ShK-Domain-Containing Protein from a Parasitic Nematode Modulates Drosophila melanogaster Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Recombinant Protein
2.2. Fly Breeding and Maintenance
2.3. Fly Injections for Toxicity Assays
2.4. Negative Geotaxis Assay
2.5. Chill Coma Recovery
2.6. Bacterial Co-Injection, Survival, and CFUs
3. Results
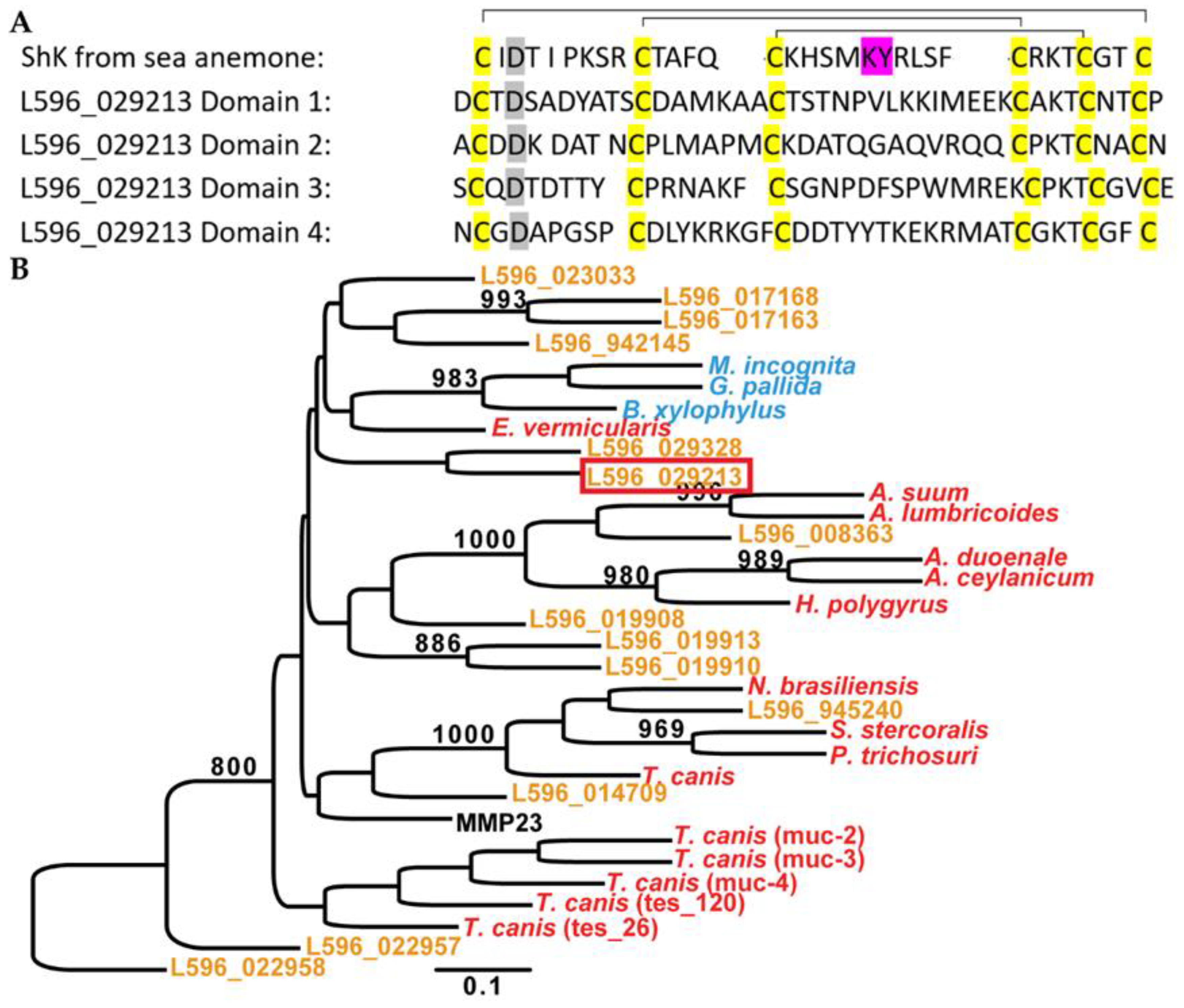
3.1. S. carpocapsae Releases Sc-ShK-1 Protein in Hosts during Infection
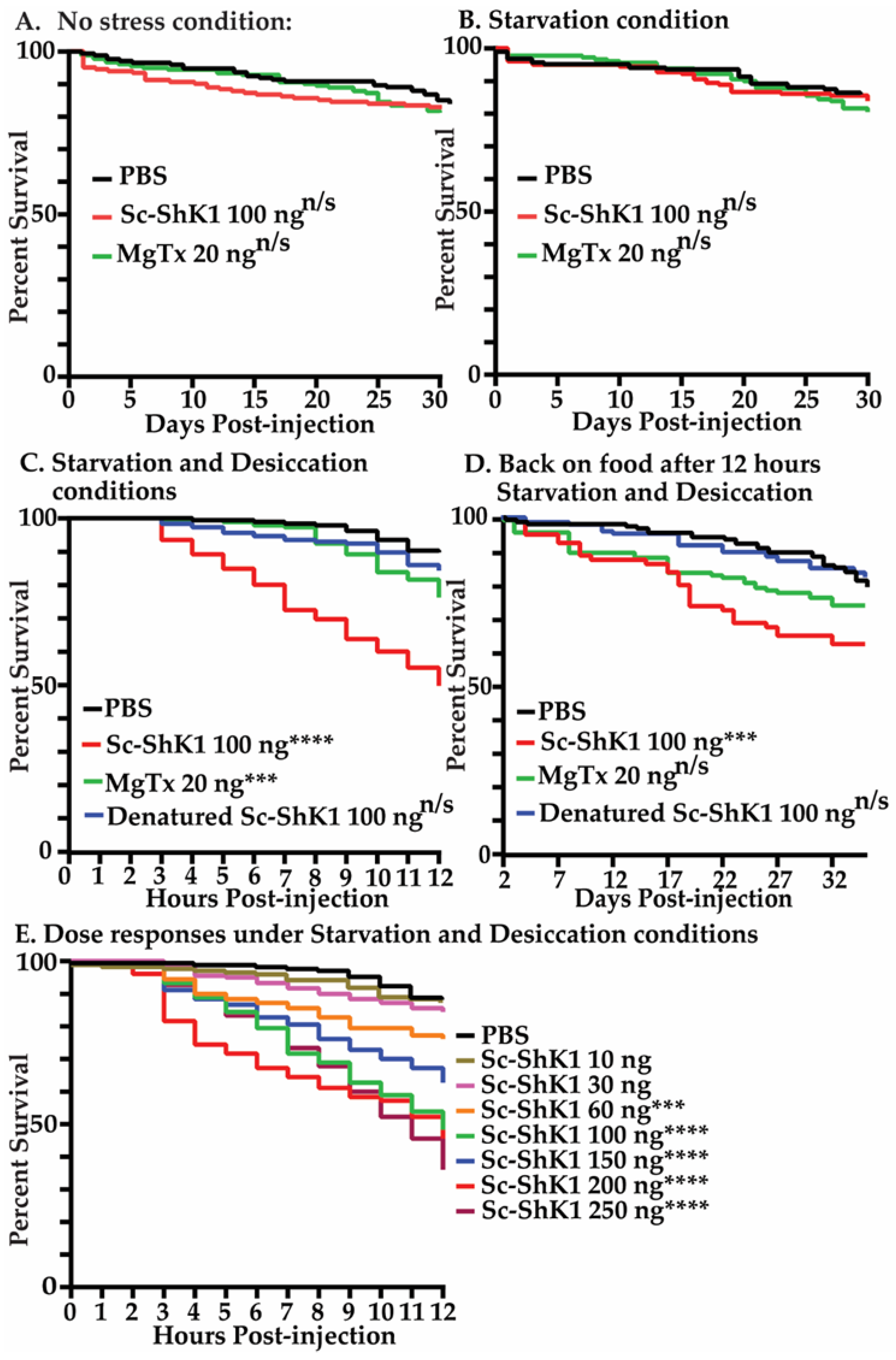
3.2. Sc-ShK-1 Is Toxic under Starvation and Desiccation Conditions
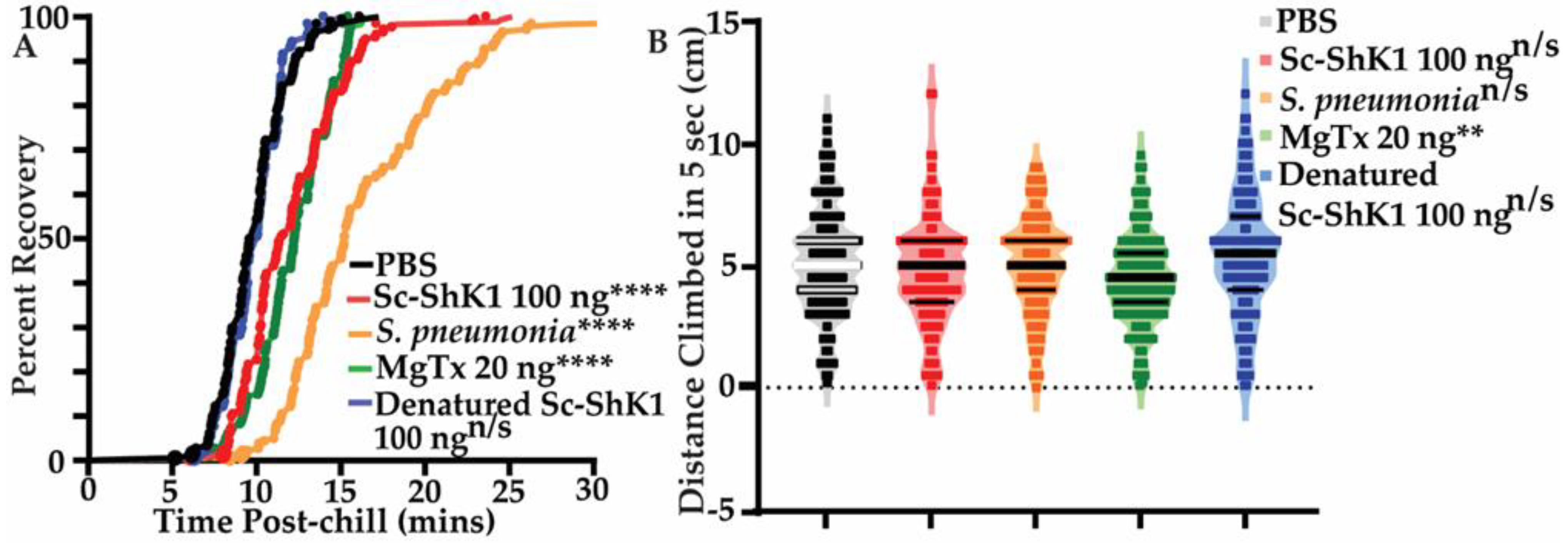
3.3. Sc-ShK-1 Negatively Affects Host Health
3.4. Sc-ShK-1 Modulates Host Immunity in a Bacterial Co-Infection
4. Discussion
4.1. EPN Model Supports the Study of Toxin Molecules
4.2. Sc-ShK-1 Toxin Increases Host Susceptibility during Stress Condition
4.3. Chill Coma Recovery and Negative Geotaxis Assay Is Useful to Measure Fly Health
4.4. Sc-ShK-1 Decreases Host Resistance to Infection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Chang, S.C.; Nguyen, H.M.; Huq, R.; Tanner, M.R.; Londono, L.M.; Estrada, R.; Dhawan, V.; Chauhan, S.; Upadhyay, S.K. Kv1. 3 channel-blocking immunomodulatory peptides from parasitic worms: Implications for autoimmune diseases. FASEB J. 2014, 28, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W.; Elliott, D.E.; Urban, J.F., Jr.; Thompson, R.A.; Weinstock, J.V. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology 2005, 128, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, T.B.; Navarro, S.; Cristofori-Armstrong, B.; Watkins, T.S.; Tungatt, K.; Ryan, R.Y.; Haigh, O.L.; Lutzky, V.P.; Mulvenna, J.P.; Rosengren, K.J. Synthetic hookworm-derived peptides are potent modulators of primary human immune cell function that protect against experimental colitis in vivo. J. Biol. Chem. 2021, 297, 100834. [Google Scholar] [CrossRef]
- Croese, J.; O’neil, J.; Masson, J.; Cooke, S.; Melrose, W.; Pritchard, D.; Speare, R. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut 2006, 55, 136–137. [Google Scholar] [CrossRef]
- Navarro, S.; Pickering, D.A.; Ferreira, I.B.; Jones, L.; Ryan, S.; Troy, S.; Leech, A.; Hotez, P.J.; Zhan, B.; Laha, T. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci. Transl. Med. 2016, 8, 362ra143. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Villegas-Moreno, J.; Mitchell, M.L.; Csoti, A.; Peigneur, S.; Amero, C.; Pennington, M.W.; Tytgat, J.; Panyi, G.; Norton, R.S. Synthesis, folding, structure and activity of a predicted peptide from the sea anemone Oulactis sp. with an ShKT fold. Toxicon 2018, 150, 50–59. [Google Scholar] [CrossRef]
- Ferreira, I.; Smyth, D.; Gaze, S.; Aziz, A.; Giacomin, P.; Ruyssers, N.; Artis, D.; Laha, T.; Navarro, S.; Loukas, A. Hookworm excretory/secretory products induce interleukin-4 (IL-4)+ IL-10+ CD4+ T cell responses and suppress pathology in a mouse model of colitis. Infect. Immun. 2013, 81, 2104–2111. [Google Scholar] [CrossRef]
- Tomar, P.; Thakur, N.; Yadav, A.N. Endosymbiotic microbes from entomopathogenic nematode (EPNs) and their applications as biocontrol agents for agro-environmental sustainability. Egypt. J. Biol. Pest Control 2022, 32, 1–19. [Google Scholar] [CrossRef]
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218. [Google Scholar]
- Lu, D.; Baiocchi, T.; Dillman, A.R. Genomics of entomopathogenic nematodes and implications for pest control. Trends Parasitol. 2016, 32, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, A.M.; Shapiro-Ilan, D.I.; Hiltpold, I. Entomopathogenic nematodes in sustainable food production. Front. Sustain. Food Syst. 2020, 4, 125. [Google Scholar] [CrossRef]
- Dillman, A.R.; Guillermin, M.L.; Lee, J.H.; Kim, B.; Sternberg, P.W.; Hallem, E.A. Olfaction shapes host-parasite interactions in parasitic nematodes. Proc. Natl. Acad. Sci. USA 2012, 109, E2324–E2333. [Google Scholar] [CrossRef]
- Lu, D.; Macchietto, M.; Chang, D.; Barros, M.M.; Baldwin, J.; Mortazavi, A.; Dillman, A.R. Activated entomopathogenic nematode infective juveniles release lethal venom proteins. PLoS Pathog. 2017, 13, e1006302. [Google Scholar] [CrossRef] [PubMed]
- Heppert, J.K.; Ransone, E.M.; Grossman, A.S.; Mauer, T.J.; Goodrich-Blair, H. 5 Nematodes as Models for Symbiosis. In Nematodes as Model Organisms; CABI GB: Wallingford, UK, 2022; pp. 58–81. [Google Scholar]
- Flores-Ponce, M.; Vallebueno-Estrada, M.; González-Orozco, E.; Ramos-Aboites, H.E.; García-Chávez, J.N.; Simões, N.; Montiel, R. Signatures of co-evolutionary host-pathogen interactions in the genome of the entomopathogenic nematode Steinernema carpocapsae. BMC Evol. Biol. 2017, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.Z.; Serra, L.; Lu, D.; Mortazavi, A.; Dillman, A.R. A core set of venom proteins is released by entomopathogenic nematodes in the genus Steinernema. PLoS Pathog. 2019, 15, e1007626. [Google Scholar] [CrossRef] [PubMed]
- Toubarro, D.; Lucena-Robles, M.; Nascimento, G.; Costa, G.; Montiel, R.; Coelho, A.V.; Simões, N. An apoptosis-inducing serine protease secreted by the entomopathogenic nematode Steinernema carpocapsae. Int. J. Parasitol. 2009, 39, 1319–1330. [Google Scholar] [CrossRef]
- McNeilly, T.N.; Frew, D.; Burgess, S.T.; Wright, H.; Bartley, D.J.; Bartley, Y.; Nisbet, A.J. Niche-specific gene expression in a parasitic nematode; increased expression of immunomodulators in Teladorsagia circumcincta larvae derived from host mucosa. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Verdes, A.; Simpson, D.; Holford, M. Are fireworms venomous? Evidence for the convergent evolution of toxin homologs in three species of fireworms (Annelida, Amphinomidae). Genome Biol. Evol. 2018, 10, 249–268. [Google Scholar] [CrossRef]
- Gerdol, M.; Cervelli, M.; Mariottini, P.; Oliverio, M.; Dutertre, S.; Modica, M.V. A recurrent motif: Diversity and evolution of ShKT domain containing proteins in the vampire snail Cumia reticulata. Toxins 2019, 11, 106. [Google Scholar] [CrossRef]
- von Reumont, B.M.; Campbell, L.I.; Richter, S.; Hering, L.; Sykes, D.; Hetmank, J.; Jenner, R.A.; Bleidorn, C. A polychaete’s powerful punch: Venom gland transcriptomics of Glycera reveals a complex cocktail of toxin homologs. Genome Biol. Evol. 2014, 6, 2406–2423. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.; Pennington, M.W.; Norton, R.S. Analogs of the sea anemone potassium channel blocker ShK for the treatment of autoimmune diseases. Inflamm. Allergy Drug Targets. 2011, 10, 313–321. [Google Scholar]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R. Kv1. 3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Bajaj, S.; Chandy, K. ShK toxin: History, structure and therapeutic applications for autoimmune diseases. WikiJ. Sci. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Tyagi, A.; Ahmed, T.; Jian, S.; Bajaj, S.; Ong, S.T.; Goay, S.S.M.; Zhao, Y.; Vorobyov, I.; Tian, C.; Chandy, K.G. Rearrangement of a unique Kv1. 3 selectivity filter conformation upon binding of a drug. Proc. Natl. Acad. Sci. USA 2022, 119, e2113536119. [Google Scholar] [CrossRef]
- Tarcha, E.J.; Olsen, C.M.; Probst, P.; Peckham, D.; Muñoz-Elías, E.J.; Kruger, J.G.; Iadonato, S.P. Safety and pharmacodynamics of dalazatide, a Kv1. 3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial. PLoS ONE 2017, 12, e0180762. [Google Scholar] [CrossRef]
- Frazão, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: An overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef]
- Linderman, J.A.; Chambers, M.C.; Gupta, A.S.; Schneider, D.S. Infection-related declines in chill coma recovery and negative geotaxis in Drosophila melanogaster. PLoS ONE 2012, 7, e41907. [Google Scholar] [CrossRef]
- Zhang, J.; Marshall, K.E.; Westwood, J.T.; Clark, M.S.; Sinclair, B.J. Divergent transcriptomic responses to repeated and single cold exposures in Drosophila melanogaster. J. Exp. Biol. 2011, 214, 4021–4029. [Google Scholar] [CrossRef]
- Parks, S.C.; Nguyen, S.; Nasrolahi, S.; Bhat, C.; Juncaj, D.; Lu, D.; Ramaswamy, R.; Dhillon, H.; Fujiwara, H.; Buchman, A. Parasitic nematode fatty acid-and retinol-binding proteins compromise host immunity by interfering with host lipid signaling pathways. PLoS Pathog. 2021, 17, e1010027. [Google Scholar] [CrossRef]
- Loukas, A.; Hintz, M.; Linder, D.; Mullin, N.P.; Parkinson, J.; Tetteh, K.K.; Maizels, R.M. A family of secreted mucins from the parasitic nematode Toxocara canis bears diverse mucin domains but shares similar flanking six-cysteine repeat motifs. J. Biol. Chem. 2000, 275, 39600–39607. [Google Scholar] [CrossRef] [PubMed]
- Prentis, P.; Pavasovic, A.; Norton, R. Sea anemones: Quiet achievers in the field of peptide toxins. Toxins 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Stehling, E.G.; Sforça, M.L.; Zanchin, N.I.; Oyama, S.r., Jr.; Pignatelli, A.; Belluzzi, O.; Polverini, E.; Corsini, R.; Spisni, A.; Pertinhez, T.A. Looking over Toxin–K+ Channel Interactions. Clues from the Structural and Functional Characterization of α-KTx Toxin Tc32, a Kv1. 3 Channel Blocker. Biochemistry 2012, 51, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Al Azad, S.; Shahriyar, S.; Mondal, K.J. Margatoxin (MgTX) and Its Effect on Immune Response and Disease Development. Eur. Acad. Res. 2016, 4, 40–57. [Google Scholar]
- Bartok, A.; Toth, A.; Somodi, S.; Szanto, T.G.; Hajdu, P.; Panyi, G.; Varga, Z. Margatoxin is a non-selective inhibitor of human Kv1. 3 K+ channels. Toxicon 2014, 87, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Tafesh-Edwards, G.; Kenney, E.; Toubarro, D.; Simões, N.; Eleftherianos, I. Excreted secreted products from the parasitic nematode Steinernema carpocapsae manipulate the Drosophila melanogaster immune response. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Hao, Y.-J.; Toubarro, D.; Nascimento, G.; Simões, N. Purification, biochemical and molecular analysis of a chymotrypsin protease with prophenoloxidase suppression activity from the entomopathogenic nematode Steinernema carpocapsae. Int. J. Parasitol. 2009, 39, 975–984. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Nascimento, G.; Ferreira, R.; Martinez, M.; Simões, N. Pepsin-like aspartic protease (Sc-ASP155) cloning, molecular characterization and gene expression analysis in developmental stages of nematode Steinernema carpocapsae. Gene 2012, 500, 164–171. [Google Scholar] [CrossRef]
- Toubarro, D.; Avila, M.M.; Hao, Y.; Balasubramanian, N.; Jing, Y.; Montiel, R.; Faria, T.Q.; Brito, R.M.; Simoes, N. A serpin released by an entomopathogen impairs clot formation in insect defense system. PLoS ONE 2013, 8, e69161. [Google Scholar]
- Balasubramanian, N.; Toubarro, D.; Simoes, N. Biochemical study and in vitro insect immune suppression by a trypsin-like secreted protease from the nematode Steinernema carpocapsae. Parasite Immunol. 2010, 32, 165–175. [Google Scholar] [CrossRef]
- Toubarro, D.; Avila, M.M.; Montiel, R.; Simões, N. A pathogenic nematode targets recognition proteins to avoid insect defenses. PLoS ONE 2013, 8, e75691. [Google Scholar] [CrossRef] [PubMed]
- Blaxter, M.; Koutsovoulos, G. The evolution of parasitism in Nematoda. Parasitology 2015, 142, S26–S39. [Google Scholar] [CrossRef] [PubMed]
- Martínez, B.A.; Hoyle, R.G.; Yeudall, S.; Granade, M.E.; Harris, T.E.; Castle, J.D.; Leitinger, N.; Bland, M.L. Innate immune signaling in Drosophila shifts anabolic lipid metabolism from triglyceride storage to phospholipid synthesis to support immune function. PLoS Genet. 2020, 16, e1009192. [Google Scholar] [CrossRef] [PubMed]
- Lissner, M.M.; Schneider, D.S. The physiological basis of disease tolerance in insects. Curr. Opin. Insect Sci. 2018, 29, 133–136. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, H.A.; Sinclair, B.J. Mechanisms underlying insect chill-coma. J. Insect Physiol. 2011, 57, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, A.K.; Dhillon, H.; Dillman, A.R. ShK-Domain-Containing Protein from a Parasitic Nematode Modulates Drosophila melanogaster Immunity. Pathogens 2022, 11, 1094. https://doi.org/10.3390/pathogens11101094
Lima AK, Dhillon H, Dillman AR. ShK-Domain-Containing Protein from a Parasitic Nematode Modulates Drosophila melanogaster Immunity. Pathogens. 2022; 11(10):1094. https://doi.org/10.3390/pathogens11101094
Chicago/Turabian StyleLima, Aklima K., Harpal Dhillon, and Adler R. Dillman. 2022. "ShK-Domain-Containing Protein from a Parasitic Nematode Modulates Drosophila melanogaster Immunity" Pathogens 11, no. 10: 1094. https://doi.org/10.3390/pathogens11101094
APA StyleLima, A. K., Dhillon, H., & Dillman, A. R. (2022). ShK-Domain-Containing Protein from a Parasitic Nematode Modulates Drosophila melanogaster Immunity. Pathogens, 11(10), 1094. https://doi.org/10.3390/pathogens11101094






