Investigation of the Potential of Heterophil/Lymphocyte Ratio as a Biomarker to Predict Colonization Resistance and Inflammatory Response to Salmonella enteritidis Infection in Chicken
Abstract
1. Introduction
2. Results
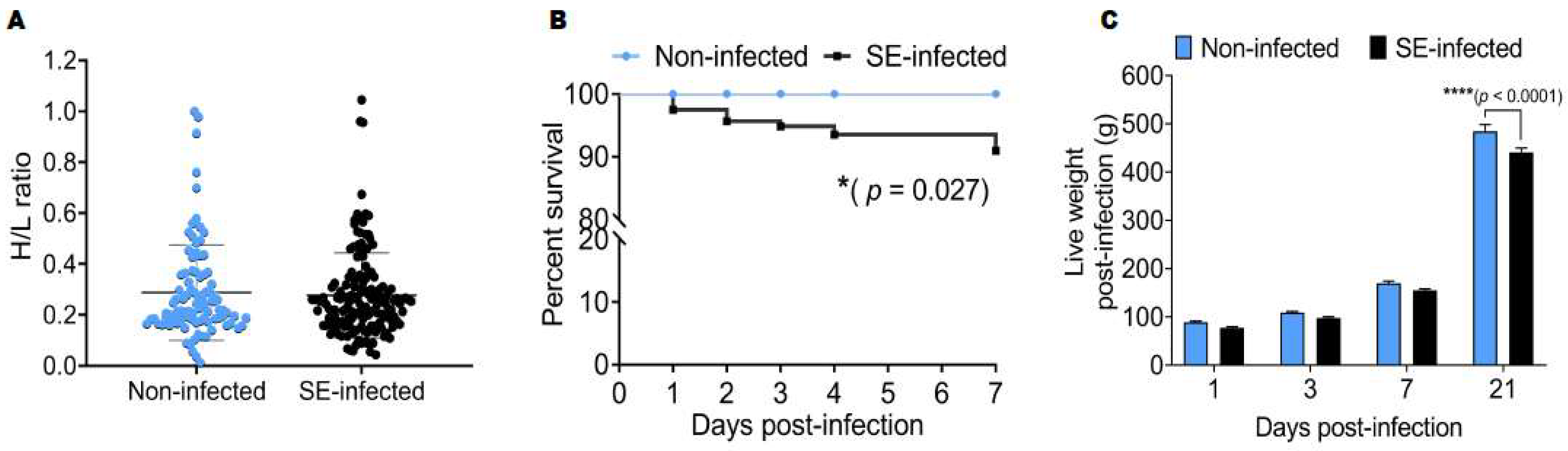
2.1. Effects of Salmonella Enteritidis Infection on the Survival Rate and Live Weight Post-Infection
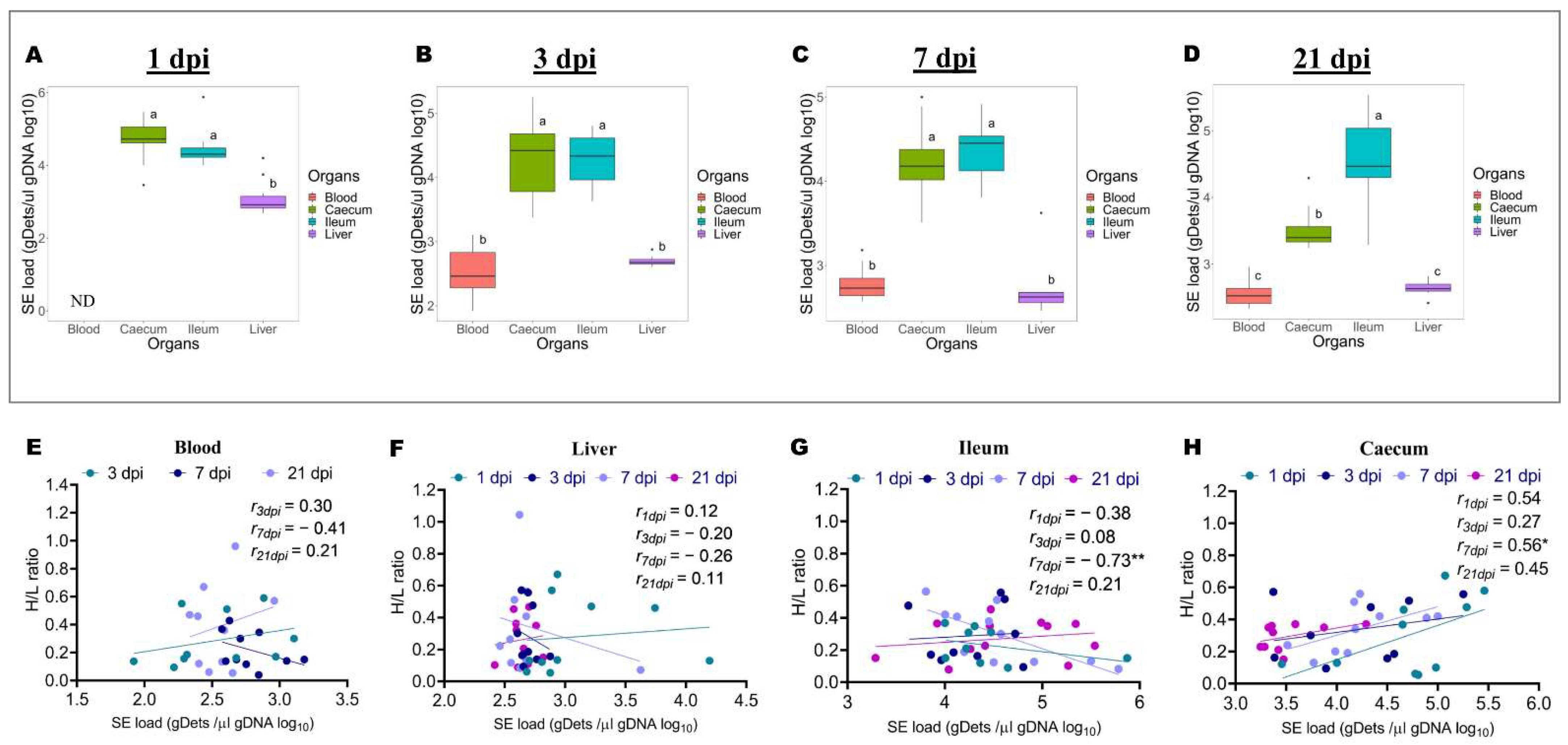
2.2. Differences in Salmonella Enteritidis Load in the Blood, Ileum, Caecum, and Liver at 1, 3, 7, and 21 Days after Infection, and Association with H/L Ratio
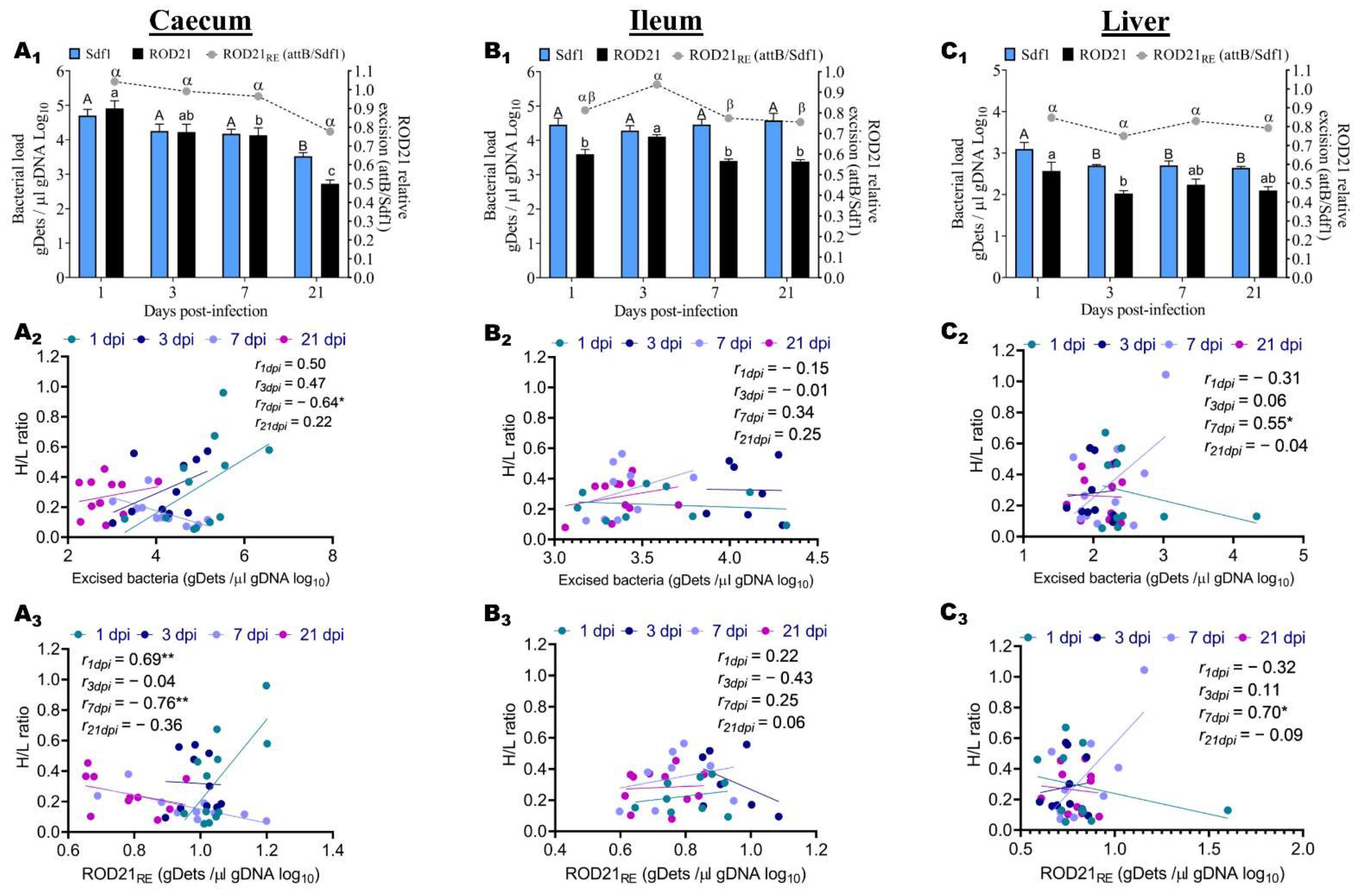
2.3. ROD21 Absolute and Relative Excision Kinetic, and Association with H/L Ratio
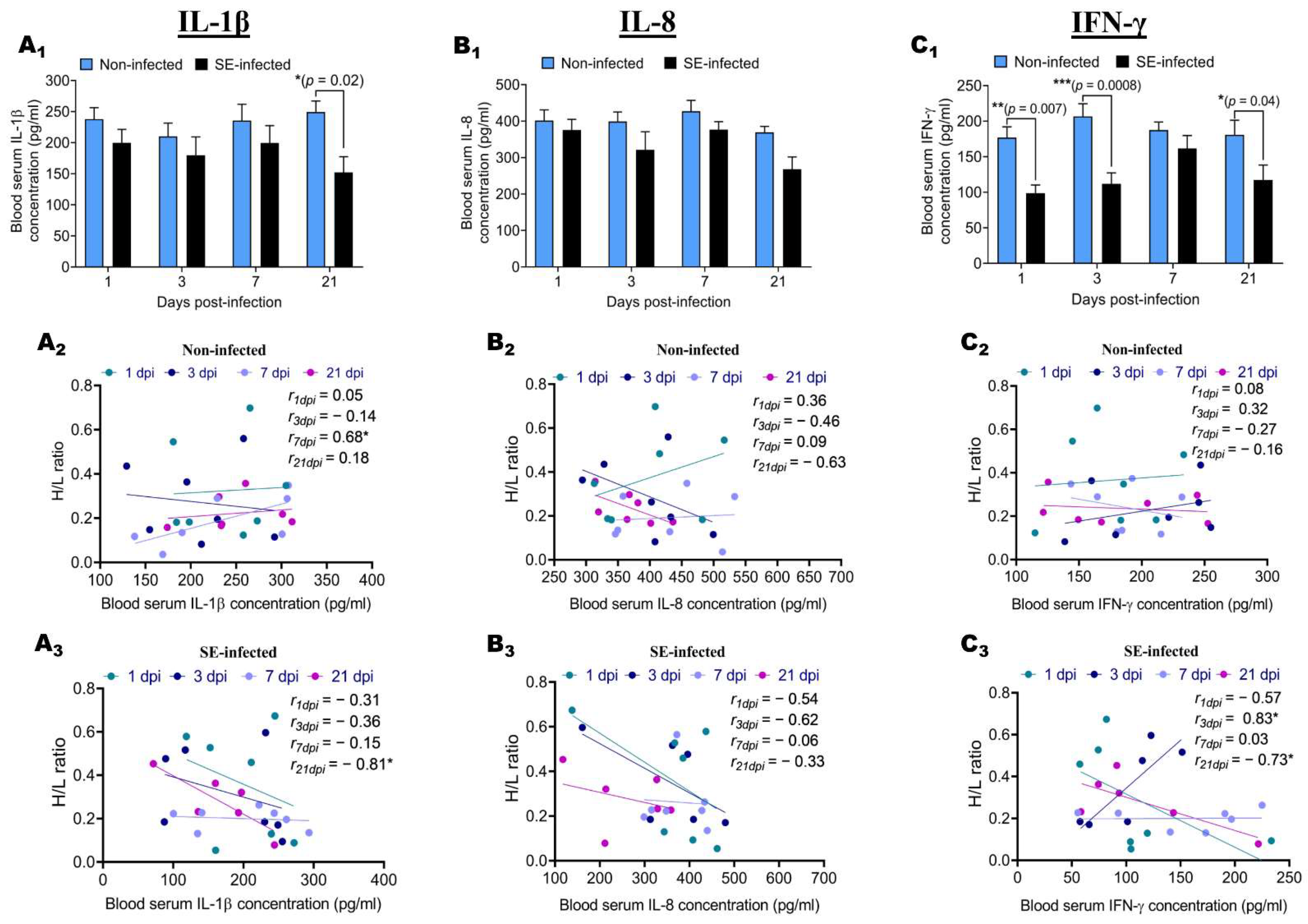
2.4. Effect of Salmonella enteritidis Infection on IL-1β, IL-8, and IFN-γ Blood Serum Concentration, and Association with H/L Ratio
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Determination of the H/L Ratio
4.3. Salmonella Challenge and Sampling
4.4. DNA Extraction
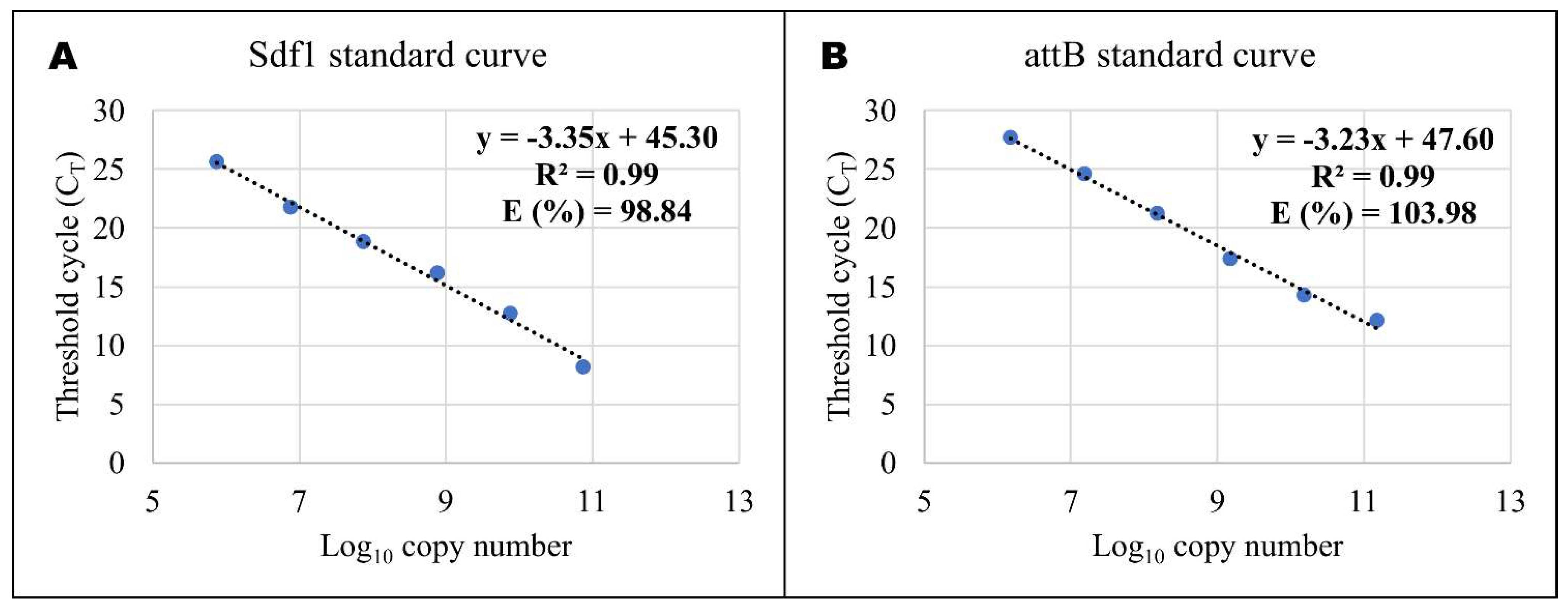
4.5. Quantitative Real-time PCR (RT-QPCR)
| Genes | Forward (F) Reverse (R) Primers and Probe (P) 5′ to 3′ | Accession no./Reference |
|---|---|---|
| Sdf1 | F: TCCCTGAATCTGAGAAAGAAAAACTC | No.AF370707.1 |
| R: TTGATGTGGTTGGTTCGTCACT | ||
| P: TGCAGCGAGCATGTTCTGGAAAGC | ||
| attB | F: GTTACTATGCGCCCCGTTCACAC | [14,50] |
| R: CCGATTAAGCCCCAAAAACTATG | ||
| P: TTCGAGTCCAGTCAGAGGA |
4.6. Blood Serum IL-1β, IL-8, and IFN-γ Concentrations
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricke, S.C. Strategies to Improve Poultry Food Safety, a Landscape Review. Annu. Rev. Anim. Biosci. 2021, 9, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef]
- Park, S.Y.; Woodward, C.L.; Kubena, L.F.; Nisbet, D.J.; Birkhold, S.G.; Ricke, S.C. Environmental Dissemination of Foodborne Salmonella in Preharvest Poultry Production: Reservoirs, Critical Factors, and Research Strategies. Crit. Rev. Environ. Sci. Technol. 2008, 38, 73–111. [Google Scholar] [CrossRef]
- Castillo, N.A.; de Moreno de LeBlanc, A.; Galdeano, C.M.; Perdigón, G. Probiotics: An alternative strategy for combating salmonellosis: Immune mechanisms involved. Food Res. Int. 2012, 45, 831–841. [Google Scholar] [CrossRef]
- Peruzy, M.F.; Capuano, F.; Proroga, Y.T.R.; Cristiano, D.; Carullo, M.R.; Murru, N. Antimicrobial Susceptibility Testing for Salmonella Serovars Isolated from Food Samples: Five-Year Monitoring (2015–2019). Antibiotics 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Cauwerts, K.; Devriese, L.A.; Haesebrouck, F.; Ducatelle, R. Feed additives to control Salmonella in poultry. World’s Poult. Sci. J. 2002, 58, 501–513. [Google Scholar] [CrossRef]
- Applegate, T.J.; Klose, V.; Steiner, T.; Ganner, A.; Schatzmayr, G. Probiotics and phytogenics for poultry: Myth or reality? J. Appl. Poult. Res. 2010, 19, 194–210. [Google Scholar] [CrossRef]
- Hume, M.E. Historic perspective: Prebiotics, probiotics, and other alternatives to antibiotics. Poult. Sci. 2011, 90, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C. Impact of Prebiotics on Poultry Production and Food Safety. Yale J. Biol. Med. 2018, 91, 151–159. [Google Scholar]
- Ricke, S.C.; Lee, S.I.; Kim, S.A.; Park, S.H.; Shi, Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020, 99, 670–677. [Google Scholar] [CrossRef]
- Festa, R.; Ambrosio, R.L.; Lamas, A.; Gratino, L.; Palmieri, G.; Franco, C.M.; Cepeda, A.; Anastasio, A. A Study on the Antimicrobial and Antibiofilm Peptide 1018-K6 as Potential Alternative to Antibiotics against Food-Pathogen Salmonella enterica. Foods 2021, 10, 1372. [Google Scholar] [CrossRef]
- Thomson, N.R.; Clayton, D.J.; Windhorst, D.; Vernikos, G.; Davidson, S.; Churcher, C.; Quail, M.A.; Stevens, M.; Jones, M.A.; Watson, M.; et al. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 2008, 18, 1624–1637. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, T.S.; Nieto, P.A.; Tobar, H.E.; Salazar-Echegarai, F.J.; Lizana, R.J.; Quezada, C.P.; Santiviago, C.A.; Araya, D.V.; Riedel, C.A.; Kalergis, A.M.; et al. Excision of an unstable pathogenicity island in Salmonella enterica serovar Enteritidis is induced during infection of phagocytic cells. PLoS ONE 2011, 6, e26031. [Google Scholar] [CrossRef]
- Pardo-Roa, C.; Salazar, G.A.; Noguera, L.P.; Salazar-Echegarai, F.J.; Vallejos, O.P.; Suazo, I.D.; Schultz, B.M.; Coronado-Arrazola, I.; Kalergis, A.M.; Bueno, S.M. Pathogenicity island excision during an infection by Salmonella enterica serovar Enteritidis is required for crossing the intestinal epithelial barrier in mice to cause systemic infection. PLoS Pathog. 2019, 15, e1008152. [Google Scholar] [CrossRef] [PubMed]
- Minias, P. Evolution of heterophil/lymphocyte ratios in response to ecological and life-history traits: A comparative analysis across the avian tree of life. J. Anim. Ecol. 2019, 88, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Lentfer, T.L.; Pendl, H.; Gebhardt-Henrich, S.G.; Frohlich, E.K.; Von Borell, E. H/L ratio as a measurement of stress in laying hens—Methodology and reliability. Br. Poult. Sci. 2015, 56, 157–163. [Google Scholar] [CrossRef]
- Thiam, M.; Barreto Sánchez, A.L.; Zhang, J.; Zheng, M.; Wen, J.; Zhao, G.; Wang, Q. Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken. Animals 2021, 11, 3498. [Google Scholar] [CrossRef]
- al-Murrani, W.K.; Kassab, A.; al-Sam, H.Z.; al-Athari, A.M. Heterophil/lymphocyte ratio as a selection criterion for heat resistance in domestic fowls. Br. Poult. Sci. 1997, 38, 159–163. [Google Scholar] [CrossRef]
- Al-Murrani, W.K.; Al-Rawi, A.J.; Al-Hadithi, M.F.; Al-Tikriti, B. Association between heterophil/lymphocyte ratio, a marker of ‘resistance’ to stress, and some production and fitness traits in chickens. Br. Poult. Sci. 2006, 47, 443–448. [Google Scholar] [CrossRef]
- Campo, J.L.; Davila, S.G. Estimation of heritability for heterophil:lymphocyte ratio in chickens by restricted maximum likelihood. Effects of age, sex, and crossing. Poult. Sci. 2002, 81, 1448–1453. [Google Scholar] [CrossRef]
- Wilcoxen, T.E.; Boughton, R.K.; Morgan, G.M.; Schoech, S.J. Heritability of immunological characteristics in Florida Scrub-Jays (Aphelocoma coerulescens). Can. J. Zool. 2013, 91, 789–794. [Google Scholar] [CrossRef]
- Dar, M.A.; Urwat, U.; Ahmad, S.M.; Ahmad, R.; Kashoo, Z.A.; Dar, T.A.; Bhat, S.A.; Mumtaz, P.T.; Shabir, N.; Shah, R.A.; et al. Gene expression and antibody response in chicken against Salmonella Typhimurium challenge. Poult. Sci. 2019, 98, 2008–2013. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Calefi, A.S.; Cruz, D.S.G.; Aloia, T.P.A.; Zager, A.; Astolfi-Ferreira, C.S.; Piantino Ferreira, J.A.; Sharif, S.; Palermo-Neto, J. Heat stress decreases expression of the cytokines, avian beta-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017, 186, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Q.; Everaert, N.; Liu, R.; Zheng, M.; Zhao, G.; Wen, J. Dietary Inulin Supplementation Modulates Short-Chain Fatty Acid Levels and Cecum Microbiota Composition and Function in Chickens Infected With Salmonella. Front. Microbiol. 2020, 11, 584380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Li, Q.; Wang, Q.; Wen, J.; Zhao, G. Comparison of the Efficiency of BLUP and GBLUP in Genomic Prediction of Immune Traits in Chickens. Animals 2020, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, Q.; Liu, R.; Zheng, M.; Wen, J.; Zhao, G. Genome-Wide Association Study of H/L Traits in Chicken. Animals 2019, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Chalghoumi, R.; Marcq, C.; Thewis, A.; Portetelle, D.; Beckers, Y. Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin Y) on Salmonella species cecal colonization and growth performances of challenged broiler chickens. Poult. Sci. 2009, 88, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Al-Murrani, W.K.; Al-Rawi, I.K.; Raof, N.M. Genetic resistance to Salmonella typhimurium in two lines of chickens selected as resistant and sensitive on the basis of heterophil/lymphocyte ratio. Br. Poult. Sci. 2002, 43, 501–507. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zheng, X.C.; Wang, T.; Zhang, T.Y. Effect of dietary oridonin supplementation on growth performance, gut health, and immune response of broilers infected with Salmonella pullorum. Ir. Vet. J. 2018, 71, 16. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Tahoun, A.; El-Gohary, A.E.A.; El-Abasy, M.; El-Khayat, F.; Gillespie, T.; Kitade, Y.; Hafez, H.M.; Neubauer, H.; El-Adawy, H. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Mon, K.K.Z.; Zhu, Y.; Chanthavixay, G.; Kern, C.; Zhou, H. Integrative analysis of gut microbiome and metabolites revealed novel mechanisms of intestinal Salmonella carriage in chicken. Sci. Rep. 2020, 10, 4809. [Google Scholar] [CrossRef]
- Chappell, L.; Kaiser, P.; Barrow, P.; Jones, M.A.; Johnston, C.; Wigley, P. The immunobiology of avian systemic salmonellosis. Vet. Immunol. Immunopathol. 2009, 128, 53–59. [Google Scholar] [CrossRef]
- Wales, A.D.; Davies, R.H. A critical review of Salmonella Typhimurium infection in laying hens. Avian Pathol. 2011, 40, 429–436. [Google Scholar] [CrossRef]
- Cummings, P.L.; Kuo, T.; Javanbakht, M.; Shafir, S.; Wang, M.; Sorvillo, F. Salmonellosis Hospitalizations in the United States: Associated Chronic Conditions, Costs, and Hospital Outcomes, 2011, Trends 2000–2011. Foodborne Pathog. Dis. 2016, 13, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for Foodborne Disease Outbreaks—United States, 2009-2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.; Chousalkar, K.K. Development of PMAxxTM-Based qPCR for the Quantification of Viable and Non-viable Load of Salmonella From Poultry Environment. Front. Microbiol. 2020, 11, 581201. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O.; Finlay, B.B. Pathogenicity islands: A molecular toolbox for bacterial virulence. Cell Microbiol. 2006, 8, 1707–1719. [Google Scholar] [CrossRef]
- Nieto, P.A.; Pardo-Roa, C.; Salazar-Echegarai, F.J.; Tobar, H.E.; Coronado-Arrazola, I.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. New insights about excisable pathogenicity islands in Salmonella and their contribution to virulence. Microbes Infect. 2016, 18, 302–309. [Google Scholar] [CrossRef]
- Porwollik, S.; Santiviago, C.A.; Cheng, P.; Florea, L.; Jackson, S.; McClelland, M. Differences in gene content between Salmonella enterica serovar Enteritidis isolates and comparison to closely related serovars Gallinarum and Dublin. J. Bacteriol. 2005, 187, 6545–6555. [Google Scholar] [CrossRef]
- Piña-Iturbe, A.; Ulloa-Allendes, D.; Pardo-Roa, C.; Coronado-Arrázola, I.; Salazar-Echegarai, F.J.; Sclavi, B.; González, P.A.; Bueno, S.M. Comparative and phylogenetic analysis of a novel family of Enterobacteriaceae-associated genomic islands that share a conserved excision/integration module. Sci. Rep. 2018, 8, 10292. [Google Scholar] [CrossRef]
- Feasey, N.A.; Hadfield, J.; Keddy, K.H.; Dallman, T.J.; Jacobs, J.; Deng, X.; Wigley, P.; Barquist, L.; Langridge, G.C.; Feltwell, T.; et al. Distinct Salmonella Enteritidis lineages associated with enterocolitis in high-income settings and invasive disease in low-income settings. Nat. Genet. 2016, 48, 1211–1217. [Google Scholar] [CrossRef]
- Salazar-Echegarai, F.J.; Tobar, H.E.; Nieto, P.A.; Riedel, C.A.; Bueno, S.M. Conjugal transfer of the pathogenicity island ROD21 in Salmonella enterica serovar Enteritidis depends on environmental conditions. PLoS ONE 2014, 9, e90626. [Google Scholar] [CrossRef]
- Kaiser, P.; Poh, T.Y.; Rothwell, L.; Avery, S.; Balu, S.; Pathania, U.S.; Hughes, S.; Goodchild, M.; Morrell, S.; Watson, M.; et al. A genomic analysis of chicken cytokines and chemokines. J. Interferon Cytokine Res. 2005, 25, 467–484. [Google Scholar] [CrossRef]
- Eckmann, L.; Kagnoff, M.F. Cytokines in host defense against Salmonella. Microbes Infect. 2001, 3, 1191–1200. [Google Scholar] [CrossRef]
- Beal, R.K.; Wigley, P.; Powers, C.; Hulme, S.D.; Barrow, P.A.; Smith, A.L. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 2004, 100, 151–164. [Google Scholar] [CrossRef]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.L.; Kogut, M.H.; Ferro, P.J.; Rothwell, L.; Pevzner, I.Y.; Kaiser, P. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology 2004, 113, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enteritidis. J. Interferon Cytokine Res. 2003, 23, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Fidan, E.D.; Nazligül, A.; Türkyilmaz, M.; Aypak, S.U.; Kilimci, F.S.; Karaarslan, S.; Kaya, M.J.R.B.D.Z. Effect of photoperiod length and light intensity on some welfare criteria, carcass, and meat quality characteristics in broilers. Rev. Bras. De Zootec. 2017, 46, 202–210. [Google Scholar] [CrossRef]
- Bueno, S.M.; Santiviago, C.A.; Murillo, A.A.; Fuentes, J.A.; Trombert, A.N.; Rodas, P.I.; Youderian, P.; Mora, G.C. Precise excision of the large pathogenicity island, SPI7, in Salmonella enterica serovar Typhi. J. Bacteriol. 2004, 186, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiam, M.; Barreto Sánchez, A.L.; Zhang, J.; Wen, J.; Zhao, G.; Wang, Q. Investigation of the Potential of Heterophil/Lymphocyte Ratio as a Biomarker to Predict Colonization Resistance and Inflammatory Response to Salmonella enteritidis Infection in Chicken. Pathogens 2022, 11, 72. https://doi.org/10.3390/pathogens11010072
Thiam M, Barreto Sánchez AL, Zhang J, Wen J, Zhao G, Wang Q. Investigation of the Potential of Heterophil/Lymphocyte Ratio as a Biomarker to Predict Colonization Resistance and Inflammatory Response to Salmonella enteritidis Infection in Chicken. Pathogens. 2022; 11(1):72. https://doi.org/10.3390/pathogens11010072
Chicago/Turabian StyleThiam, Mamadou, Astrid Lissette Barreto Sánchez, Jin Zhang, Jie Wen, Guiping Zhao, and Qiao Wang. 2022. "Investigation of the Potential of Heterophil/Lymphocyte Ratio as a Biomarker to Predict Colonization Resistance and Inflammatory Response to Salmonella enteritidis Infection in Chicken" Pathogens 11, no. 1: 72. https://doi.org/10.3390/pathogens11010072
APA StyleThiam, M., Barreto Sánchez, A. L., Zhang, J., Wen, J., Zhao, G., & Wang, Q. (2022). Investigation of the Potential of Heterophil/Lymphocyte Ratio as a Biomarker to Predict Colonization Resistance and Inflammatory Response to Salmonella enteritidis Infection in Chicken. Pathogens, 11(1), 72. https://doi.org/10.3390/pathogens11010072






