No Evidence for the Presence of SARS-CoV-2 in Bank Voles and Other Rodents in Germany, 2020–2022
Abstract
:1. Introduction
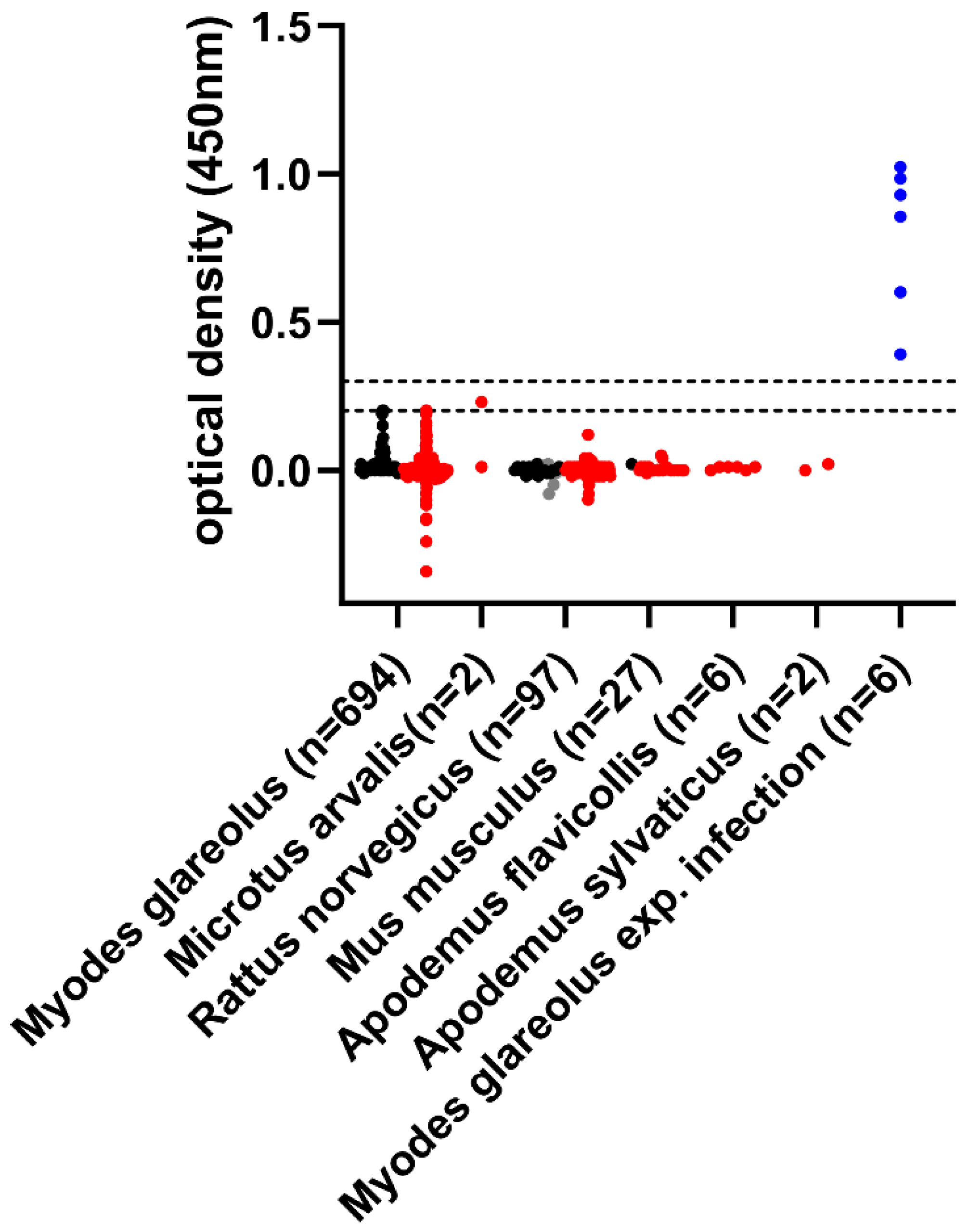
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordes, F.; Blasdell, K.; Morand, S. Transmission ecology of rodent-borne diseases: New frontiers. Integr. Zool. 2015, 10, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Wasberg, A.; Raghwani, J.; Li, J.; Pettersson, J.H.; Lindahl, J.F.; Lundkvist, Å.; Ling, J. Discovery of a novel coronavirus in Swedish bank voles (Myodes glareolus). Viruses 2022, 14, 1205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- WHO. COVID-19 Strategy Update—14 April 2020. 2020. Available online: https://www.who.int/publications-detail/covid-19-strategy-update---14-april-2020 (accessed on 16 May 2020).
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Michelitsch, A.; Wernike, K.; Ulrich, L.; Mettenleiter, T.C.; Beer, M. SARS-CoV-2 in animals: From potential hosts to animal models. Adv. Virus Res. 2021, 110, 59–102. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Lauth, A.M.; Root, J.J.; Porter, S.M.; Walker, A.E.; Guilbert, L.; Hawvermale, D.; Pepper, A.; Maison, R.M.; Hartwig, A.E.; Gordy, P.; et al. Peridomestic mammal susceptibility to severe acute respiratory syndrome coronavirus 2 infection. Emerg. Infect. Dis. 2021, 27, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Tarrés-Freixas, F.; Trinité, B.; Pons-Grífols, A.; Romero-Durana, M.; Riveira-Muñoz, E.; Ávila-Nieto, C.; Pérez, M.; Garcia-Vidal, E.; Perez-Zsolt, D.; Muñoz-Basagoiti, J.; et al. Heterogeneous infectivity and pathogenesis of SARS-CoV-2 variants beta, delta and omicron in transgenic K18-hACE2 and wildtype mice. Front. Microbiol. 2022, 13, 840757. [Google Scholar] [CrossRef] [PubMed]
- Montagutelli, X.; Prot, M.; Levillayer, L.; Salazar, E.; Jouvion, G.; Conquet, L.; Beretta, M.; Donati, F.; Albert, M.; Gambaro, F.; et al. Variants with the N501Y mutation extend SARS-CoV-2 host range to mice, with contact transmission. bioRxiv 2021. [Google Scholar] [CrossRef]
- Trimpert, J.; Vladimirova, D.; Dietert, K.; Abdelgawad, A.; Kunec, D.; Dökel, S.; Voss, A.; Gruber, A.D.; Bertzbach, L.D.; Osterrieder, N. The Roborovski dwarf hamster is a highly susceptible model for a rapid and fatal course of SARS-CoV-2 infection. Cell Rep. 2020, 33, 108488. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, N.; Bertzbach, L.D.; Dietert, K.; Abdelgawad, A.; Vladimirova, D.; Kunec, D.; Hoffmann, D.; Beer, M.; Gruber, A.D.; Trimpert, J. Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters. Viruses 2020, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.F.; Yan, L.M.; Chin, A.W.H.; Fung, K.; Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Vladimirova, D.; Dietert, K.; Abdelgawad, A.; Gruber, A.D.; Osterrieder, N.; Trimpert, J. SARS-CoV-2 infection of Chinese hamsters (Cricetulus griseus) reproduces COVID-19 pneumonia in a well-established small animal model. Transbound. Emerg. Dis. 2020, 68, 1075–1079. [Google Scholar] [CrossRef]
- Rosenke, K.; Meade-White, K.; Letko, M.; Clancy, C.; Hansen, F.; Liu, Y.; Okumura, A.; Tang-Huau, T.L.; Li, R.; Saturday, G.; et al. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg. Microbes Infect. 2020, 9, 2673–2684. [Google Scholar] [CrossRef]
- Yen, H.L.; Sit, T.H.C.; Brackman, C.J.; Chuk, S.S.Y.; Gu, H.; Tam, K.W.S.; Law, P.Y.T.; Leung, G.M.; Peiris, M.; Poon, L.L.M. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: A case study. Lancet 2022, 399, 1070–1078. [Google Scholar] [CrossRef]
- Chan, J.F.; Siu, G.K.; Yuan, S.; Ip, J.D.; Cai, J.P.; Chu, A.W.; Chan, W.M.; Abdullah, S.M.U.; Luo, C.; Chan, B.P.; et al. Probable animal-to-human transmission of SARS-CoV-2 delta variant AY.127 causing a pet shop-related COVID-19 outbreak in Hong Kong. Clin. Infect. Dis. 2022, 75, e76–e81. [Google Scholar] [CrossRef]
- Ulrich, L.; Michelitsch, A.; Halwe, N.; Wernike, K.; Hoffmann, D.; Beer, M. Experimental SARS-CoV-2 infection of bank voles. Emerg. Infect. Dis. 2021, 27, 1193–1195. [Google Scholar] [CrossRef]
- Griffin, B.D.; Chan, M.; Tailor, N.; Mendoza, E.J.; Leung, A.; Warner, B.M.; Duggan, A.T.; Moffat, E.; He, S.; Garnett, L.; et al. SARS-CoV-2 infection and transmission in the North American deer mouse. Nat. Commun. 2021, 12, 3612. [Google Scholar] [CrossRef]
- Fagre, A.; Lewis, J.; Eckley, M.; Zhan, S.; Rocha, S.M.; Sexton, N.R.; Burke, B.; Geiss, B.; Peersen, O.; Bass, T.; et al. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLoS Pathog. 2021, 17, e1009585. [Google Scholar] [CrossRef]
- Badiola, J.J.; Otero, A.; Sevilla, E.; Marín, B.; García Martínez, M.; Betancor, M.; Sola, D.; Pérez Lázaro, S.; Lozada, J.; Velez, C.; et al. SARS-CoV-2 outbreak on a Spanish mink farm: Epidemiological, molecular, and pathological studies. Front. Vet. Sci. 2021, 8, 805004. [Google Scholar] [CrossRef] [PubMed]
- Chaintoutis, S.C.; Thomou, Z.; Mouchtaropoulou, E.; Tsiolas, G.; Chassalevris, T.; Stylianaki, I.; Lagou, M.; Michailidou, S.; Moutou, E.; Koenen, J.J.H.; et al. Outbreaks of SARS-CoV-2 in naturally infected mink farms: Impact, transmission dynamics, genetic patterns, and environmental contamination. PLoS Pathog. 2021, 17, e1009883. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sikkema, R.S.; Velkers, F.C.; Nieuwenhuijse, D.F.; Fischer, E.A.J.; Meijer, P.A.; Bouwmeester-Vincken, N.; Rietveld, A.; Wegdam-Blans, M.C.A.; Tolsma, P.; et al. Adaptation, spread and transmission of SARS-CoV-2 in farmed minks and associated humans in the Netherlands. Nat. Commun. 2021, 12, 6802. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, R.J.; Vreman, S.; Hakze-van der Honing, R.W.; Zwart, R.; de Rond, J.; Weesendorp, E.; Smit, L.A.M.; Koopmans, M.; Bouwstra, R.; Stegeman, A.; et al. Clinical and pathological findings in SARS-CoV-2 disease outbreaks in farmed mink (Neovison vison). Vet. Pathol. 2020, 57, 653–657. [Google Scholar] [CrossRef]
- Oude Munnink, B.B.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; van der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2020, 371, 172–177. [Google Scholar] [CrossRef]
- Sila, T.; Sunghan, J.; Laochareonsuk, W.; Surasombatpattana, S.; Kongkamol, C.; Ingviya, T.; Siripaitoon, P.; Kositpantawong, N.; Kanchanasuwan, S.; Hortiwakul, T.; et al. Suspected cat-to-human transmission of SARS-CoV-2, Thailand, July–September 2021. Emerg. Infect. Dis. 2022, 28, 1485–1488. [Google Scholar] [CrossRef]
- Robert-Koch-Institut. Anzahl und Anteile von VOC und VOI in Deutschland. 2022. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/VOC_VOI_Tabelle.html (accessed on 15 August 2022).
- Zhu, W.; Yang, J.; Lu, S.; Lan, R.; Jin, D.; Luo, X.L.; Pu, J.; Wu, S.; Xu, J. Beta- and novel delta-coronaviruses are identified from wild animals in the Qinghai-Tibetan Plateau, China. Virol. Sin. 2021, 36, 402–411. [Google Scholar] [CrossRef]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef]
- Weiss, S.R.; Leibowitz, J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011, 81, 85–164. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, H.; Li, E.; Guo, Z.; Wang, T.; Yan, F.; Liu, L.; Li, Y.; Chen, D.; Meng, K.; et al. The SARS-CoV-2 B.1.351 variant can transmit in rats but not in mice. Front. Immunol. 2022, 13, 869809. [Google Scholar] [CrossRef]
- Colombo, V.C.; Sluydts, V.; Mariën, J.; Broecke, B.V.; Van Houtte, N.; Leirs, W.; Jacobs, L.; Iserbyt, A.; Hubert, M.; Heyndrickx, L.; et al. SARS-CoV-2 surveillance in Norway rats (Rattus norvegicus) from Antwerp sewer system, Belgium. Transbound. Emerg. Dis. 2021, 69, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Bonanno Ferraro, G.; Veneri, C.; Mancini, P.; Iaconelli, M.; Suffredini, E.; Bonadonna, L.; Lucentini, L.; Bowo-Ngandji, A.; Kengne-Nde, C.; Mbaga, D.S.; et al. A state-of-the-art scoping review on SARS-CoV-2 in sewage focusing on the potential of wastewater surveillance for the monitoring of the COVID-19 pandemic. Food Environ. Virol. 2021, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Barut, T.; Halwe, N.J.; Taddeo, A.; Kelly, J.N.; Schön, J.; Ebert, N.; Ulrich, L.; Devisme, C.; Steiner, S.; Trüeb, B.S.; et al. The spike gene is a major determinant for the SARS-CoV-2 Omicron-BA.1 phenotype. bioRxiv 2022. [Google Scholar] [CrossRef]
- Corleis, B.; Hoffmann, D.; Rauch, S.; Fricke, C.; Roth, N.; Gergen, J.; Kovacikova, K.; Schlottau, K.; Halwe, N.J.; Ulrich, L.; et al. Low-dose bivalent mRNA vaccine is highly effective against different SARS-CoV-2 variants in a transgenic mouse model. bioRxiv 2022. [Google Scholar] [CrossRef]
- Schmidt, E.; Obiegala, A.; Imholt, C.; Drewes, S.; Saathoff, M.; Freise, J.; Runge, M.; Jacob, J.; Mayer-Scholl, A.; Ulrich, R.G.; et al. Influence of season, population and individual characteristics on the prevalence of Leptospira spp. in bank voles in North-West Germany. Biology 2021, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Aebischer, A.; Michelitsch, A.; Hoffmann, D.; Freuling, C.; Balkema-Buschmann, A.; Graaf, A.; Müller, T.; Osterrieder, N.; Rissmann, M.; et al. Multi-species ELISA for the detection of antibodies against SARS-CoV-2 in animals. Transbound. Emerg. Dis. 2021, 68, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Corleis, B.; Rauch, S.; Roth, N.; Mühe, J.; Halwe, N.J.; Ulrich, L.; Fricke, C.; Schön, J.; Kraft, A.; et al. CVnCoV and CV2CoV protect human ACE2 transgenic mice from ancestral B BavPat1 and emerging B.1.351 SARS-CoV-2. Nat. Commun. 2021, 12, 4048. [Google Scholar] [CrossRef] [PubMed]
| Rodent Species | Sample Material | Federal State | Year | Number of Samples |
|---|---|---|---|---|
| Myodes glareolus | blood | NW | 2021 | 614 |
| Myodes glareolus | chest cavity lavage | BB, BW, NI | 2020/21 | 80 |
| Microtus arvalis | chest cavity lavage | BW | 2021 | 2 |
| Mus musculus | chest cavity lavage | HE, SH | 2021/22 | 27 |
| Rattus norvegicus | chest cavity lavage | BB, BW, MV, SH | 2020/21/22 | 97 |
| Apodemus flavicollis | chest cavity lavage | BW, SH | 2021/22 | 6 |
| Apodemus sylvaticus | chest cavity lavage | BW, HE | 2021 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wernike, K.; Drewes, S.; Mehl, C.; Hesse, C.; Imholt, C.; Jacob, J.; Ulrich, R.G.; Beer, M. No Evidence for the Presence of SARS-CoV-2 in Bank Voles and Other Rodents in Germany, 2020–2022. Pathogens 2022, 11, 1112. https://doi.org/10.3390/pathogens11101112
Wernike K, Drewes S, Mehl C, Hesse C, Imholt C, Jacob J, Ulrich RG, Beer M. No Evidence for the Presence of SARS-CoV-2 in Bank Voles and Other Rodents in Germany, 2020–2022. Pathogens. 2022; 11(10):1112. https://doi.org/10.3390/pathogens11101112
Chicago/Turabian StyleWernike, Kerstin, Stephan Drewes, Calvin Mehl, Christin Hesse, Christian Imholt, Jens Jacob, Rainer G. Ulrich, and Martin Beer. 2022. "No Evidence for the Presence of SARS-CoV-2 in Bank Voles and Other Rodents in Germany, 2020–2022" Pathogens 11, no. 10: 1112. https://doi.org/10.3390/pathogens11101112
APA StyleWernike, K., Drewes, S., Mehl, C., Hesse, C., Imholt, C., Jacob, J., Ulrich, R. G., & Beer, M. (2022). No Evidence for the Presence of SARS-CoV-2 in Bank Voles and Other Rodents in Germany, 2020–2022. Pathogens, 11(10), 1112. https://doi.org/10.3390/pathogens11101112






