Amoxicillin-Resistant Streptococci Carriage in the Mouths of Children: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
| Reference/ Country Quality Score | Sample Size/ Study Design | Collection/Isolation/Identification Methods | Results | |
|---|---|---|---|---|
| Resistance Value Considered (RVC)/ AMPC-RS Found | Number of Children Carrying AMPC-RS | |||
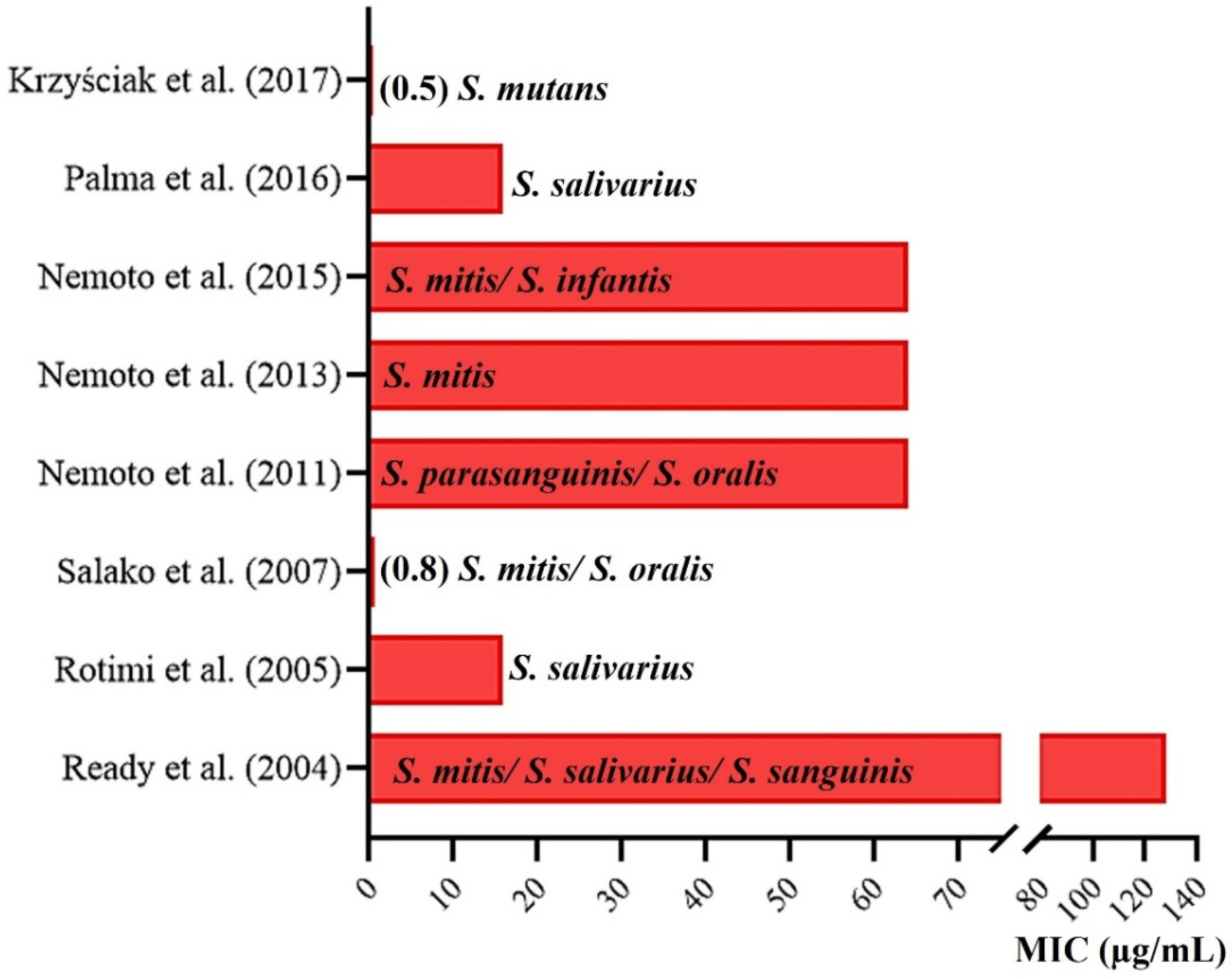
| Ready et al. (2004) [20] United Kingdom 1:S; 2:S; 3:N; 4:S; 5:S; 6:S; 7:S; Score: 6 | A total of 40 children. Group 1 consisted of 25 who had not used any class of ATB in the 3 months prior to sampling, and group 2 consisted of 15 who had used AMPC in the previous 3 months. | Supragingival dental biofilm removed from tooth surfaces. Calcium alginate swab. Iso-Sensitest with 5% defibrinated horse blood used for the isolation. Identification by using molecular biology techniques. | RVC: ≥8 µg/mL. All the children harbored AMPC-resistant bacteria. Of 224 isolates (MIC range, 8 to 128 μg/mL), 128 AMPC-resistant bacteria were isolated from group 1, and 96 isolated from group 2. The median percentage of the total cultivable oral microbiota resistant to AMPC (mainly Haemophilus spp., Streptococcus spp. and Veillonella spp.) was 2.4% in children without AMPC use and 10.9% in children with AMPC use. | The work does not present this data. However, it points out that the 40 (100%) children carried different strains of bacteria resistant to different ATBs. |
| Rotimi et al. (2005) [22] Kuwait 1:S; 2:S; 3:N; 4:S; 5:N; 6:S; 7:S; Score: 5 | A total of 102 children between 5 and 12 years old of which 88 were from Kuwait and 14 were from other countries. | Supragingival biofilm of deciduous and permanent molars beyond the tongue’s surface. Sterile curettes. Mitis Salivarius agar used for the isolation. Identification by using API 20 Strep test kits (BioMerieux). | RVC: >0.25 µg/mL. In total, 540 strains were isolated. S. salivarius was found most frequently (21.5%), followed by S. sanguis (16.3%). S. mitis (55.9%), S. oralis (55.0%), S. intermedius (52.3%), S. anginosus (45.0%), S. sanguinis (45.2%), S. bovis (41.7%), S. salivarius (39.6%) and S. mutans (36.8%) showed high resistance to AMPC. | The work does not present this data. |
| Salako et al. (2007) [21] Kuwait 1:S; 2:S; 3:N; 4:S; 5:N; 6:S; 7: S; Score: 5 | A total of 102 healthy and 102 mentally disabled children between 5 and 12 years old. | Supragingival dental biofilm of all primary and permanent molars. Sterile curettes. Mitis Salivarius agar used for the isolation. Identification by using API 20 Strep test kits (BioMerieux). | RVC: >0.25 µg/mL. In total, 741 strains, 330 from healthy children and 411 from those with disabilities. A higher prevalence of S. salivarius in healthy individuals (27.3%) and S. sanguis in disabled individuals (22.6%) was found. A high frequency of AMPC-RS, except S. mutans, was observed in 18% of healthy children and 21% of children with disabilities. | The work does not present this data. |
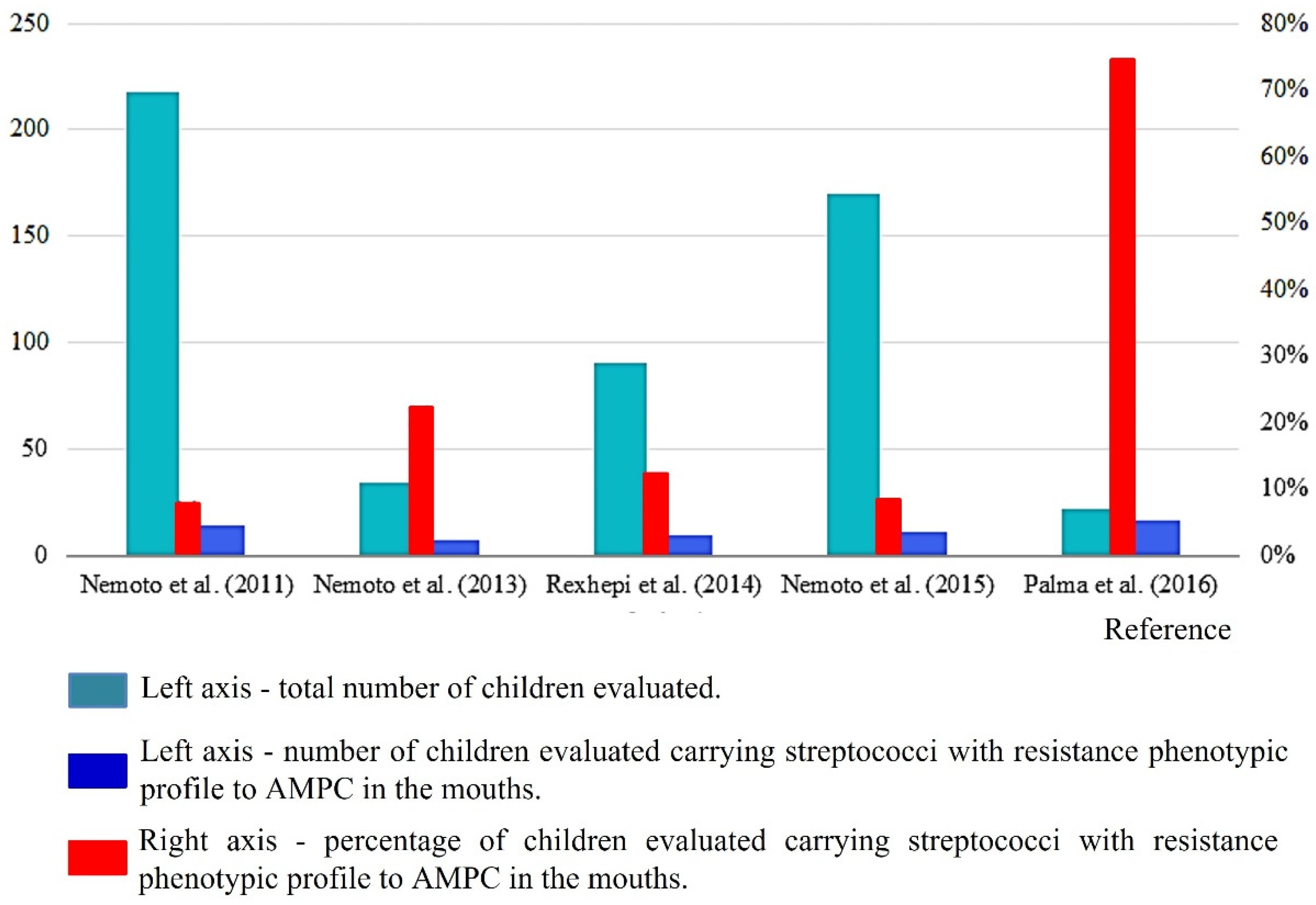
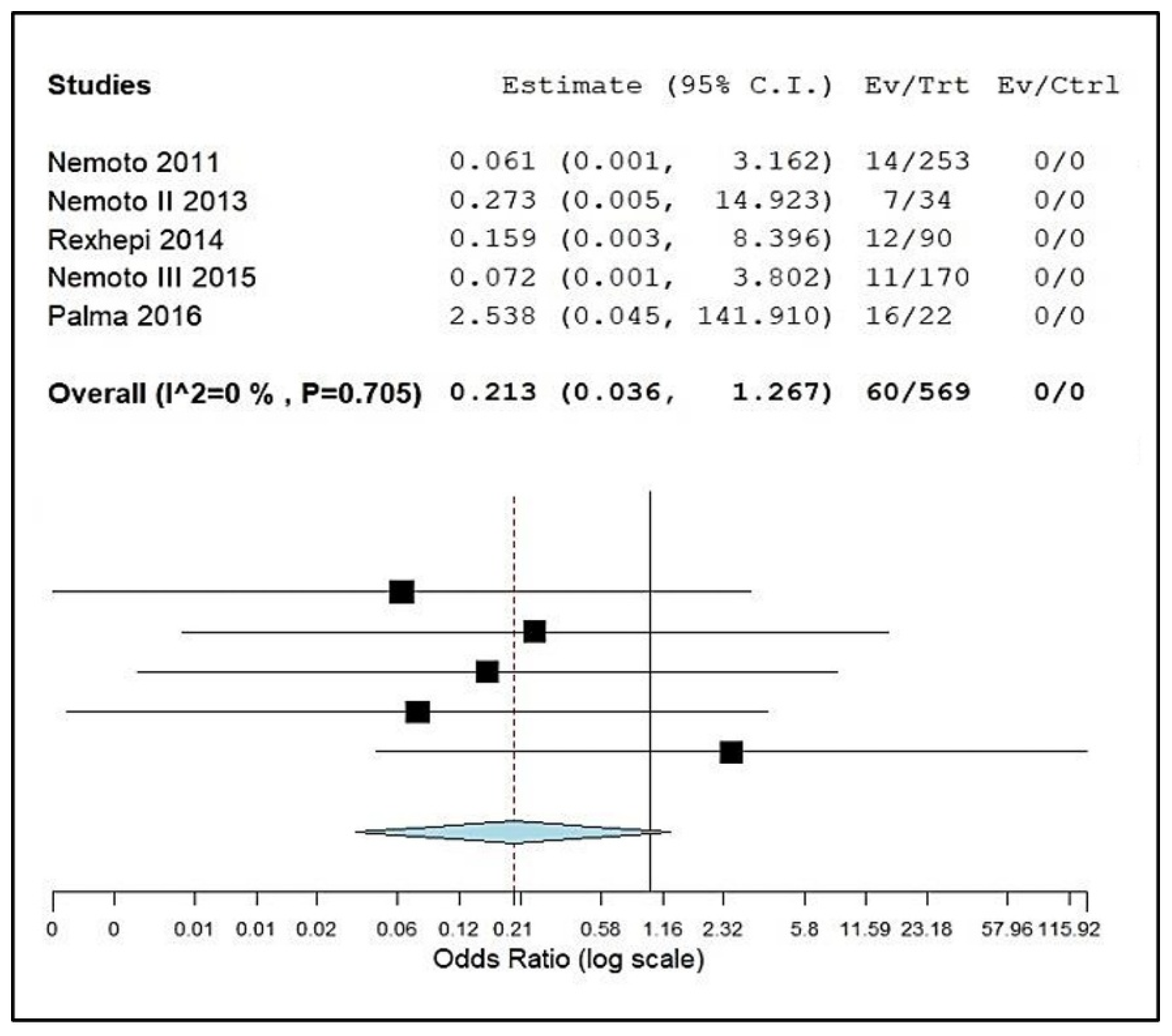
| Nemoto et al. (2011) [13] Japan 1:S; 2:S; 3:N; 4:S; 5:S; 6:S; 7:S; Score: 6 | A total of 253 systemically healthy children, teenagers and young adults (2–22 years). | Supra and subgingival biofilm were collected from all teeth. Sterile instrument. Mitis Salivarius agar used for the isolation. Identification by using molecular biology techniques. | RVC: ≥8 µg/mL. Resistant bacteria were selected with selective streptococcal agar with 32 µg/mL of AMPC. In total, 344 strains were isolated; 18 streptococcal strains from 14 patients were highly resistant to AMPC. MICs for these strains ranged from 16 to 64 µg/mL. | A total of 14 children aged between 3 and 9 years. |
| Nemoto et al. (2013) [18] Japan 1:S; 2:S; 3:S; 4:S; 5:S; 6:S; 7:S; Score: 7 | A total of 34 children and adolescents aged 4–19 years with a high risk of developing IE. | Supra and subgingival biofilm was collected from all teeth. Sterile instrument. Mitis Salivarius agar used for the isolation. Identification by using molecular biology techniques. | RVC: ≥8 µg/mL. Resistant bacteria were selected with selective streptococcal agar with 32 µg/mL of AMPC. In total, 9 resistant strains (MIC of 16–64 μg/mL) were found in 7 individuals, each of which was also resistant to other analyzed ATBs, except for new quinolones. | A total of 6 children with AMPC-RS and 1 individual aged 18 years. |
| Fysal et al. (2013) [16] India 1:S; 2:S; 3:N; 4:S; 5:N; 6:S; 7:S; Score: 5 | A total of 50 children aged 6 to 12. | Contents of the oral cavity. Swabs, probes and curettes. Blood agar used for the isolation. Identification by using biochemical tests. | RVC: <20 mm (inhibition halo). In total, 3 strains of S. mutans were isolated. All had a diameter > 24 mm, showing no resistance. | The work does not present this data. |
| Rexhepi et al. (2014) [23] Kosovo 1:S; 2:N; 3:N; 4:S; 5:S; 6:N; 7:N; Score: 4 | A total of 90 patients aged 6 to 15 years divided into 3 groups: (n = 30) healthy; (n = 30) with CHA without using ATB in the last 3 months, and (n = 30) with CHA who used ATB in the last 3 months. | Supragingival dental biofilm of dental surfaces. Sterile swab. Nutrient or Blood agar used for the isolation. Identification by using VITEK 2 (BioMerieux) and colorimetric GP card. | The RVC for the research is not mentioned. The disk diffusion method was carried out. In total, S. mitis was more present (37.2%), followed by other cocci (8.6%) and then S. sanguinis (7.8%). High resistance to AMPC was observed for S. sanguinis (20%), then S. oralis (13.6%), S. mitis (12.9%) and S. salivarius (11.1%). | 9 children. |
| Loyola-Rodriguez et al. (2014) [24] Mexico 1:S; 2:S; 3:N; 4:S; 5:N; 6:S; 7:S; Score: 5 | A total of 60 children that needed dental treatment for infections and acute symptoms in the primary dentition. | Content inside the canals of deciduous teeth. Endodontic file. BHI agar used for the isolation. Identification by using molecular biology techniques. | RVC: ≥8 µg/mL. Collected samples were inoculated in a culture medium with clindamycin or AMPC at 8 or 16 μg/mL. S. oralis and S mutans highly resistant to ATBs were found in 75% and 45% of the samples, respectively. The authors do not associate to which ATB these proportions are related. | The work does not present this data. |
| Nemoto et al. (2015) [27] Japan 1:S; 2:S; 3:N; 4:S; 5:S; 6:S; 7:S; Score: 6 | A total of 170 children (4–13 years) and their mothers (150) aged 26 to 49 years, systematically healthy and without ATB use in the last 3 months. | Unstimulated expectorated saliva. Sterile plastic tube. Mitis Salivarius agar used for the isolation. Identification by using molecular biology techniques. | RCV: ≥8 µg/mL. Resistant bacteria were selected with selective streptococcal agar with 32 µg/mL of AMPC. MICs ranged from 16–64 µg/mL. Streptococci highly resistant to AMPC were isolated from 11 children and 7 mothers, which included four mother–child pairs. | 11 children. |
| Palma et al. (2016) [28] Brazil 1:S; 2:S; 3:N; 4:S; 5:S; 6:S; 7:S; Score: 6 | A total of 22 oral and systemically healthy infants aged 2 to 16 months were included. | Samples were collected from the oral mucosa. Sterile swab. BHI agar with 5% sheep defibrinated blood and Mitis Salivarius agar were used for the isolation. Identification by using molecular biology techniques. | RCV: ≥4 µg/mL. In total, 95 S. salivarius strains were evaluated. High frequencies of infants carrying strains with intermediate resistance to AMPC (n = 16, 72.7%) were found. Among the 95 isolates tested, 75 strains (78.9%) were resistant to at least one ATB among those tested, and 27 (28.4%) were resistant to two or more classes of ATB. MIC of AMPC ranged from 0.03–16 µg/mL. | 19 infants. |
| Krzyściak et al. (2017) [26] Poland 1:S; 2:S; 3:N; 4:S; 5:N; 6:S; 7:N; Score: 4 | A total of 143 children with an average age of 4.6 years old. Sample collection in children with and without cavitations due to dental caries. | Dental biofilm on the surface of deciduous molars with early caries. Sterile curettes. HLR-S agar used for the isolation. Identification by using biochemical tests (STREPTOtest, Lachema). | An RCV was not presented for the evaluated ATBs. In total, 142 S. mutans strains were divided into four clusters that showed different sensitivity profiles to different ATBs. It was observed that 13% of the isolates were resistant to penicillin, while all were sensitive to vancomycin. The highest MIC of AMPC for S. mutans found was 0.5 µg/mL. | The work does not present this data. |
| Ali Mahmood et al. (2018) [17] Iraq 1:N; 2:N; 3:N; 4:S; 5:N; 6:S; 7:S; Score: 3 | A total of 60 dental plaque samples were collected from children aged 3 to 5. | Material collected from caries lesions and supragingival biofilm. Sterile swab. Unclear isolation method. Identification by using biochemical tests. | RVC was not presented for the ATBs evaluated. Of the 120 isolates, 50% were Streptococcus spp. The authors tested 100 µL of a 125 mg/5 mL suspension of marketed AMPC from four brands. The diameters of the inhibition zones were observed for S. mutans, that were considered slightly susceptible to these drugs. | The work does not present this data. |
| Al-Shami et al. (2019) [25] Yemen 1:N; 2:N; 3:N; 4:S; 5:N; 6:S; 7:S; Score: 3 | A total of 87 biofilm samples from children (2–5 years old) and 87 from their mothers (33–44 years old) with active caries. It was not clear how many mothers and children were evaluated. | Supragingival dental biofilm from sites with active caries. Unclear collection. Mitis Salivarius agar with potassium tellurite, bacitracin and 20% sucrose used for the isolation. Unclear identification method. | An RCV was not presented for the ATBs evaluated by the halo formation method. In total, 174 specimens of S. mutans were evaluated. The resistance rate of S. mutans to AMPC was 14.9% in isolates from mothers and 12.6% in children. | The work does not present this data. |
3. Discussion
4. Materials and Methods
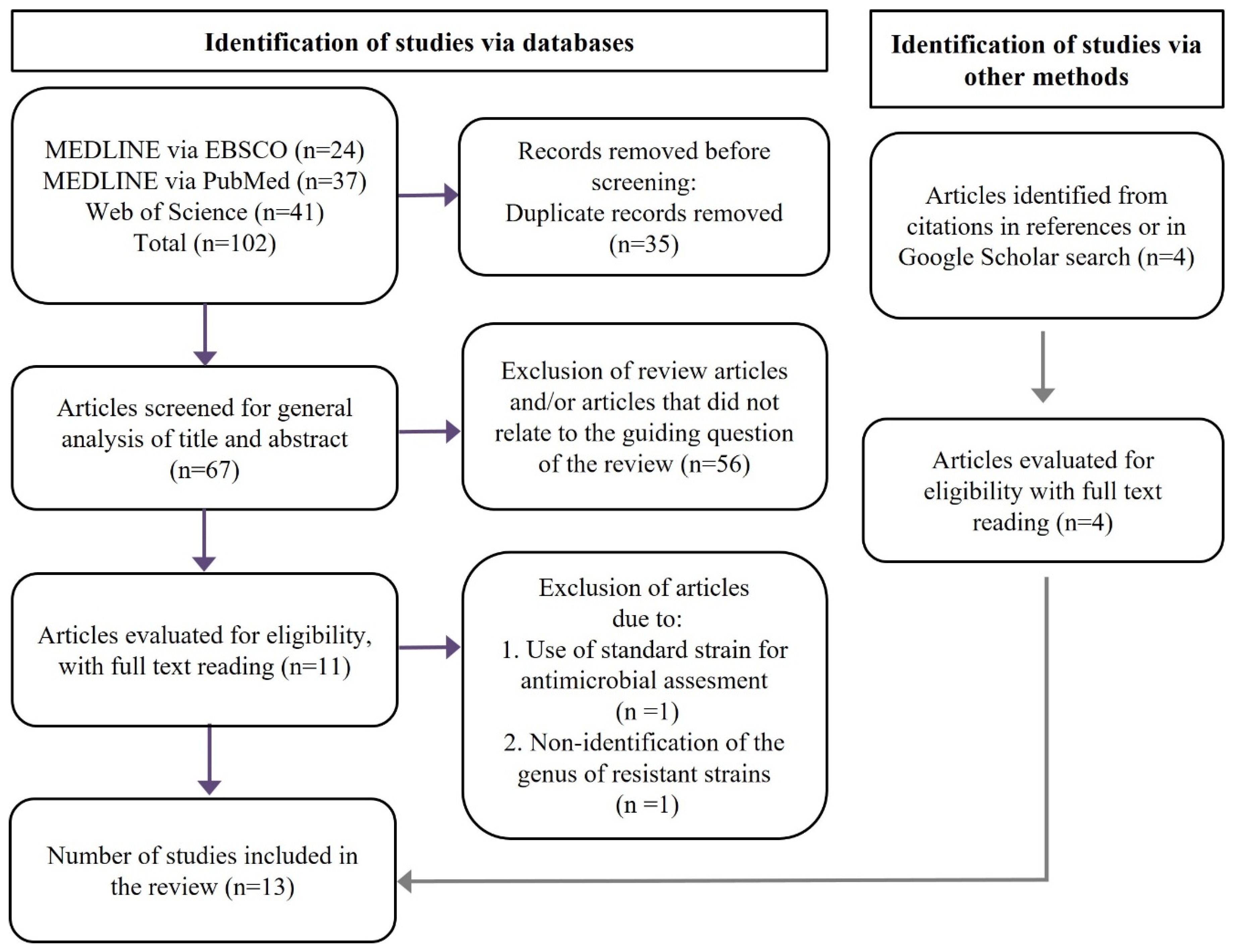
4.1. Selection of Studies
4.2. Data Extraction and Quality Assessment of Included Studies
4.3. Evidence Synthesis and Statistical Analysis (Meta-Analysis)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, L.K.K.; Lacey, S.; Mandalia, S.; Melzer, M. Hospital-based study of viridans streptococcal bacteremia in children and adults. J. Infect. 2008, 56, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Paz, D.L.; Lakbar, I.; Tattevin, P. A review of current treatment strategies for infective endocarditis. Expert Rev. Anti-Infect. Ther. 2021, 19, 297–307. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Duval, X. Infective endocarditis. N. Engl. J. Med. 2013, 368, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Abolfathi, G.; Mahmoudi, E.; Mohammadzadeh, Z. Evaluation of Salivary Streptococcus mutans and Dental Caries in Children with Heart Diseases. J. Dent. Res. Dent. Clin. Dent. Prospects. 2015, 9, 105–108. [Google Scholar] [CrossRef]
- Yumoto, H.; Hirota, K.; Hirao, K.; Ninomiya, M.; Murakami, K.; Fujii, H.; Miyake, Y. The Pathogenic Factors from Oral Streptococci for Systemic Diseases. Int. J. Mol. Sci. 2019, 20, 4571. [Google Scholar] [CrossRef]
- Wilson, W.R.; Taubert, K.A.; Gewitz, M.H.; Lockhart, P.B.; Baddour, L.M.; E Levison, M.; Bolger, A.F.; Cabell, C.H.; Takahashi, M.; Baltimore, R.S.; et al. Prevention of infective endocarditis: Guidelines from the American Heart Association. Circulation 2007, 116, 1736–1754. [Google Scholar] [CrossRef]
- Baltimore, R.S.; Gewitz, M.; Baddour, L.M.; Beerman, L.B.; Jackson, M.A.; Lockhart, P.B.; Pahl, E.; Schutze, G.E.; Shulman, S.T.; Willoughby, R. Infective Endocarditis in Childhood: 2015 Update—A Scientific Statement from the American Heart Association. Circulation 2015, 132, 1487–1515. [Google Scholar] [CrossRef]
- Wilson, W.R.; Gewitz, M.; Lockhart, P.B.; Bolger, A.F.; DeSimone, D.C.; Kazi, D.S.; Couper, D.J.; Beaton, A.; Kilmartin, C.; Miro, J.M.; et al. Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement From the American Heart Association. Circulation 2021, 143, 963–978. [Google Scholar] [CrossRef]
- Drawz, S.M.; Bonomo, R.A. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef]
- Ware, A.L.; Tani, L.Y.; Weng, H.Y.; Wilkes, J.; Menon, S.C. Resource utilization and out-comes of infective endocarditis in children. J. Pediatr. 2014, 165, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Bryskier, A. Viridans group streptococci: A reservoir of resistant bacteria in oral cavities. Clin. Microbiol. Infect. 2002, 8, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Nakano, K.; Masuda, K.; Wada, K.; Ardin, A.C.; Nomura, R.; Ooshima, T. Distribution of oral streptococci highly resistant to amoxicillin in dental plaque specimens from Japanese children and adolescents. J. Med. Microbiol. 2011, 60, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Nemoto, H.; Nakano, K.; Naka, S.; Nomura, R.; Ooshima, T. Amoxicillin-resistant oral streptococci identified in dental plaque specimens from healthy Japanese adults. J. Cardiol. 2012, 59, 285–290. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Clinical and Laboratory Standards Institute: M100 Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2021; Volume 31, p. 103. [Google Scholar]
- Fysal, N.; Jose, S.; Kulshrestha, R.; Arora, D.; Abdul Hafiz, K.A.; Vasudevan, S. Antibiogram pattern of oral microflora in periodontic children of age group 6 to 12 years: A clinicomicro-biological study. J. Contemp. Dent. Pract. 2013, 14, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ali Mahmood, R.; Abidali, A.-S.Z. Effect of amoxicillin use on Streptococcus mutans in children. Antimicrob Agents Chemother. 2018, 54, 1148–1151. [Google Scholar]
- Nemoto, H.; Nomura, R.; Ooshima, T.; Nakano, K. Distribution of amoxicillin-resistant oral streptococci in dental plaque specimens obtained from Japanese children and adolescents at risk for infective endocarditis. J. Cardiol. 2013, 62, 296–300. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ready, D.; Lancaster, H.; Qureshi, F.; Bedi, R.; Mullany, P.; Wilson, M. Effect of amoxicillin use on oral microbiota in young children. Antimicrob. Agents Chemother. 2004, 48, 2883–2887. [Google Scholar] [CrossRef]
- Salako, N.O.; Rotimi, V.; Philip, L.; Haidar, H.A.; Hamdan, H.M. The prevalence and anti-biotic sensitivity of oral Viridans streptococci in healthy children and children with disabilities in Kuwait. Spec. Care Dent. 2007, 27, 67–72. [Google Scholar] [CrossRef]
- Rotimi, V.O.; Salako, N.O.; Mokaddas, E.; Philip, L.; Rajan, P. High frequency of isolation of ATB-resistant oral viridans streptococci from children in Kuwait. J. Chemother. 2005, 17, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Rexhepi, A.; Niko, R.; Begzati, A.; Loxha, M.P.; Krasniqi, V.; Agani, Z.; Xhemajli, B.; Kutllovci, T.; Tahiri, U. Dental Plaque Streptococci and Their Amoxicillin Resistance in Children with Congenital Heart Anomalies: Results of a Prospective Study. Open J. Stomatol. 2014, 4, 345–351. [Google Scholar] [CrossRef][Green Version]
- Loyola-Rodriguez, J.P.; Garcia-Cortes, J.O.; Martinez-Martinez, R.E.; Patiño-Marin, N.; Mar-tinez-Castañon, G.A.; Zavala-Alonso, N.V.; Amano, A. Molecular identification and ATB resistant bacteria isolated from primary dentition infections. Aust. Dent. J. 2014, 59, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Al-shami, I.Z.; Al-Hamzi, M.A.; Al-Shamahy, H.A.; Majeed, A.L.A.A. Efficacy of some ATBs against Streptococcus Mutans Associated with Tooth decay in Children and their Mothers. Online J. Dent. Oral Health 2019, 2, 1–4. [Google Scholar] [CrossRef]
- Krzyściak, W.; Kościelniak, D.; Papiez, M.; Jurczak, A.; Vyhouskaya, P. Methods of Biotyping of Streptococcus mutans Species with the Routine Test as a Prognostic Value in Early Childhood Caries. Evid. -Based Complement. Altern Med. 2017, 2017, 6859543. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Nomura, R.; Nakano, K. Isolation of amoxicillin-resistant oral streptococci from children and their mothers. Pediatr. Dent. J. 2015, 25, 8–13. [Google Scholar] [CrossRef]
- Palma, T.H.; Harth-Chú, E.N.; Scott, J.; Stipp, R.N.; Boisvert, H.; Salomão, M.F.; Theobaldo, J.D.; Possobon, R.F.; Nascimento, L.C.; McCafferty, J.W.; et al. Oral cavities of healthy infants harbour high proportions of Streptococcus salivarius strains with phenotypic and genotypic resistance to multiple classes of ATBs. J. Med. Microbiol. 2016, 65, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Kavvoura, F.K.; Ioannidis, J.P. Methods for meta-analysis in genetic association studies: A review of their potential and pitfalls. Hum. Genet. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Lannes-Costa, P.S.; de Oliveira, J.S.S.; da Silva Santos, G.; Nagao, P.E. A current review of pathogenicity determinants of Streptococcus sp. J. Appl. Microbiol. 2021, 131, 1600–1620. [Google Scholar] [CrossRef]
- Dulon, M.; Peters, C.; Schablon, A.; Nienhaus, A. MRSA carriage among healthcare workers in non-outbreak settings in Europe and the United States: A systematic review. BMC Infect. Dis. 2014, 14, 363. [Google Scholar] [CrossRef]
- Zango, U.U.; Ibrahim, M.; Shawai, S.A.A.; Shamsuddin, I.M. A review on β-lactam antibiotic drug resistance. MOJ Drug Des. Develop. Ther. 2019, 3, 52–58. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Health 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
| Item | Criteria | Question | Yes | No |
|---|---|---|---|---|
| 01 | Population specification | Are the subjects and population studied adequately described? | ||
| 02 | Collection method | Is there a description of the collection instrument, the selected locations and the form of transport to the analysis? | ||
| 03 | Sources of bias | Are risks of bias and/or methodological limitations presented and/or discussed? | ||
| 04 | Information about species of resistant strains | Are resistant strains presented at the species level? | ||
| 05 | Inference on the epidemiological prevalence | Do the results present data from which the prevalence of individuals carrying strains with some degree of resistance can be inferred? | ||
| 06 | Controls for reproducibility | Are the methods (MIC *, inhibition halos, biochemical and molecular tests) properly used to determine ATB sensitivity or not? | ||
| 07 | Interpretation of results | Are antibiotic sensitivity or non-susceptibility standards presented and/or discussed following internationally recognized criteria? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo Júnior, A.G.; Costa, M.L.V.A.; Silva, F.R.P.; Arcanjo, D.D.R.; Moura, L.F.A.D.; Oliveira, F.A.A.; Soares, M.J.S.; Quelemes, P.V. Amoxicillin-Resistant Streptococci Carriage in the Mouths of Children: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1114. https://doi.org/10.3390/pathogens11101114
Araújo Júnior AG, Costa MLVA, Silva FRP, Arcanjo DDR, Moura LFAD, Oliveira FAA, Soares MJS, Quelemes PV. Amoxicillin-Resistant Streptococci Carriage in the Mouths of Children: A Systematic Review and Meta-Analysis. Pathogens. 2022; 11(10):1114. https://doi.org/10.3390/pathogens11101114
Chicago/Turabian StyleAraújo Júnior, Ayrton G., Marina L. V. A. Costa, Felipe R. P. Silva, Daniel D. R. Arcanjo, Lúcia F. A. D. Moura, Felipe A. A. Oliveira, Maria J. S. Soares, and Patrick V. Quelemes. 2022. "Amoxicillin-Resistant Streptococci Carriage in the Mouths of Children: A Systematic Review and Meta-Analysis" Pathogens 11, no. 10: 1114. https://doi.org/10.3390/pathogens11101114
APA StyleAraújo Júnior, A. G., Costa, M. L. V. A., Silva, F. R. P., Arcanjo, D. D. R., Moura, L. F. A. D., Oliveira, F. A. A., Soares, M. J. S., & Quelemes, P. V. (2022). Amoxicillin-Resistant Streptococci Carriage in the Mouths of Children: A Systematic Review and Meta-Analysis. Pathogens, 11(10), 1114. https://doi.org/10.3390/pathogens11101114







