IgA-Based Secretory Response in Tears of COVID-19 Patients: A Potential Biomarker of Pro-Inflammatory State in Course of SARS-CoV-2 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Medical Examination and Detailed Questionnaires
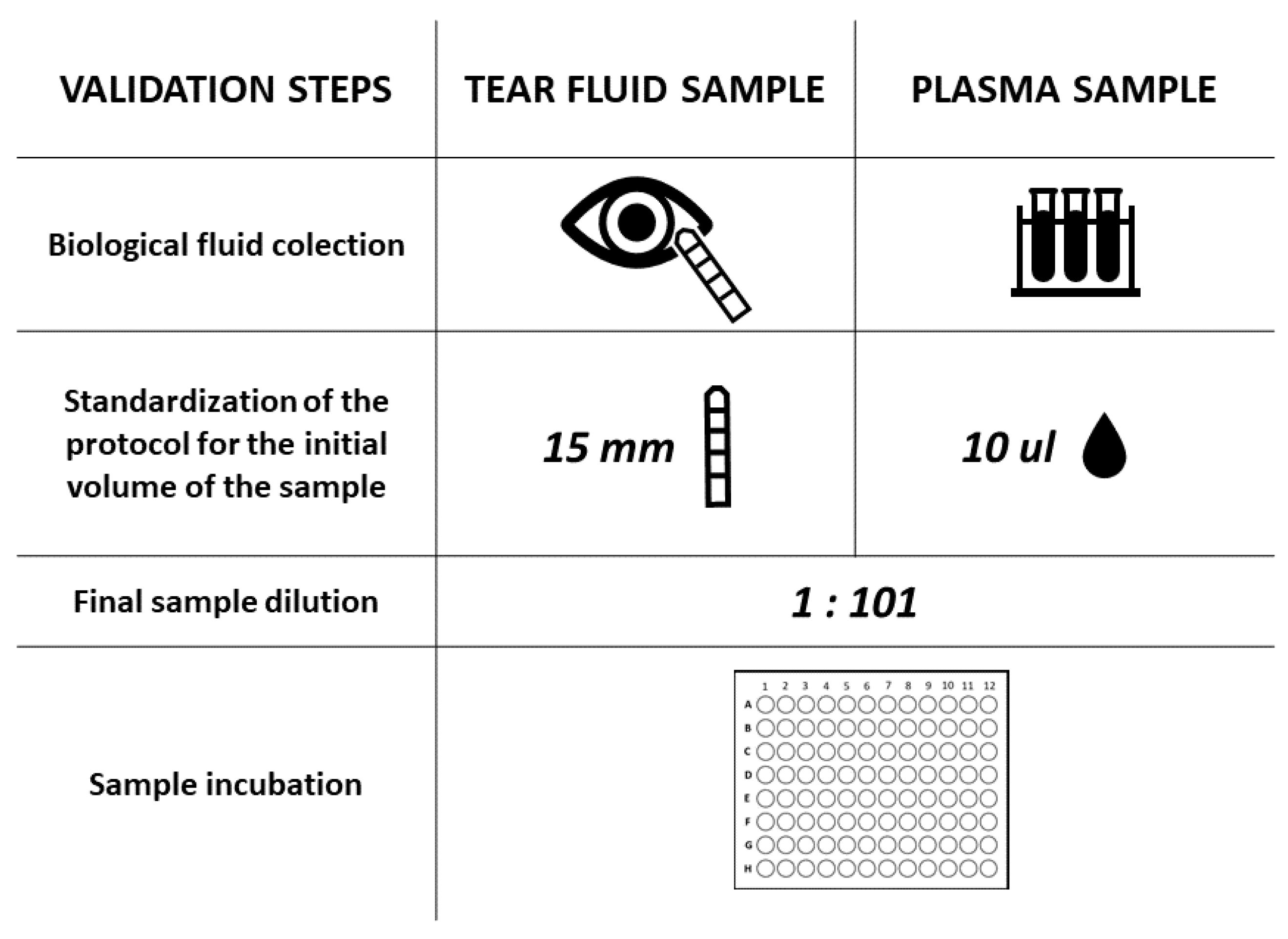
2.3. Tear Sample and Conjunctival Swab Collection
2.4. Plasma Collection
2.5. Serological Assay for Specific Anti-SARS-CoV-2 IgA Antibody Detection
2.6. Luminex Assay
2.7. Identification of COVID-19 Positive Patients
2.7.1. Viral RNA Isolation
2.7.2. qRT–PCR Assays for Detecting SARS-CoV-2 RNA
2.8. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Group
3.2. Detection of Specific Anti-SARS-CoV-2 IgA Antibodies in Tears
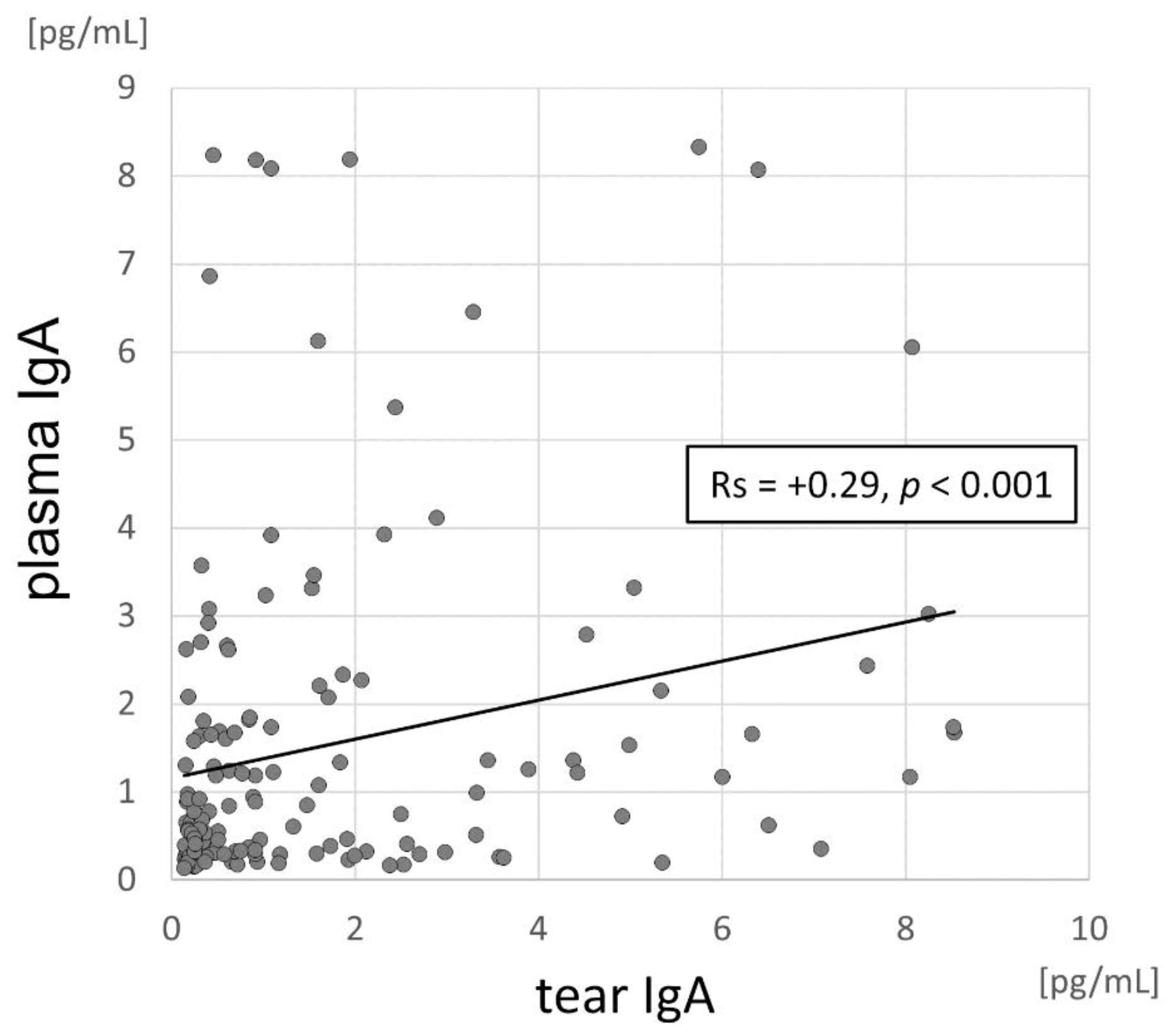
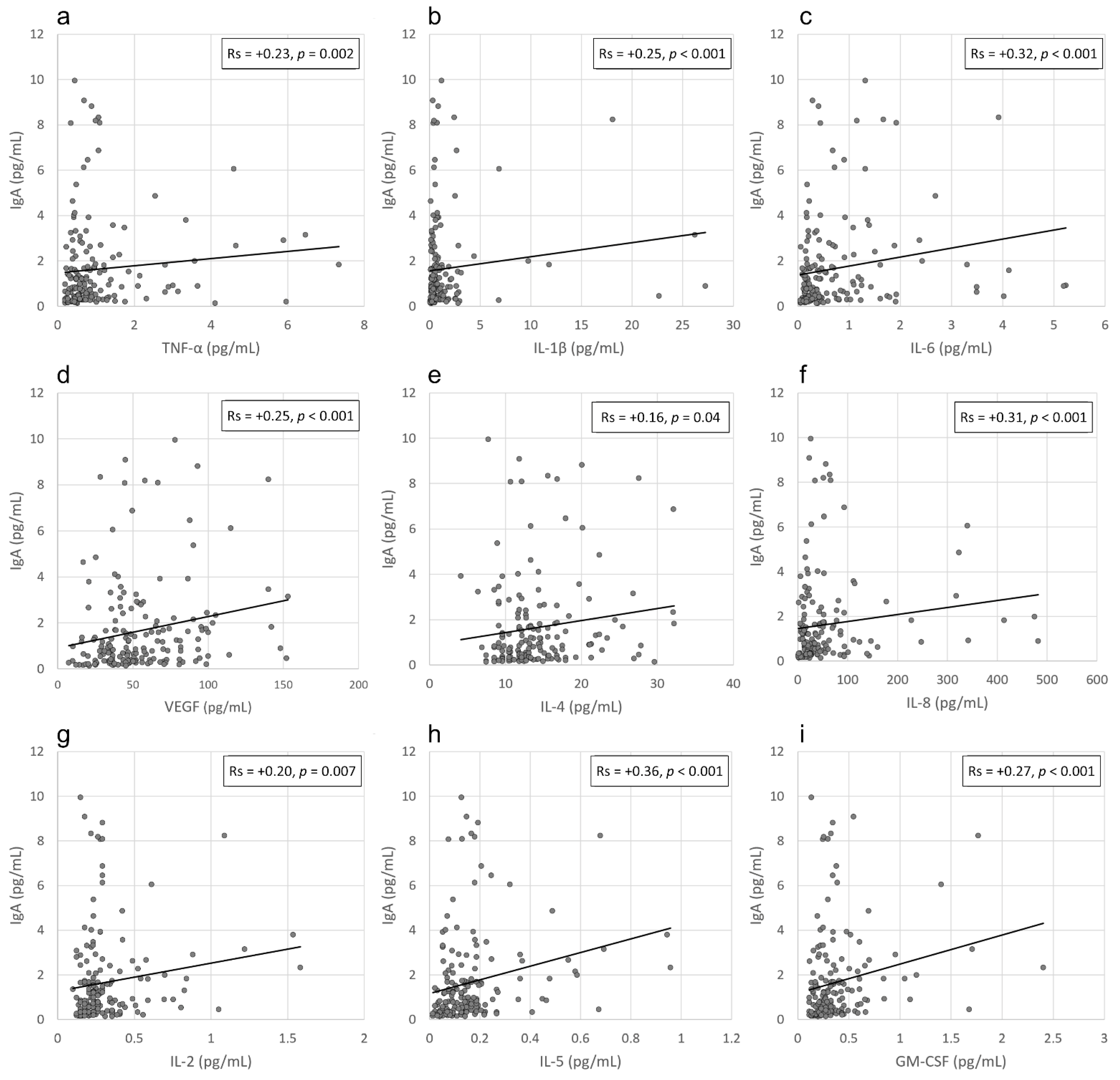
3.3. Correlation between IgA Levels and Viral Load in the Conjunctival Sac and the Local Inflammatory Response
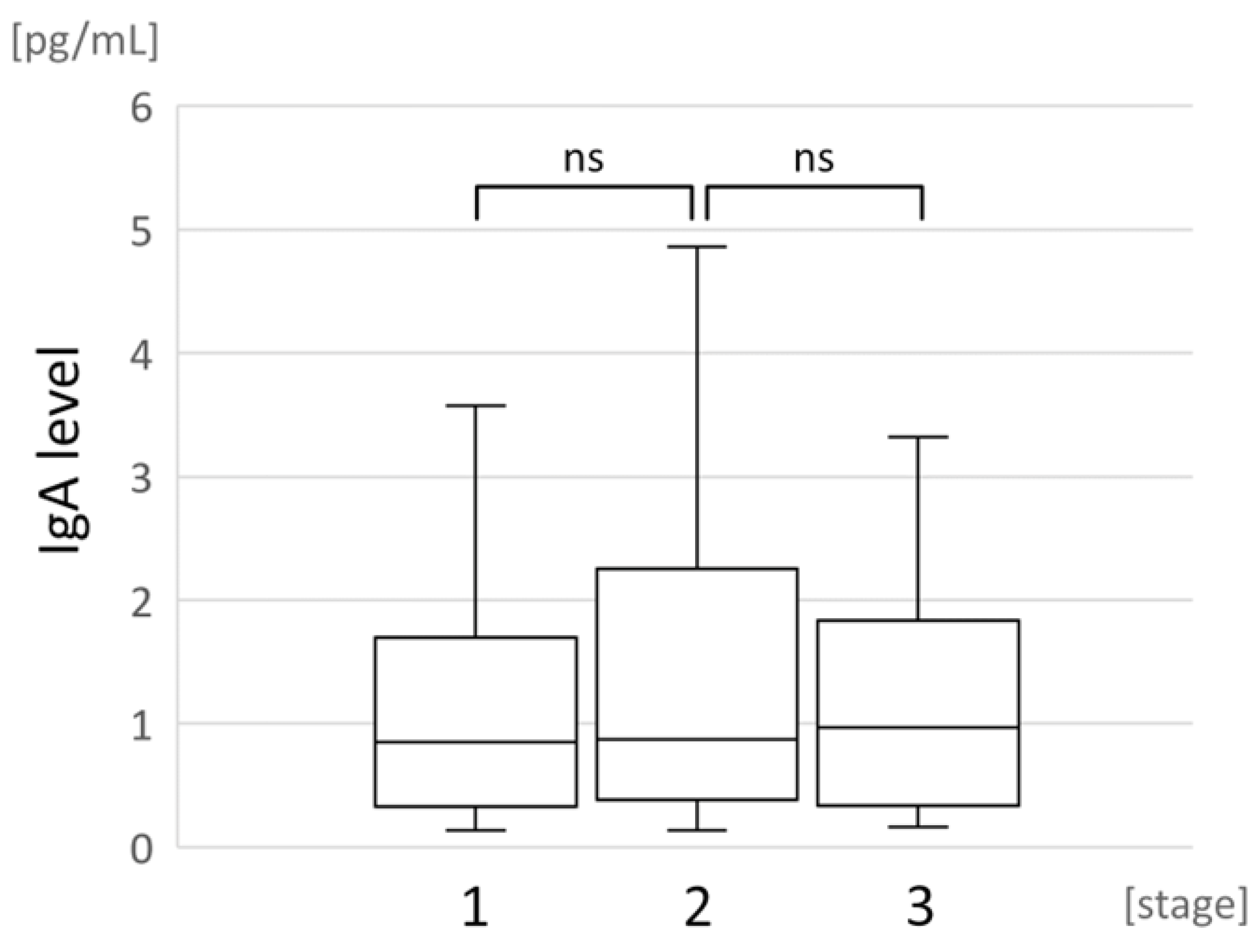
3.4. Correlation between Local IgA Levels and COVID-19 Severity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cervia, C.; Nilsson, J.; Zurbuchen, Y.; Valaperti, A.; Schreiner, J.; Wolfensberger, A.; Raeber, M.E.; Adamo, S.; Weigang, S.; Emmenegger, M.; et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2021, 147, 545–557.e9. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Martini, F.; Maritati, M.; Caselli, E.; Gallenga, C.E.; Guarino, M.; De Giorgio, R.; Mazziotta, C.; Tramarin, M.L.; Badiale, G.; et al. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms 2022, 10, 1193. [Google Scholar] [CrossRef]
- Collin, J.; Queen, R.; Zerti, D.; Dorgau, B.; Georgiou, M.; Djidrovski, I.; Hussain, R.; Coxhead, J.M.; Joseph, A.; Rooney, P.; et al. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul. Surf. 2021, 19, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, H.; Mei, T.; Chen, B.; Chen, L.; Li, S.; Zhang, X.; Sun, X. SARS-CoV-2 on the ocular surface: Is it truly a novel transmission route? Br. J. Ophthalmol. 2021, 105, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Santoro, D.; de Sousa, L.B.; Câmara, N.O.S.; de Freitas, D.; de Oliveira, L.A. SARS-COV-2 and Ocular Surface: From Physiology to Pathology, a Route to Understand Transmission and Disease. Front. Physiol. 2021, 12, 612319. [Google Scholar] [CrossRef] [PubMed]
- Maurin, C.; He, Z.; Mentek, M.; Verhoeven, P.; Pillet, S.; Bourlet, T.; Rogues, F.; Pugniet, J.L.; Peyragrosse, T.; Barallon, M.; et al. Exploration of the ocular surface infection by SARS-CoV-2 and implications for corneal donation: An ex vivo study. PLoS Med. 2022, 19, e1003922. [Google Scholar] [CrossRef]
- Kiryanov, S.A.; Levina, T.A.; Kadochnikova, V.V.; Konopleva, M.V.; Suslov, A.P.; Trofimov, D.Y. Clinical Evaluation of Nasopharyngeal, Oropharyngeal, Nasal Swabs, and Saliva for the Detection of SARS-CoV-2 by Direct RT-PCR. Diagnostics 2022, 12, 1091. [Google Scholar] [CrossRef]
- Sabage, L.E.; Mazzo, A.; Sabage, J.; Olivo, T.E.T.; Santos, C.F.; Lourençone, L.F.M. Use of Schirmer strips and conjunctival swabs for virus detection on the ocular surface of adults: A scoping review. Arq. Bras. Oftalmol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Bertram, M.; Allbaugh, R.A.; Mochel, J.P.; Peraza, J.; Page, L.; Sebbag, L. Influence of Schirmer strip wetness on volume absorbed, volume recovered, and total protein content in canine tears. Vet. Ophthalmol. 2021, 24, 425–428. [Google Scholar] [CrossRef]
- Sebbag, L.; McDowell, E.M.; Hepner, P.M.; Mochel, J.P. Effect of tear collection on lacrimal total protein content in dogs and cats: A comparison between Schirmer strips and ophthalmic sponges. BMC Vet. Res. 2018, 14, 61. [Google Scholar] [CrossRef]
- Niedźwiedź, A.; Kawa, M.; Pius-Sadowska, E.; Kuligowska, A.; Ziontkowska, A.; Wrzałek, D.; Wiącek, M.P.; Parczewski, M.; Ossowski, A.; Zielińska, G.; et al. Increased proinflammatory cytokines in tears correspond with conjunctival SARS-CoV-2 positivity in symptomatic COVID-19 patients. Sci. Rep. 2022, 12, 7225. [Google Scholar] [CrossRef]
- Niedźwiedź, A.; Kawa, M.; Pius-Sadowska, E.; Kuligowska, A.; Ziontkowska, A.; Wrzałek, D.; Parczewski, M.; Safranow, K.; Kozłowski, K.; Machaliński, B.; et al. Evaluating Ocular Symptoms and Tear Film Cytokine Profiles in Symptomatic COVID-19 Patients. J. Clin. Med. 2022, 11, 2647. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Parczewski, M.; Piekarska, A.; Simon, K.; To-masiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of March 31, 2020. Pol. Arch. Intern. Med. 2020; 130, 352–357, Update in Pol. Arch. Intern. Med. 2020, 130, 557–558. [Google Scholar] [CrossRef]
- Flisiak, R.; Parczewski, M.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists. Annex no. 2 as of October 13, 2020. Pol. Arch. Intern. Med. 2020, 130, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Torriani, G.; Yerly, S.; Mazza, L.; Calame, A.; Arm-Vernez, I.; Zimmer, G.; Agoritsas, T.; Stirnemann, J.; Spechbach, H.; et al. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin. Microbiol. Infect. 2020, 26, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- VanDerMeid, K.R.; Su, S.P.; Krenzer, K.L.; Ward, K.W.; Zhang, J.Z. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol. Vis. 2011, 17, 1056–1063. [Google Scholar] [PubMed]
- Dietzen, D.J. Amino acids, peptides, and proteins. In Principles and Applications of Molecular Diagnostics; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–380. [Google Scholar]
- Olas, K.; Butterweck, H.; Teschner, W.; Schwarz, H.P.; Reipert, B. Immunomodulatory properties of human serum immunoglobulin A: Anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin. Exp. Immunol. 2005, 140, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021, 20, 593–605. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef]
- Muyldermans, A.; Bjerke, M.; Demuyser, T.; De Geyter, D.; Wybo, I.; Soetens, O.; Weets, I.; Kuijpers, R.; Allard, S.D.; Piérard, D.; et al. SARS-CoV-2 RNA and antibodies in tear fluid. BMJ Open. Ophthalmol. 2021, 6, e000733. [Google Scholar] [CrossRef]
- Selva, K.J.; Davis, S.K.; Haycroft, E.R.; Lee, W.S.; Lopez, E.; Reynaldi, A.; Davenport, M.P.; Kent, H.E.; Juno, J.A.; Chung, A.W.; et al. Tear antibodies to SARS-CoV-2: Implications for transmission. Clin. Transl. Immunol. 2021, 10, e1354. [Google Scholar] [CrossRef]
- Mahmoud, H.; Hamody, A.; MHefny, H.; Tohamy, D.; Awny, I. Evaluation of Anti-SARS-CoV-2 IgA in the Conjunctival Secretions of COVID-19 Patients. Clin. Ophthalmol. 2021, 15, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Dolar-Szczasny, J.; Toro, M.D.; Dworzańska, A.; Wójtowicz, T.; Korona-Glowniak, I.; Sawicki, R.; Boguszewska, A.; Polz-Dacewicz, M.; Tomasiewicz, K.; Załuska, W.; et al. Ocular Involvement of SARS-CoV-2 in a Polish Cohort of COVID-19-Positive Patients. Int. J. Environ. Res. Public Health 2021, 18, 2916. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.C. Role of IgA and IgA fc receptors in inflammation. J. Clin. Immunol. 2010, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saha, C.; Das, M.; Patil, V.; Stephen-Victor, E.; Sharma, M.; Wymann, S.; Jordi, M.; Vonarburg, C.; Kaveri, S.V.; Bayry, J. Monomeric Immunoglobulin A from Plasma Inhibits Human Th17 Responses In Vitro Independent of FcαRI and DC-SIGN. Front. Immunol. 2017, 8, 275. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ogura, H.; Shimizu, K.; Ikeda, M.; Hirose, T.; Matsuura, H.; Kang, S.; Takahashi, K.; Tanaka, T.; Shimazu, T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018, 8, 13995. [Google Scholar] [CrossRef]
- Steffen, U.; Koeleman, C.A.; Sokolova, M.V.; Bang, H.; Kleyer, A.; Rech, J.; Unterweger, H.; Schicht, M.; Garreis, F.; Hahn, J.; et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 2020, 11, 120. [Google Scholar] [CrossRef]
| Parameter | Number of Patients | % |
|---|---|---|
| Sex (male/female) | 102/77 | 56.98/43.02 |
| Stage of the disease (according to the PAoEaI guidelines): | ||
| 1 | 45 | 25.14 |
| 2 | 98 | 54.75 |
| 3 | 36 | 20.11 |
| 4 | 0 | 0 |
| Medical history: | ||
| Need for the hospitalization | 134 | 74.86 |
| COVID-19 symptoms: | ||
| Fever above 38 °C | 125 | 69.83 |
| Dyspnoea | 82 | 45.81 |
| Cough | 135 | 75.42 |
| Chest pain | 50 | 27.93 |
| Smell/taste disorders | 64 | 35.75 |
| Headache | 69 | 38.55 |
| Diarrhoea | 48 | 26.82 |
| Pneumonia | 161 | 90.96 |
| Conjunctivitis * | 2 | 1.12 |
| Ophthalmic Symptom | IgA Positive | IgA Negative | p | |
|---|---|---|---|---|
| % of patients with a given ophthalmic symptom at the time of enrolment | Eyelids swelling | 1.30 | 1.28 | 1.00 |
| Eye itching | 2.60 | 3.85 | 1.00 | |
| Eye burning | 3.90 | 7.69 | 0.49 | |
| Eye tearing | 3.90 | 10.26 | 0.21 | |
| Eye redness | 3.90 | 2.56 | 0.68 | |
| Sand sensation under the eyelid | 1.30 | 3.85 | 0.62 | |
| Presence of the discharge | 2.60 | 1.28 | 0.62 | |
| Gluing of the eyelids | 3.90 | 0.00 | 0.12 | |
| Light sensitivity | 2.60 | 3.85 | 1.00 | |
| Eye stiffness | 0.00 | 1.28 | 1.00 | |
| Eye pain | 2.60 | 6.41 | 0.44 | |
| Visual impairment | 2.60 | 2.56 | 0.28 | |
| Misty vision | 2.60 | 2.56 | 1.00 | |
| Blurry vision | 3.90 | 3.85 | 1.00 | |
| % of patients with a given ophthalmic symptom during the preceding 7 days | Eyelids swelling | 2.60 | 0.00 | 0.25 |
| Eye itching | 2.60 | 3.85 | 1.00 | |
| Eye burning | 5.19 | 6.41 | 1.00 | |
| Eye tearing | 7.79 | 10.26 | 0.78 | |
| Eye redness | 2.60 | 5.13 | 0.68 | |
| Sandy sensation under the eyelid | 2.60 | 2.56 | 1.00 | |
| Presence of discharge | 3.90 | 1.28 | 0.37 | |
| Gluing of the eyelids | 2.60 | 2.56 | 1.00 | |
| Light sensitivity | 5.19 | 2.56 | 0.44 | |
| Eye stiffness | 1.30 | 1.28 | 1.00 | |
| Eye pain | 2.60 | 7.69 | 0.28 | |
| Visual impairment | 5.19 | 2.56 | 0.44 | |
| Misty vision | 6.49 | 0.00 | 0.03 | |
| Blurry vision | 5.19 | 0.00 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedźwiedź, A.; Pius-Sadowska, E.; Kawa, M.; Kuligowska, A.; Parczewski, M.; Safranow, K.; Kozłowski, K.; Machaliński, B.; Machalińska, A. IgA-Based Secretory Response in Tears of COVID-19 Patients: A Potential Biomarker of Pro-Inflammatory State in Course of SARS-CoV-2 Infection. Pathogens 2022, 11, 1098. https://doi.org/10.3390/pathogens11101098
Niedźwiedź A, Pius-Sadowska E, Kawa M, Kuligowska A, Parczewski M, Safranow K, Kozłowski K, Machaliński B, Machalińska A. IgA-Based Secretory Response in Tears of COVID-19 Patients: A Potential Biomarker of Pro-Inflammatory State in Course of SARS-CoV-2 Infection. Pathogens. 2022; 11(10):1098. https://doi.org/10.3390/pathogens11101098
Chicago/Turabian StyleNiedźwiedź, Anna, Ewa Pius-Sadowska, Miłosz Kawa, Agnieszka Kuligowska, Miłosz Parczewski, Krzysztof Safranow, Krzysztof Kozłowski, Bogusław Machaliński, and Anna Machalińska. 2022. "IgA-Based Secretory Response in Tears of COVID-19 Patients: A Potential Biomarker of Pro-Inflammatory State in Course of SARS-CoV-2 Infection" Pathogens 11, no. 10: 1098. https://doi.org/10.3390/pathogens11101098
APA StyleNiedźwiedź, A., Pius-Sadowska, E., Kawa, M., Kuligowska, A., Parczewski, M., Safranow, K., Kozłowski, K., Machaliński, B., & Machalińska, A. (2022). IgA-Based Secretory Response in Tears of COVID-19 Patients: A Potential Biomarker of Pro-Inflammatory State in Course of SARS-CoV-2 Infection. Pathogens, 11(10), 1098. https://doi.org/10.3390/pathogens11101098






