Abstract
Hepatitis B virus (HBV) is a hepatotropic virus with the potential to cause chronic infection, and it is one of the common causes of liver disease worldwide. Chronic HBV infection leads to liver cirrhosis and, ultimately, hepatocellular carcinoma (HCC). The persistence of covalently closed circular DNA (cccDNA) and the impaired immune response in patients with chronic hepatitis B (CHB) has been studied over the past few decades. Despite advances in the etiology of HBV and the development of potent virus-suppressing regimens, a cure for HBV has not been found. Both the innate and adaptive branches of immunity contribute to viral eradication. However, immune exhaustion and evasion have been demonstrated during CHB infection, although our understanding of the mechanism is still evolving. Recently, the successful use of an antiviral drug for hepatitis C has greatly encouraged the search for a cure for hepatitis B, which likely requires an approach focused on improving the antiviral immune response. In this review, we discuss our current knowledge of the immunopathogenic mechanisms and immunobiology of HBV infection. In addition, we touch upon why the existing therapeutic approaches may not achieve the goal of a functional cure. We also propose how combinations of new drugs, and especially novel immunotherapies, contribute to HBV clearance.
1. Introduction
Hepatitis B virus (HBV) is a prototypical member of the Hepadnaviridae family. HBV is a hepatotropic virus that generates covalently closed circular (ccc) DNA, a plasmid-like episome, in the nucleus of host cells [1,2,3]. According to the estimated number of the world’s population with serological evidence of current or past HBV infection, around 2 billion people may have been infected with HBV at some point in their life [4,5,6]. Many of these infections are acquired in infancy or early childhood and could lead to chronic hepatitis B (CHB), which is highly prevalent in some parts of Africa and Asia [6,7]. There are more than 250 million individuals infected with CHB worldwide (at the time of writing) [7]. Furthermore, ~700,000 deaths per year are caused by complications of persistent HBV infection, liver cirrhosis, as well as hepatocellular carcinoma (HCC) [2,8,9,10,11,12]. In China, the burden of HBV is considerable [13,14]. It was reported that, in 21–49-year-old men, the seroprevalence was ~6% [15]. For younger individuals (aged between 1 and 29 years old), the reported incidence was ~2.6% [16]. This lower figure can be attributed to the success of vaccination policy, which caused the seroprevalence of hepatitis B surface antigen (HBsAg) in younger people to decline rapidly.
In patients with CHB, a high HBV load, and serum hepatitis B e antigen (HBeAg) and HBsAg levels may play a key role in the impaired antiviral immune response [17]. However, the mechanism has not been fully explored for various reasons. Preventive treatments, including a prophylactic vaccine, have a significant effect on HBV control [18]. However, the vaccine does not appear to be beneficial for people with existing CHB. In addition, current hepatitis B therapies are limited to immunoregulatory drugs, including IFN-α, or several direct-acting antivirals (DAAs) such as tenofovir and entecavir (ETV) [10]. Moreover, the drugs rarely achieve HBV clearance from the liver, meaning that the majority of patients need lifelong treatment [19]. The target of any new treatment regimen is to increase the possibility of a functional cure [20]. Current therapies have improved the quality of life and the survival of patients with CHB, and reduced the incidence of HCC, cirrhosis, and other complications through the suppression of HBV replication and/or by reducing hepatic necroinflammation. However, the ultimate goal of a functional cure is not frequently achieved.
In this review, we have summarized the key milestones of HBV research that has been performed over the last 30 years and focused on recent findings relating to advances in the etiology of HBV and immunologic assumptions. As the important challenge of achieving an HBV functional cure is likely to be overcome by improving the HBV-specific immune response, we have also reviewed the current strategies aimed at restoring the function of HBV-specific immune cells.
2. The Etiology for Hepatitis B
In China, prior to vaccination, hepatitis B was typically spread through vertical transmission. Nearly 20% of HBsAg-positive families contain at least two HBsAg carriers [13,21]. In Asia, since hepatitis B occurs at an early age, CHB and viral persistence seem more frequent, complicating the selection of effective treatment options. The HBV genotypes vary across different geographical regions. In Europe and the United States, genotypes A and D are the most frequently occurring HBV genotypes, while genotypes B and C are predominately found in China [22]. Many important factors, such as the genotype, the age of the infected individual, as well as the stage of the disease, could influence the immune response to therapy.
The entry of HBV into host cells is a complex process. Parenchymal liver cells are susceptible to infection upon HBV entering the circulatory system. Features of human liver microcirculation, including slow blood flow, have been demonstrated to increase the possibility of HBV interacting with the sodium taurocholate co-transporting polypeptide (NTCP), which is expressed on the surface of hepatocytes. This interaction is thought to initiate viral entry into host cells and its subsequent replication [23,24,25]. Additionally, platelets are always recruited to the liver microcirculation after viral infection. Their activation correlates with severely reduced microcirculation, and delayed viral elimination. Lack of platelet-produced serotonin contributes to accelerating viral clearance in the liver.
HBV then spreads rapidly throughout the liver. Studies performed in chimpanzees verified that, during the initial spreading stage, HBV can easily escape recognition by the innate immune system. This immune escape is probably due to the unique replication strategy of HBV, which involves the cccDNA molecule [2]. Furthermore, cccDNA has been confirmed to be the source of the circulating antigens HBsAg and HBeAg in human peripheral blood. This replicative feature enables HBV to produce a viral load of over a billion particles per milliliter [17]. Moreover, studies of human liver biopsies did not find significant innate immune responses in the early phase of CHB. In addition, it was reported that HBV may have evolved a hidden strategy to evade recognition by the innate immune system and rapidly infect and replicate in the hepatocytes [26,27].
HBV DNA is considered to be a major biomarker of viral replication and has been regarded as an important endpoint of clinical trials using nucleoside analog therapy [28,29]. Hepatitis B virions have an envelope containing three viral gene products, including HBsAg determinant [2,30]. The HBV envelope has been found to enclose an inner nucleocapsid particle, which in most virions, is composed of 120 core protein (i.e., HBcAg) homodimers. Furthermore, it has been shown that the nucleocapsid particles include a copy of a partially double-stranded relaxed circular DNA (rcDNA) genome (Figure 1) [31,32]. Approximately 10% of the nucleocapsid particles are thought to contain double-stranded linear DNA (dslDNA) in place of an rcDNA genome [2,33,34]. After HBV entry, the nucleocapsids (with their rcDNA) are transported into the nucleus, where the host enzymes participate in the repair of the viral genome and its conversion into the cccDNA [35,36,37].
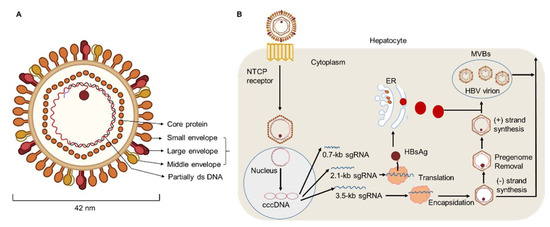
Figure 1.
HBV particle and life cycle. (A) Hepatitis B virions are about 42 nm in diameter. The envelope of HBV virion contains three forms of HBsAg: large (L), middle (M), and small (S) envelope proteins. The capsid encapsidates a partially double stranded (ds) DNA. The HBV envelope has an inner nucleocapsid particle that always consists of 120 core protein. (B) Firstly, HBV attaches to the host cell membrane through its envelope proteins and the sodium taurocholate co-transporting polypeptide (NTCP). Next, the viral genome reaches the cytoplasm of hepatocytes and enters the nucleus, where host enzymes will repair the genome into the covalently closed circular DNA (cccDNA). In addition, transcription and nuclear export of mRNA to the hepatocellular cytoplasm for translation are observed. HBsAg are produced via the endoplasmic reticulum (ER)-Golgi complex and then assembled in the cytoplasm, while HBV virions are formed by budding from multivesicular bodies (MVBs). The new virions will exit the host and infect new hepatocytes.
3. Mechanisms of HBV Immune Evasion
HBV can avoid elimination by the immune system via a process called immune evasion, which is a major concern in CHB. There are various mechanisms of HBV immune evasion, including reduced TNF-α production by T cells and Kupffer cells, impaired IFN-α production by plasmacytoid DC (pDC) cells, low induction of interferon-stimulated genes (ISGs), inhibition of TLR signaling [38,39,40,41,42,43,44,45,46], and so on. Although immune evasion may implicate both the innate and adaptive branches of immunity, the exact mechanisms remain unknown.
3.1. Evasion of the Adaptive Immune Response
Over the last 30 years, numerous studies in humans and animal models have demonstrated that the outcome of HBV infection is strongly determined by the dynamics and the effector functions of the HBV-specific adaptive immune response. Adaptive immunity is a complex branch of the human immune system, and the main force against HBV [2]. During HBV infection, both the number and fitness of HBV-specific T and B lymphocytes increase significantly [47,48,49]. The indispensable role played by HBV-specific CD8+ T cells in the clearance of HBV has been well recognized [38,48,50,51,52,53]. Moreover, CD4+ T cells are required to promote the activation and function of these CD8+ T cells. However, these adaptive immune responses are typically functionally impaired in patients with CHB [2,47,53,54,55,56,57]. It has been reported that HBV-specific CD8+ T cells in both the peripheral blood and liver microenvironment of patients with CHB always exhibit an exhausted phenotype [58,59]. Furthermore, in patients with CHB, suppressive mechanisms, such as regulatory T cells (Tregs), and the increased expression of co-inhibitory receptors such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), T cell immunoglobulin and mucin domain 3 (Tim-3), and programmed cell death protein 1 (PD-1) on CD8+ T cells dampen the antiviral response [10,53,60,61,62].
Although the mechanisms that regulate the above co-inhibitory factors are still to be elucidated, hepatocytes and other hepatic cell populations might contribute to the impaired function and exhaustion of HBV-specific CD8+ T cells. It was reported that the hepatocytes of patients with CHB did not express certain classical costimulatory molecules (e.g., CD80 and CD86), meaning that infected hepatocytes may not be able to transfer the second signal required for CD8+ T cell activation [63,64]. In addition, Benechet et al. found that naïve HBV-specific CD8+ T cells primed by infected hepatocytes could not differentiate into interferon (IFN)-γ-expressing effector T cells in an HBV transgenic mouse model [65]. Tregs and macrophages may also contribute to the immunosuppressive environment through the production of immunomodulatory cytokines such as interleukin (IL)-10 and tumor growth factor (TGF)-β1 [39,66,67]. Furthermore, myeloid-derived suppressor cells (MDSC) in the liver microenvironment were shown to suppress T cell signaling partially through the production of arginase, which could degrade arginine and significantly inhibit T cell effector function [68,69,70].
Antibodies secreted by B cells recognize antigens by directly binding to the components of the pathogen or interacting with the proteins expressed on the surface of hepatocytes [48]. However, only antibodies targeting the HBV envelope can prevent the spread of HBV infection [57,71]. In addition, the presence of HBsAg in the serum causes B cell exhaustion and impedes the maturation of HBsAg-specific B cells, which might be associated with the high expression of PD-1 [72]. Indeed, PD-1 blockade or HBsAg clearance were shown to restore the antibody-producing ability of HBsAg-specific B cells [73,74]. Moreover, persistent IFN-α therapy was able to induce large numbers of CD24+CD38hi regulatory B cells (Bregs) and promote an immunosuppressive response, which resulted in the downregulation of CD8+ T cell and natural killer (NK) cell effector functions (Figure 2). In addition, patients with fewer Bregs exhibited improved therapeutic effects [53]. Some other types of cells also pay a role during HBV infection such as the liver-resident macrophages, the Kupffer cells [75]. Kupffer cells are regarded as one of the predominant populations in the liver and they secrete immunomodulatory cytokines, for example, TGF-β1 and IL-10 [38]. Additionally, Kupffer cells in the liver highly expressed PD-L1 or PD-L2 during CHB infection, thus suppressing antiviral immune responses and leading to immune tolerance [38,75]. However, Kupffer cells could also present antigens to CD8+ T cell and induce their activation. Moreover, Kupffer cells have been found to recruit monocytes via chemokines in the liver [75]. Tacke et al. reported that the pathogenic macrophage subsets were a potential target for treating liver disease in mouse models [75]. Furthermore, there are enriched NKT cells in the human liver, which also play important roles in CHB and could modulate both innate and adaptive immunity [40,76]. The MHC-like molecule CD1d is important for NKT cells to recognize the lipid-based antigen [40,77,78]. However, until now, the characterization of NKT cells in the liver of CHB patients has still been poorly verified. It has been reported that, in the HBV-infected liver, the proportion of NKT cells were obviously decreased and had lost a-galactosylceramide (a-GalCer)-induced IFN-γ production, which may contribute to immune evasion. Importantly, when PBMCs were stimulated with α-GalCer plus IL-2 and IL-15, the ratio and the IFN-γ production of NKT cell were restored [76,77], which indicated that protective immunity might be partially recovered in patients with CHB. Taken together, these findings imply that the mechanisms that impair T and B cell responses during CHB likely contribute to the evasion of the adaptive immune response.
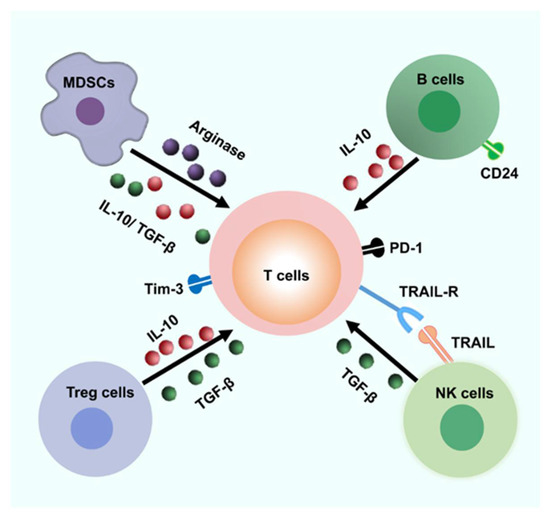
Figure 2.
Possible inhibitory mechanisms result in immune escape. The liver microenvironment is enriched with various cells that can inhibit the T cell responses. Abnormal PD-1, Tim-3, and other negative signaling pathways probably result in the T cell exhaustion. MDSCs and Tregs could be a great source of arginase and suppress T cell responses through arginase and secreting several immune-modulatory cytokines such as TGF-β1 and IL-10. In addition, CD24+ Breg cells may suppress T cell function by IL-10. Furthermore, HBV-specific CD8+ T cells could be lysed by activated NK cells via a contact-dependent manner (for example, TRAIL/TRAIL-R). MDSC, Myeloid-derived suppressor cells; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
3.2. Evasion of the Innate Immune Response
Although T cell dysfunction may be the most fascinating immune change leading to HBV persistence, the interactions between HBV and different types of innate immune cells should not be ignored. However, the relationship between the virus and innate immune response remains highly controversial, and the mechanisms of innate immune escape have not been fully understood [17].
HBV can disrupt antiviral responses within infected cells and evade the innate immune response. Pattern recognition receptor (PRR) distribution has been identified as an important factor for identifying drugs targeting the innate immune system [79,80]. During chronic HBV infection, interactions between HBV antigens, PRRs, as well as various innate immune cell types, have been reported [17]. For instance, dendritic cells (DCs) promote the adaptive immune response through antigen presentation and the production of several cytokines, including IL-12 [81]. HBV may suppress DCs by downregulating the expression of co-stimulatory molecules such as CD80 and CD86, and by inducing the high expression of PD-L1 [82,83,84]. Additionally, it has been reported that the proportion of plasmacytoid DCs (pDCs) was markedly decreased in patients with CHB [85,86,87]. It was also reported that monocytes inhibit the production of IL-12 by DCs when exposed to HBsAg [88]. Therefore, targeting innate immunity may be a potential novel approach to developing a functional cure for HBV infection in the future.
4. Progress in Hepatitis-B-Specific Immunotherapy
Existing treatment regimens have achieved remarkable cure rates for patients infected with the hepatitis C virus (HCV). However, the current regimens for treating HBV remain suboptimal. Current therapeutic approaches include nucleoside analogues (NA) and nucleotide drugs (NUCs), which both efficiently inhibit HBV replication. Lamivudine, the first nucleoside reverse transcriptase inhibitor (NRTI), obtained Food and Drug Administration (FDA) approval in 1998. Since then, other NRTIs such as adefovir and telbivudine have been developed but these are not used as first-line therapies due to drug-associated resistance. Currently, entecavir (ETV), tenofovir alafenamide (TAF), and tenofovir disoproxil fumarate (TDF) are used as the first-line oral drugs against HBV infection [89,90]. These agents can optimally lower HBV DNA levels in the serum of patients and reduce liver failure. However, current antiviral agents have minimal impact directly on cccDNA in primary human hepatocytes [80]. With the persistence of long-lived cccDNA, the potential for relapse of HBV exists, even after the clearance of viremia [91]. In addition, integrated viral DNA may survive immune clearance and the potential for relapse also exists in patients with resolved HBV infection. To date, the study of HBV cccDNA is still hampered by the lack of an appropriate model [92,93]. A deeper understanding of cccDNA might provide new perspectives to find a functional cure. Additionally, the loss of HBeAg or HBsAg with prolonged therapy occurs in very few patients [17,27]. For HBeAg-positive patients, oral antiviral drugs are regarded as the most common treatment strategy because of their effectiveness and ability to provide sustained viral suppression. The decision to treat HBeAg-positive CHB patients with one of the NUCs (such as lamivudine, entecavir, or tenofovir) should be individualized [94,95]. Additionally, the number of HBeAg-negative CHB patients is increasing and these patients have become the majority in terms of the form of chronic HBV, especially in Middle Eastern and north African countries. Indeed, few patients who are HBeAg-negative would achieve the loss of HBsAg, and a large quantity of these patients may experience HBV recurrence after discontinuation of therapy. Therefore, most guidelines suggest lifelong treatment, with the goal of achieving high rates of viral suppression [96]. Marcellin et al. carried out a study of HBeAg-negative patients with TDF treatment for up to 10 years and demonstrated that TDF therapy resulted in persistent maintenance of viral suppression and was well tolerated [97]. Furthermore, through a study from 17 countries, Maria Buti found that more than 90% of patients who were HBeAg-negative and receiving TDF had an HBV DNA of less than 29 IU/mL after treatment for 48 weeks [98].
IFN-α has antiviral properties and can regulate immune function. To date, IFN-α has been regarded as the first-choice therapy for treating CHB [10]. A multi-center study reported that, after 48 weeks of treatment with a combination of PEG-IFN-α plus TDF, a 9.1% HBsAg loss was observed at week 72 post treatment initiation [99,100,101]. In addition, Fu et al. reported that, after PEG-IFN-α-2b treatment, approximately 30% of patients with CHB underwent HBeAg seroconversion by week 72 [53,54]. However, there are some limitations of therapeutic IFN-α administration. For example, IFN-α therapy may have some side effects and contraindications, especially in patients with advanced liver disease [102]. To achieve a functional cure for HBV, a properly orchestrated activation of anti-HBV immunity is required. As patients with CHB have low numbers of HBV-specific CD8+ T cells, which are frequently exhausted, existing immunotherapeutic approaches designed to promote antiviral immunity may not be adequate [103,104,105]. After analyzing the characteristics of innate and adaptive immune response during HBV infection, some promising immuno-dependent therapeutic strategies to achieve a functional cure for CHB were proposed. These included IL-2, checkpoint inhibitors (e.g., anti-PD-1 and anti-CTLA-4), and therapeutic vaccines. We demonstrated that non-responder (NR) patients who failed to respond to PEG-IFN-α treatment, benefited from a sequential low dose of IL-2 (1 × 106 IU) therapy, which caused a decrease in the frequency of PD-1+ CD8+ T cells and Tregs [9]. Furthermore, we found sequential IL-2 therapy significantly restored the frequency of HBV-specific CD8+ T cells and the HBsAg-specific effector function of CD8+ T cells. Importantly, we found that, in the majority of NR patients, HBeAg levels were markedly decreased after sequential IL-2 therapy [10] (Figure 3).
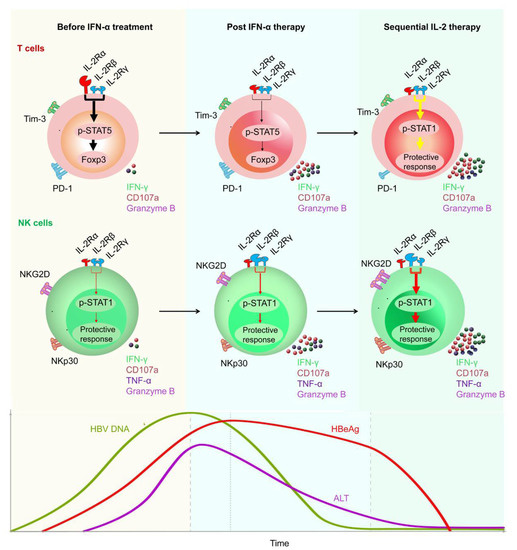
Figure 3.
Restoration of HBV-specific CD8+ T cell and NK cell responses by sequential IL-2 treatment in non-responder patients after IFN-α therapy. IL-2Rα was high expressed, and NKp30 was low expressed on T cells and NK cells, respectively, of non-responder (NR) patients, in whom IFN-α therapy had failed. Those NR patients were treated with low-dose IL-2 for 24 weeks. A decrease in IL-2Rα expression on their CD4+ T cells was verified, suggesting that IFN-α therapy may provide a rationale for sequential IL-2 treatment without increasing regulatory T cells (Tregs). In addition, non-responders experienced a decrease in the numbers of PD-1 expression. Furthermore, sequential IL-2 administration restored effective immune function, involving STAT1 activation in both T cells and NK cells. Moreover, IL-2 therapy increased the function of HBV-specific T cells and NK cells, which translated into improved clinical outcomes, including HBeAg seroconversion, among the non-responder CHB patients.
Checkpoint inhibitors and therapeutic vaccination have also been proposed to restore the antiviral immune response of patients with CHB [106]. However, so far, treatments with checkpoint inhibitors have only been applied usefully in some solid malignancies including melanoma and renal cell carcinoma. In vitro studies have shown that anti-PD1/PDL-1 blockade could partially restore the function of exhausted HBV-specific CD8+ T cells [62,107,108]. Gane et al. found that nivolumab (a PD-1 inhibitor) with or without GS-4774 (a therapeutic vaccine) was well-tolerated and would contribute to an HBsAg decrease in virally suppressed HBeAg-negative patients in a phase Ib study [109]. In addition, trials of several vaccine candidates have been carried out in patients with CHB [18,110,111]. Boni et al. reported that the administration of tenofovir plus GS-4774 therapy was well tolerated and could improve HBV-specific T cell responses in CHB patients. In addition, the production of TNF-α, IFN-γ, as well as IL-2, obviously increased. Furthermore, data have suggested that combination treatments including vaccines may be regarded as sequential administration that is able to increase the antiviral immune response in the future [112].
The role of the innate immune system should not be ignored in the process of HBV eradication. However, in the context of HBV infection, the innate immune response is often poorly activated due to immune evasion. RIG-I or Toll-like receptor (TLR) agonists, such as TLR-7 and TLR-8 agonists, have been used to induce the activation of innate immunity. GS-9620, a TLR-7 agonist, has been found to induce the production of IFN-α, especially by pDCs. In addition, the treatment of RO7020531 triggered obvious immune activation in patients with CHB [113,114,115]. Moreover, recently developed TLR-8 agonists may contribute to the activation of PRRs present in the liver, and GS-9688 has been shown to promote the production of IL-12 and IL-18 from monocytes or DCs [116,117,118]. Furthermore, as cytokines such as IL-12 also contribute to NK cell activation, which have been demonstrated to kill both HBV-infected hepatocytes and HBV-specific CD8+ T cells, it is necessary to comprehensively evaluate the function of activated innate immunity in the process of HBV eradication. Furthermore, there are a large quantity of new therapeutic drugs for patients with CHB under investigation. GLS4 is a core protein allosteric modulator. A total of 20 weeks of treatment with GLS4 resulted in reduced DNA levels (1.48–6.09 log decrease) [20]. Several capsid assembly modulators have been under development for CHB therapy. For example, ABI-H0731, was found to cause a significant decrease in HBV DNA levels at 12 weeks, when combined with entecavir [119]. In addition, the administration of RO7049389 not only reduced HBV DNA levels, but also decreased HBsAg, as well as HBeAg levels in the serum [20,120]. Additionally, the effects of siRNAs in clinical trials also appear encouraging. Treatment with JNJ3989 achieved a 1.3–3.8 log decrease in HBsAg levels [20]. Furthermore, several other new therapies that have been investigated have been reported as safe and well tolerated in healthy volunteers, such as GSK3389404 [19] (Table 1).

Table 1.
Select new therapeutic strategies for patients with CHB under development.
Furthermore, immunological approaches against HBV infection, which involve the use of T cells engineered with a classical T cell receptor (TCR) specific for human leukocyte antigens (HLA)-class I restricted HBV epitopes or a chimeric antigen receptor (CAR), have shown some promise [106]. Despite the encouraging preliminary results of such T cell therapies, they are associated with a risk of inducing fatal hepatic inflammation. Thus, the adoptive transfer of engineered T cells and the manufacturing techniques used must be evaluated with more caution. In addition, more robust experimental and clinical trial data are needed. Platelets play important roles in inflammatory and immune-mediated disorders. Aiolfi et al. reported that platelets contributed to the pathogenesis of HBV-related liver disease by their ability to promote the homing of effector CD8+ T cells in the liver, expression of pro-angiogenic mediators (such as VEGF and TGF-β1) and the production of pro-inflammatory cytokines (such as IFN-γ) [130]. Aspirin is a widely used anti-platelet drug. Notably, the suppression of platelet activation using aspirin would significantly reduce the number of HBV-specific CD8+ T cells and the recruitment of inflammatory cells in the liver, which contributes to alleviating liver injury and the likelihood of HCC [130,131]. Therefore, anti-platelet therapy might be another promising approach for the treatment of patients with CHB. Collectively, to achieve the goal of developing a functional cure, more knowledge derived from the accumulation of experiment and clinical trials is needed.
5. Conclusions and Perspectives
Based on the findings presented, it is clear that the future of HBV treatment requires the direct suppression of cccDNA replication. However, achieving the ultimate goal of finding a functional cure for CHB will be challenging. Although the molecular biology of HBV is becoming gradually understood and novel DAAs are being developed, it is still unclear whether these agents are safe and would be able to provide a long-term functional cure. Immunotherapy is receiving increasing attention from scientists and clinicians in many fields of research. Additionally, the investigation of curative strategies for patients with CHB will benefit greatly from the knowledge of immunological features and mechanisms that govern HBV pathogenesis and immunobiology. Despite ongoing challenges in the quest for HBV eradication, there remains much promise and optimism on the way to achieving the goal of an HBV functional cure.
Author Contributions
D.W. drafted the manuscript and figures. H.W. and B.F. edited/reviewed the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82100230), the Research Fund for the Key Laboratory of Anhui Province (KLICD-2022-Z2), the Fundamental Research Funds for the Central Universities (WK9110000168), the China Postdoctoral Science Foundation (2020T130112ZX), and the Postdoctoral Foundation of Hefei (2020130).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data referred to this study are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.; Li, F.; Xie, L.; Crane, J.; Zhen, G.; Mishina, Y.; Deng, R.; Gao, B.; Chen, H.; Liu, S.; et al. Inhibition of overactive TGF-β attenuates progression of heterotopic ossi-fication in mice. Nat. Commun. 2018, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Iannacone, M.; Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat. Rev. Immunol. 2022, 22, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Locarnini, S.; Hatzakis, A.; Chan, D.-S.; Lok, A. Strategies to control hepatitisHepatitis B: Public policy, epidemiology, vaccine and drugs. J. Hepatol. 2015, 62, S76–S86. [Google Scholar] [CrossRef]
- Trepo, C.; Chan, H.L.Y.; Lok, A. Hepatitis B Virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.-L. Hepatitis B virus infection. Nat. Rev. Dis. Primers. 2018, 4, 18035. [Google Scholar] [CrossRef]
- Revill, P.A.; Chisari, F.V.; Block, J.M.; Dandri, M.; Gehring, A.J.; Guo, H.; Hu, J.; Kramvis, A.; Lampertico, P.; Janssen, H.L.A.; et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterolhepatol 2019, 4, 545–558. [Google Scholar] [CrossRef]
- Chua, C.G.; Mehrotra, A.; Mazzulli, T.; Wong, D.K.; Feld, J.J.; Janssen, H.L.A.; Gehring, A.J. Optimized ex vivo stimulation identifies multi-functional HBV-specific T cells in a majority of chronic hepatitis B patients. Sci. Rep. 2020, 10, 11344. [Google Scholar] [CrossRef]
- Wang, F.S.; Fan, J.G.; Zhang, Z.; Gao, B.; Wang, H.Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef]
- Wang, D.; Fu, B.; Shen, X.; Guo, C.; Liu, Y.; Zhang, J.; Sun, J.; Ye, Y.; Li, J.; Tian, Z.; et al. Restoration of HBV-specific CD8 (+) T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-α therapy. Signal Transduct. Target Ther. 2021, 6, 376. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, X.; Fu, B.; Nian, Z.; Qian, Y.; Sun, R.; Tian, Z.; Wei, H. Hepatectomy promotes recurrence of liver cancer by enhancing IL-11-STAT3 signaling. EBioMedicine 2019, 46, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Cai, D.; Yan, R.; Li, L.; Zong, Y.; Guo, L.; Mercier, A.; Zhou, Y.; Tang, A.; Henne, K.; et al. Preclinical Profile and Characterization of the Hepatitis B Virus Core Protein Inhibitor ABI-H0731. Antimicrob. Agents Chemother. 2020, 64, e01463-20. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.J.; Stevens, G.A.; Groeger, J.; Wiersma, S.T. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012, 30, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bi, S.; Yang, W.; Wang, L.; Cui, G.; Cui, F.; Zhang, Y.; Liu, J.; Gong, X.; Chen, Y.; et al. Reprint of: Epidemiological serosurvey of Hepatitis B in China--declining HBV prevalence due to Hepatitis B vaccination. Vaccine 2013, 31, J21–J28. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Wang, Q.; Shen, H.; Zhang, M.; Zhang, Y.; Yan, D.; Liu, M. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21–49 years in rural China: A population-based, cross-sectional study. Lancet Infect. Dis. 2016, 16, 80–86. [Google Scholar] [CrossRef]
- Cui, F.; Shen, L.; Li, L.; Wang, H.; Wang, F.; Bi, S.; Liu, J.; Zhang, G.; Wang, F.; Zheng, H.; et al. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. Emerg. Infect. Dis. 2017, 23, 765–772. [Google Scholar] [CrossRef]
- Kuipery, A.; Gehring, A.J.; Isogawa, M. Mechanisms of HBV immune evasion. Antivir. Res. 2020, 179, 104816. [Google Scholar] [CrossRef]
- Mancini-Bourgine, M.; Fontaine, H.; Scott-Algara, D.; Pol, S.; Bréchot, C.; Michel, M.-L. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology 2004, 40, 874–882. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, J.S.; Ahn, S.H. Hepatitis B Virus Cure: Targets and Future Therapies. Int. J. Mol. Sci. 2020, 22, 213. [Google Scholar] [CrossRef]
- Fanning, G.C.; Zoulim, F.; Hou, J.; Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: Towards a cure. Nat. Rev. Drug Discov. 2019, 18, 827–844. [Google Scholar] [CrossRef]
- Liang, X.; Bi, S.; Yang, W.; Wang, L.; Cui, G.; Cui, F.; Zhang, Y.; Liu, J.; Gong, X.; Chen, Y.; et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J. Infect. Dis. 2009, 200, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Velkov, S.; Ott, J.J.; Protzer, U.; Michler, T. The Global Hepatitis B Virus Genotype Distribution Approximated from Available Gen-otyping Data. Genes 2018, 9, 495. [Google Scholar] [CrossRef]
- Park, J.H.; Iwamoto, M.; Yun, J.H.; Uchikubo-Kamo, T.; Son, D.; Jin, Z.; Yoshida, H.; Ohki, M.; Ishimoto, N.; Mizutani, K.; et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 2022, 606, 1027–1031. [Google Scholar] [CrossRef]
- Asami, J.; Kimura, K.T.; Fujita-Fujiharu, Y.; Ishida, H.; Zhang, Z.; Nomura, Y.; Liu, K.; Uemura, T.; Sato, Y.; Ono, M.; et al. Structure of the bile acid transporter and HBV re-ceptor NTCP. Nature 2022, 606, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Lempp, F.A.; Mehrle, S.; Nkongolo, S.; Kaufman, C.; Fälth, M.; Stindi, J.; Koniger, C.; Nassal, M.; Kubitz, R.; et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 2014, 146, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, W. Mechanisms of immune escape in viral hepatitis. Gut 1999, 44, 759–764. [Google Scholar] [CrossRef]
- Zhou, L.; He, R.; Fang, P.; Li, M.; Yu, H.; Wang, Q.; Yu, Y.; Wang, F.; Zhang, Y.; Chen, A.; et al. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recog-nition. Nat. Commun. 2021, 12, 98. [Google Scholar] [CrossRef]
- Mommeja-Marin, H.; Mondou, E.; Blum, M.R.; Rousseau, F. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: Analysis and review of the literature. Hepatology 2003, 37, 1309–1319. [Google Scholar] [CrossRef]
- Crignis, E.D.; Hossain, T.; Romal, S.; Carofiglio, F.; Moulos, P.; Khalid, M.M.; Rao, S.; Bazrafshan, A.; Verstegen, M.M.; Pourfarzad, F.; et al. Application of human liver organoids as a pa-tient-derived primary model for HBV infection and related hepatocellular carcinoma. Elife 2021, 10, e60747. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Chisari, F.V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 2006, 1, 23–61. [Google Scholar] [CrossRef]
- Lucifora, J.; Delphin, M. Current knowledge on Hepatitis Delta Virus replication. Antivir. Res. 2020, 179, 104812. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, S.; Watashi, K. Hepatitis B virus biology and life cycle. Antivir. Res. 2020, 182, 104925. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.; Yang, C.C.; Choijilsuren, G.; Chang, C.H.; Liou, A.T. Hepatitis B Virus. Trends Microbiol. 2018, 26, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Blondot, M.L.; Bruss, V.; Kann, M. Intracellular transport and egress of hepatitis B virus. J. Hepatol. 2016, 64, S49–S59. [Google Scholar] [CrossRef]
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Zarifyan, D.; Goptar, I.; Chulanov, V. Orthologous CRISPR/Cas9 systems for specific and efficient degradation of covalently closed circular DNA of hepatitis B virus. Cell Mol. Life Sci. 2019, 76, 1779–1794. [Google Scholar] [CrossRef]
- Wei, L.; Ploss, A. Core components of DNA lagging strand synthesis machinery are essential for hepatitis B virus cccDNA for-mation. Nat. Microbiol. 2020, 5, 715–726. [Google Scholar] [CrossRef]
- Wei, L.; Ploss, A. Mechanism of Hepatitis B Virus cccDNA Formation. Viruses 2021, 13, 1463. [Google Scholar] [CrossRef]
- De Simone, G.; Andreata, F.; Bleriot, C.; Fumagalli, V.; Laura, C.; Garcia-Manteiga, J.M.; Lucia, P.D.; Gilotto, S.; Ficht, X.; Ponti, F.F.D.; et al. Identification of a Kupffer cell subset ca-pable of reverting the T cell dysfunction induced by hepatocellular priming. Immunity 2021, 54, 2089–2100.e8. [Google Scholar] [CrossRef]
- Breous, E.; Somanathan, S.; Vandenberghe, L.H.; Wilson, J.M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 2009, 50, 612–621. [Google Scholar] [CrossRef]
- Ortega-Prieto, A.M.; Dorner, M. Immune Evasion Strategies during Chronic Hepatitis B and C Virus Infection. Vaccines 2017, 5, 24. [Google Scholar] [CrossRef]
- Zhu, X.; He, Z.; Yuan, J.; Wen, W.; Huang, X.; Hu, Y.; Lin, C.; Pan, J.; Li, R.; Deng, H.; et al. IFITM3-containing exosome as a novel mediator for anti-viral response in dengue virus infection. Cell Microbiol. 2015, 17, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013, 14, 793–803. [Google Scholar] [CrossRef]
- Dreux, M.; Garaigorta, U.; Boyd, B.; Décembre, E.; Chung, J.; Whitten-Bauer, C.; Wieland, S.; Chisari, F.V. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012, 12, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Oshiumi, H.; Azuma, M.; Kato, N.; Matsumoto, M.; Seya, T. IPS-1 is essential for type III IFN production by hepato-cytes and dendritic cells in response to hepatitis C virus infection. J. Immunol. 2014, 192, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- McKinney, E.F.; Smith, K.G. T cell exhaustion and immune-mediated disease-the potential for therapeutic exhaustion. Curr. Opin. Immunol. 2016, 43, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.E.; Zannetti, C.; Lucifora, J.; Norder, H.; Protzer, U.; Hainaut, P.; Zoulim, F.; Tommasino, M.; Trepo, C.; Hasan, U.; et al. Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS ONE 2011, 6, e26315. [Google Scholar] [CrossRef]
- Chiale, C.; Marchese, A.M.; Robek, M.D. Innate immunity and HBV persistence. Curr. Opin. Virol. 2021, 49, 13–20. [Google Scholar] [CrossRef]
- Bertoletti, A.; Ferrari, C. Adaptive immunity in HBV infection. J. Hepatol. 2016, 64, S71–S83. [Google Scholar] [CrossRef]
- Bertoletti, A.; Tan, A.T.; Koh, S. T-cell therapy for chronic viral hepatitis. Cytotherapy 2017, 19, 1317–1324. [Google Scholar] [CrossRef]
- Bertoletti, A.; Bert, N.L. Immunotherapy for Chronic Hepatitis B Virus Infection. Gut Liv. 2018, 12, 497–507. [Google Scholar] [CrossRef]
- Baudi, I.; Kawashima, K.; Isogawa, M. HBV-Specific CD8+ T-Cell Tolerance in the Liver. Front. Immunol. 2021, 12, 721975. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Wieland, S.; Steiger, C.; Ghrayeb, J.; Reimann, K.A.; Purcell, R.H.; Chisari, F.V. CD8 (+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 2003, 77, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Wang, D.; Shen, X.; Guo, C.; Liu, Y.; Ye, Y.; Sun, R.; Li, J.; Tian, Z.; Wei, H. Immunomodulation Induced During Interferon-α Therapy Impairs the Anti-HBV Immune Response Through CD24 (+) CD38 (hi) B Cells. Front. Immunol. 2020, 11, 591269. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, D.; Huang, M.; Zheng, X.; Shen, Y.; Fu, B.; Zhao, H.; Chen, X.; Peng, P.; Zhu, Q.; et al. Transcriptomic characteristics and impaired immune function of pa-tients who retest positive for SARS-CoV-2 RNA. J. Mol. Cell Biol. 2021, 13, 748–759. [Google Scholar] [CrossRef]
- Bertoletti, A.; Ferrari, C. Innate and adaptive immune responses in chronic hepatitis B virus infections: Towards restoration of immune control of viral infection. Gut 2012, 61, 1754–1764. [Google Scholar] [CrossRef]
- Hayashi, S.; Murakami, S.; Omagari, K.; Matsui, T.; Iio, E.; Isogawa, M.; Watanabe, T.; Karino, Y.; Tanaka, Y. Characterization of novel entecavir resistance mutations. J. Hepatol. 2015, 63, 546–553. [Google Scholar] [CrossRef]
- Rehermann, B.; Nascimbeni, M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 2005, 5, 215–229. [Google Scholar] [CrossRef]
- Bengsch, B.; Thimme, R. Balance lost: T cell immunity in progressive HBV infection. Hepatol. Int. 2014, 8, 7–9. [Google Scholar] [CrossRef]
- Raziorrouh, B.; Heeg, M.; Kurktschiev, P.; Schraut, W.; Zachoval, R.; Wendtner, C.; Wachtler, M.; Spannagl, M.; Denk, G.; Ulsenheimer, A.; et al. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS ONE 2014, 9, e105703. [Google Scholar] [CrossRef]
- Lopes, A.R.; Kellam, P.; Das, A.; Dunn, C.; Kwan, A.; Turner, J.; Peppa, D.; Gilson, R.J.; Gehring, A.; Bertoletti, A.; et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J. Clin. Investig. 2008, 118, 1835–1845. [Google Scholar] [CrossRef]
- Raziorrouh, B.; Schraut, W.; Gerlach, T.; Nowack, D.; Grüner, N.H.; Ulsenheimer, A.; Zachoval, R.; Wachtler, M.; Spannagl, M.; Haas, J.; et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 2010, 52, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Schurich, A.; Khanna, P.; Lopes, A.R.; Han, K.J.; Peppa, D.; Micco, L.; Nebbia, G.; Kennedy, P.T.F.; Geretti, A.-M.; Dusheiko, G.; et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011, 53, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Gehring, A. Immune response and tolerance during chronic hepatitis B virus infection. Hepatol. Res. 2007, 37, S331–S338. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Mederacke, I.; Herzog-Hauff, S.; Glebe, D.; Grün, S.; Strand, D.; Urban, S.; Gehring, A.; Galle, P.R.; Bocher, W.O. Peripheral blood dendritic cells are phenotypically and functionally intact in chronic hepatitis B virus (HBV) infection. Clin. Exp. Immunol. 2008, 151, 61–70. [Google Scholar] [CrossRef]
- Bénéchet, A.P.; Simone, G.D.; Lucia, P.D.; Cilenti, F.; Barbiera, G.; Bert, N.L.; Fumagalli, V.; Lusito, E.; Moalli, F.; Bianchessi, V.; et al. Dynamics and genomic landscape of CD8 (+) T cells undergoing hepatic priming. Nature 2019, 574, 200–205. [Google Scholar] [CrossRef]
- Sun, C.; Fu, B.; Gao, Y.; Liao, X.; Sun, R.; Tian, Z.; Wei, H. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012, 8, e1002594. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Z.; Zhu, X.; Zheng, X.; Zhou, Y.; Lu, Y.; Yan, P.; Wang, H.; Liu, H.; Jin, J.; et al. GARP-mediated active TGF-β1 induces bone marrow NK cell dysfunction in AML patients with early relapse post-allo-HSCT. Blood 2022. [Google Scholar] [CrossRef]
- Pallett, L.J.; Gill, U.S.; Quaglia, A.; Sinclair, L.V.; Jover-Cobos, M.; Schurich, A.; Singh, K.P.; Thomas, N.; Das, A.; Chen, A.; et al. Metabolic regulation of hepatitis B immunopathol-ogy by myeloid-derived suppressor cells. Nat. Med. 2015, 21, 591–600. [Google Scholar] [CrossRef]
- Heiberg, I.L.; Pallett, L.J.; Winther, T.N.; Høgh, B.; Maini, M.K.; Peppa, D. Defective natural killer cell anti-viral capacity in paediatric HBV infection. Clin. Exp. Immunol. 2015, 179, 466–476. [Google Scholar] [CrossRef]
- Dai, X.K.; Ding, Z.X.; Tan, Y.Y.; Bao, H.R.; Wang, D.Y.; Zhang, H. Neutrophils inhibit CD8 (+) T cells immune response by arginase-1 sig-naling in patients with sepsis. World J. Emerg. Med. 2022, 13, 266–273. [Google Scholar] [CrossRef]
- Hwang, G.Y.; Huang, C.J.; Lin, C.Y.; Wu, C.C. Dominant mutations of hepatitis B virus variants in hepatoma accumulate in B-cell and T-cell epitopes of the HBx antigen. Virus Res. 2003, 92, 157–164. [Google Scholar] [CrossRef]
- Salimzadeh, L.; Bert, N.L.; Dutertre, C.A.; Gill, U.S.; Newell, E.W.; Frey, C.; Hung, M.; Novikov, N.; Fletcher, S.; Kennedy, P.T.; et al. PD-1 blockade partially recovers dysfunctional vi-rus-specific B cells in chronic hepatitis B infection. J. Clin. Investig. 2018, 128, 4573–4587. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.R.; Pallett, L.J.; McCoy, L.E.; Suveizdyte, K.; Amin, O.E.; Swadling, L.; Alberts, E.; Davidson, B.R.; Kennedy, P.T.; Gill, U.S.; et al. Circulating and intrahepatic antiviral B cells are de-fective in hepatitis B. J. Clin. Investig. 2018, 128, 4588–4603. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 796–829. [Google Scholar] [CrossRef]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Ding, Y.; Zhu, P.; Dou, R.; Liang, Z.; Yang, D.; Huang, Z.; Wang, W.; Wu, X.; Weng, X. Elevated Hepatic CD1d Levels Coincide with Invariant NKT Cell Defects in Chronic Hepatitis B Virus Infection. J. Immunol. 2018, 200, 3530–3538. [Google Scholar] [CrossRef]
- Zeissig, S.; Murata, K.; Sweet, L.; Publicover, J.; Hu, Z.; Kaser, A.; Bosse, E.; Iqbal, J.; Hussain, M.M.; Balschun, K.; et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat. Med. 2012, 18, 1060–1068. [Google Scholar] [CrossRef]
- Godfrey, D.I.; MacDonald, H.R.; Kronenberg, M.; Smyth, M.J.; Kaer, L.V. NKT cells: What’s in a name? Nat. Rev. Immunol. 2004, 4, 231–237. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, Y.; Lu, M. Advances in Targeting the Innate and Adaptive Immune Systems to Cure Chronic Hepatitis B Virus Infection. Front. Immunol. 2019, 10, 3127. [Google Scholar] [CrossRef]
- Fung, S.; Choi, H.S.J.; Gehring, A.; Janssen, H.L.A. Getting to HBV cure: The promising paths forward. Hepatology 2022, 76, 233–250. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, R.; Liu, W.; Si, L.; Li, L.; Li, X.; Yao, Z.; Liao, H.; Wang, J.; Li, Y.; et al. Investigation of multidrug-resistance mutations of hepatitis B virus (HBV) in a large cohort of chronic HBV-infected patients with treatment of nucleoside/nucleotide analogs. Antivir. Res. 2021, 189, 105058. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.M.; Kuo, Y.H.; Wang, J.H.; Hung, C.H.; Hu, T.-H.; Lu, S.-N.; Chen, C.-H. Associations of HBV Genotype B vs C Infection with Relapse After Cessation of Entecavir or Tenofovir Therapy. Clin. Gastroenterol. Hepatol. 2020, 18, 2989–2997.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, J.; Gluud, C. Bile acids for viral hepatitis. Cochrane Database Syst. Rev. 2007, 2007, Cd003181. [Google Scholar] [CrossRef] [PubMed]
- Ouaguia, L.; Leroy, V.; Dufeu-Duchesne, T.; Durantel, D.; Decaens, T.; Hubert, M.; Valladeau-Guilemond, J.; Bendriss-Vermare, N.; Chaperot, L.; Aspord, C. Circulating and Hepatic BDCA1+, BDCA2+, and BDCA3+ Dendritic Cells Are Differentially Subverted in Patients with Chronic HBV Infection. Front. Immunol. 2019, 10, 112. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Z.; Xu, R.; Lin, H.; Fu, J.; Zou, Z.; Zhang, A.; Shi, J.; Chen, L.; Lv, S.; et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl. Med. 2012, 1, 725–731. [Google Scholar] [CrossRef]
- Duan, X.Z.; Wang, M.; Li, H.W.; Zhuang, H.; Xu, D.; Wang, F.S. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J. Clin. Immunol. 2004, 24, 637–646. [Google Scholar] [CrossRef]
- Chang, J.J.; Thompson, A.J.; Visvanathan, K.; Kent, S.J.; Cameron, P.U.; Wightman, F.; Desmond, P.; Locarnini, S.A.; Lewin, S.R. The phenotype of hepatitis B virus-specific T cells differ in the liver and blood in chronic hepatitis B virus infection. Hepatology 2007, 46, 1332–1340. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Atsukawa, M.; Ishikawa, T.; Yasuda, S.; Yokohama, K.; Trinh, H.N.; Arai, T.; Fukunishi, S.; Ogawa, E.; Hsu, Y.-C.; et al. Outcomes of Sequential Therapy with Tenofovir Alafenamide After Long-term Entecavir. Am. J. Gastroenterol. 2021, 116, 1264–1273. [Google Scholar] [CrossRef]
- Charlton, M.R.; Alam, A.; Shukla, A.; Dashtseren, B.; Lesmana, C.R.A.; Duger, D.; Payawal, D.A.; Cuong, D.D.; Jangalsaikhan, G.; Cua, I.H.Y.; et al. An expert review on the use of tenofovir ala-fenamide for the treatment of chronic hepatitis B virus infection in Asia. J. Gastroenterol. 2020, 55, 811–823. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, M. Hepatitis B virus persistence and reactivation. BMJ 2020, 370, m2200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Zai, W.; Hu, K.; Zhu, Y.; Deng, Q.; Wu, M.; Li, Y.; Chen, J.; Yuan, Z. HBV covalently closed circular DNA minichromosomes in distinct epigenetic transcriptional states differ in their vulnerability to damage. Hepatology 2022, 75, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, W.; Zheng, Y.; Wang, W.; Bai, L.; Chen, L.; Feng, Y.; Zhang, Z.; Yuan, Z. In situ analysis of intrahepatic virological events in chronic hepatitis B virus infection. J. Clin. Investig. 2016, 126, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Leung, N. Treatment of HBeAg-positive chronic hepatitis B with nucleos(t)ide analogues. Liv. Int. 2011, 31, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Zarski, J.P.; Marcellin, P.; Cohard, M.; Lutz, J.M.; Bouche, C.; Rais, A. Comparison of anti-HBe-positive and HBe-antigen-positive chronic hepatitis B in France. French Multicentre Group. J. Hepatol. 1994, 20, 636–640. [Google Scholar] [CrossRef]
- García-López, M.; Lens, S.; Pallett, L.J.; Testoni, B.; Rodríguez-Tajes, S.; Mariño, Z.; Bartres, C.; Gracia-Pras, E.; Leonol, T.; Perpinan, E.; et al. Viral and immune factors associated with suc-cessful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J. Hepatol. 2021, 74, 1064–1074. [Google Scholar] [CrossRef]
- Marcellin, P.; Wong, D.K.; Sievert, W.; Buggisch, P.; Petersen, J.; Flisiak, R.; Manns, M.; Kaita, K.; Krastev, Z.; Lee, S.S.; et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liv. Int. 2019, 39, 1868–1875. [Google Scholar] [CrossRef]
- Buti, M.; Gane, E.; Seto, W.K.; Chan, H.L.; Chuang, W.L.; Stepanova, T.; Hui, A.-J.; Lim, Y.-S.; Mehta, R.; Janssen, H.L.A.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 196–206. [Google Scholar] [CrossRef]
- Perrillo, R. Benefits and risks of interferon therapy for hepatitis B. Hepatology 2009, 49, S103–S111. [Google Scholar] [CrossRef]
- Dienstag, J.L. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology 2009, 49, S112–S121. [Google Scholar] [CrossRef]
- Marcellin, P.; Ahn, S.H.; Ma, X.; Caruntu, F.A.; Tak, W.Y.; Elkashab, M.; Chuang, W.-L.; Lim, S.-G.; Tabak, F.; Mehta, R.; et al. Combination of Tenofovir Disoproxil Fumarate and Pegin-terferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients with Chronic Hepatitis, B. Gastroenterology 2016, 150, 134–144.e110. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannakos, J.; Papatheodoridis, G.V. HBeAg-negative chronic hepatitis B: Why do I treat my patients with pegylated inter-feron-alfa? Liv. Int. 2014, 34, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, Y.; Li, S.; Zhang, Y.; Liu, Y.; Wu, Y.; Chen, Z. Blockade of Tim-3 signaling restores the virus-specific CD8⁺ T-cell response in patients with chronic hepatitis B. Eur. J. Immunol. 2012, 42, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Kurktschiev, P.D.; Raziorrouh, B.; Schraut, W.; Backmund, M.; Wächtler, M.; Wendtner, C.M.; Bengsch, B.; Thimme, R.; Denk, G.; Zachoval, R.; et al. Dysfunctional CD8+ T cells in hepa-titis B and C are characterized by a lack of antigen-specific T-bet induction. J. Exp. Med. 2014, 211, 2047–2059. [Google Scholar] [CrossRef] [PubMed]
- Bengsch, B.; Martin, B.; Thimme, R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier pa-tients is linked to T cell differentiation. J. Hepatol. 2014, 61, 1212–1219. [Google Scholar] [CrossRef]
- Bertoletti, A.; Tan, A.T. HBV as a target for CAR or TCR-T cell therapy. Curr. Opin. Immunol. 2020, 66, 35–41. [Google Scholar] [CrossRef]
- Boni, C.; Fisicaro, P.; Valdatta, C.; Amadei, B.; Di, P.; Giuberti, T.; Laccabue, D.; Zerbini, A.; Cavalli, A.; Missale, G.; et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007, 81, 4215–4225. [Google Scholar] [CrossRef]
- Fisicaro, P.; Valdatta, C.; Massari, M.; Loggi, E.; Biasini, E.; Sacchelli, L.; Cavallo, M.C.; Silini, E.M.; Andreone, P.; Missale, G.; et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010, 138, 682–693, 693.e1–4. [Google Scholar] [CrossRef]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef]
- Michel, M.L.; Pol, S.; Brechot, C.; Tiollais, P. Immunotherapy of chronic hepatitis B by anti HBV vaccine: From present to future. Vaccine 2001, 19, 2395–2399. [Google Scholar] [CrossRef]
- Vandepapelière, P.; Lau, G.K.; Leroux-Roels, G.; Horsmans, Y.; Gane, E.; Tawandee, T.; Merican, M.I.B.; Win, K.M.; Trepo, C.; Cooksley, G.; et al. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: A randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 2007, 25, 8585–8597. [Google Scholar] [CrossRef] [PubMed]
- Boni, C.; Janssen, H.L.A.; Rossi, M.; Yoon, S.K.; Vecchi, A.; Barili, V.; Yoshida, E.M.; Trinh, H.; Rodell, T.C.; Laccabue, D.; et al. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients with Chronic Hepatitis. Gastroenterology 2019, 157, 227–241.e227. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Li, L.; Daffis, S.; Lucifora, J.; Bonnin, M.; Maadadi, S.; Salas, E.; Chu, R.; Ramos, H.; Livingston, C.M.; et al. Toll-like receptor 7 agonist GS-9620 induces prolonged inhibition of HBV via a type I interferon-dependent mechanism. J. Hepatol. 2018, 68, 922–931. [Google Scholar] [CrossRef]
- Lanford, R.E.; Guerra, B.; Chavez, D.; Giavedoni, L.; Hodara, V.L.; Brasky, K.M.; Fosdick, A.; Frey, C.R.; Zheng, J.; Wolfgang, G.; et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013, 144, 1508–1517, 1517.e1–10. [Google Scholar] [CrossRef] [PubMed]
- Block, T.M.; Chang, K.M.; Guo, J.T. Prospects for the Global Elimination of Hepatitis, B. Annu. Rev. Virol. 2021, 8, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Tan, A.T.; Ussher, J.E.; Sandalova, E.; Tang, X.Z.; Tan-Garcia, A.; To, N.; Hong, M.; Chia, A.; Gill, U.S.; et al. Toll-like receptor 8 agonist and bacteria trigger potent activa-tion of innate immune cells in human liver. PLoS Pathog. 2014, 10, e1004210. [Google Scholar] [CrossRef]
- Xun, Z.; Yao, X.; Zhu, C.; Ye, Y.; Wu, S.; Chen, T.; Zeng, Y.; Lin, C.; Yang, B.; Ou, Q.; et al. Proteomic characterization of the natural history of chronic HBV infection re-vealed by tandem mass tag-based quantitative proteomics approach. Mater. Today Bio. 2022, 15, 100302. [Google Scholar] [CrossRef]
- Perrillo, R.; Lin, H.S.; Schwarz, K.B.; Rosenthal, P.; Lisker-Melman, M.; Chung, R.T.; Prokunina-Olsson, L.; Coherty, G.; Feld, J.; Hepatitis B Research Network (HBRN). Changes in serum hepatitis B surface and e antigen, interferon-inducible protein 10, and aminotransferase levels during combination therapy of immune-tolerant chronic hepatitis B. Hepatology 2022, 76, 775–787. [Google Scholar] [CrossRef]
- Yuen, M.F.; Agarwal, K.; Gane, E.J.; Schwabe, C.; Ahn, S.H.; Kim, D.J.; Lim, Y.-S.; Cheng, W.; Sievert, W.; Visvanathan, K.; et al. Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: A randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol. Hepatol. 2020, 5, 152–166. [Google Scholar] [CrossRef]
- Lahlali, T.; Berke, J.M.; Vergauwen, K.; Foca, A.; Vandyck, K.; Pauwels, F.; Zoulim, F.; Durantel, D. Novel Potent Capsid Assembly Modulators Regulate Multiple Steps of the Hepatitis B Virus Life Cycle. Antimicrob. Agents Chemother. 2018, 62, e00835-18. [Google Scholar] [CrossRef]
- Yuen, M.F.; Zhou, X.; Gane, E.; Schwabe, C.; Tanwandee, T.; Feng, S.; Jin, Y.; Triyani, M.; Lemenuel-Diot, A.; Cosson, V.; et al. Safety, pharmacokinetics, and antiviral activity of RO7049389, a core protein allosteric modulator, in patients with chronic hepatitis B virus infection: A multicentre, random-ised, placebo-controlled, phase 1 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 723–732. [Google Scholar] [CrossRef]
- Bazinet, M.; Pântea, V.; Placinta, G.; Moscalu, I.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Larovoi, L.; Smesnoi, V.; Musteata, T.; et al. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients with Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology 2020, 158, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, A. HBsAg, Subviral Particles, and Their Clearance in Establishing a Functional Cure of Chronic Hepatitis B Virus Infec-tion. ACS Infect. Dis. 2021, 7, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Zoulim, F.; Fournier, C.; Habersetzer, F.; Sprinzl, M.; Pol, S.; Coffin, C.S.; Leroy, V.; MA, M.; Wedemeyer, H.; Lohse, A.W.; et al. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: A phase 1b placebo-controlled trial. Hum. Vaccin. Immunother. 2020, 16, 388–399. [Google Scholar] [CrossRef]
- Luk, A.; Jiang, Q.; Glavini, K.; Triyatni, M.; Zhao, N.; Racek, T.; Zhu, Y.; Grippo, J. A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers. Clin. Transl. Sci. 2020, 13, 985–993. [Google Scholar] [CrossRef]
- Daffis, S.; Balsitis, S.; Chamberlain, J.; Zheng, J.; Santos, R.; Rowe, W.; Ramakrishnan, D.; Pattabiraman, D.; Spurlock, S.; Chu, R.; et al. Toll-Like Receptor 8 Agonist GS-9688 Induces Sustained Efficacy in the Woodchuck Model of Chronic Hepatitis, B. Hepatology 2021, 73, 53–67. [Google Scholar] [CrossRef]
- Pan, J.L.; Xu, X.Y. Research progress of siRNA in reducing serum HBsAg levels in patients with chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi 2020, 28, 179–182. [Google Scholar] [CrossRef]
- Gallay, P.; Ure, D.; Bobardt, M.; Chatterji, U.; Ou, J.; Trepanier, D.; Foster, R. The cyclophilin inhibitor CRV431 inhibits liver HBV DNA and HBsAg in transgenic mice. PLoS ONE 2019, 14, e0217433. [Google Scholar] [CrossRef]
- Yuen, M.F.; Heo, J.; Kumada, H.; Suzuki, F.; Suzuki, Y.; Xie, Q.; Jia, J.; Karino, Y.; Hou, J.; Chayama, K.; et al. Phase IIa, randomised, double-blind study of GSK3389404 in pa-tients with chronic hepatitis B on stable nucleos(t)ide therapy. J. Hepatol. 2022, 77, 967–977. [Google Scholar] [CrossRef]
- Aiolfi, R.; Sitia, G. Chronic hepatitis B: Role of anti-platelet therapy in inflammation control. Cell Mol. Immunol. 2015, 12, 264–268. [Google Scholar] [CrossRef]
- Iannacone, M.; Sitia, G.; Isogawa, M.; Marchese, P.; Castro, M.G.; Lowenstein, P.R.; Chisari, F.V.; Ruggeri, Z.M.; Guidotti, L.G. Platelets mediate cytotoxic T lympho-cyte-induced liver damage. Nat. Med. 2005, 11, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).