGAAGCTATTGATGAATT | |
| flaA R | TGCGGTGTTTGGTTTGCTTGA | |
| motB F | AATCGCCAAAGAAATCGGCG | |
| motB R | CGCCGGGGTTTACTTCACTA | |
| flgE F | AATGCCAACACGACAGGATA | |
| flgE R | TTTGTTCCAGCGTAAAGTCC | |
| degU F | GAGGTCGTAGCGGAAGCTGA | |
| degU R | CTGTTACATATTCATCTGTATCA | |
| InlB F | CTGAAAAAAATGGTGGGCATGAG | |
| InlB R | CCGCCATTTCGGGCTTCTCT | |
| PrfA F | ACGGGAAGCTTGGCTCTATT | |
| PrfA R | TGCGATGCCACTTGAATATC | |
| Lap F | GCGGCCCAAGAAGTATTAGAA | |
| Lap R | CTGTCGCGAAGATGTTTTTAATACA | |
| InlA F | TGTGACTGGCGCTTTAATTG | |
| InlA R | TCCAATAGTGACAGGTTGGCTA | |
| actA F | ATGCGGGGAAATGGGTACGT | |
| actA R | CCAAGAAGCATTGGTGTCTCTGG | |
| P3 | GGATCCAACATTCTCCACTCCTTAAAAA | Dual plasmid reporter system |
| P4 | GGTACCATCATTTAACGGCTGAAACCCT | |
| P5 | CCAAGCTTGCTTTTTCATCGTTTTTTCA | |
| P6 | GGGGTACCATGTCTAGCAGGTTCTGATTCT | |
| P7 | GGATCCCATTCTCCACTCCTTAAAAATA | |
| P8 | AACTACATATATGTTTGGGGTTAAATCAGCTGCC | |
| P9 | GGCAGCTGATTTAACCCCAAACATATATGTAGTT | |
| P10 | GGGGTACCCAAGGGTTTCAGCCGTTAAATGA | |
| P11 | ATGGACGAGAAAGAAAAGAATT | |
| P12 | AATAACACCAATAAGTGCTGTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ji, C.; Xia, X.; Cai, X.; Meng, Q.; Qiao, J. A Regulatory SRNA Rli43 Is Involved in the Modulation of Biofilm Formation and Virulence in Listeria monocytogenes. Pathogens 2022, 11, 1137. https://doi.org/10.3390/pathogens11101137
Wang L, Ji C, Xia X, Cai X, Meng Q, Qiao J. A Regulatory SRNA Rli43 Is Involved in the Modulation of Biofilm Formation and Virulence in Listeria monocytogenes. Pathogens. 2022; 11(10):1137. https://doi.org/10.3390/pathogens11101137
Chicago/Turabian StyleWang, Lixia, Chunhui Ji, Xianzhu Xia, Xuepeng Cai, Qingling Meng, and Jun Qiao. 2022. "A Regulatory SRNA Rli43 Is Involved in the Modulation of Biofilm Formation and Virulence in Listeria monocytogenes" Pathogens 11, no. 10: 1137. https://doi.org/10.3390/pathogens11101137
APA StyleWang, L., Ji, C., Xia, X., Cai, X., Meng, Q., & Qiao, J. (2022). A Regulatory SRNA Rli43 Is Involved in the Modulation of Biofilm Formation and Virulence in Listeria monocytogenes. Pathogens, 11(10), 1137. https://doi.org/10.3390/pathogens11101137





