Regulatory Effect of Irresistin-16 on Competitive Dual-Species Biofilms Composed of Streptococcus mutans and Streptococcus sanguinis
Abstract
:1. Introduction
2. Results
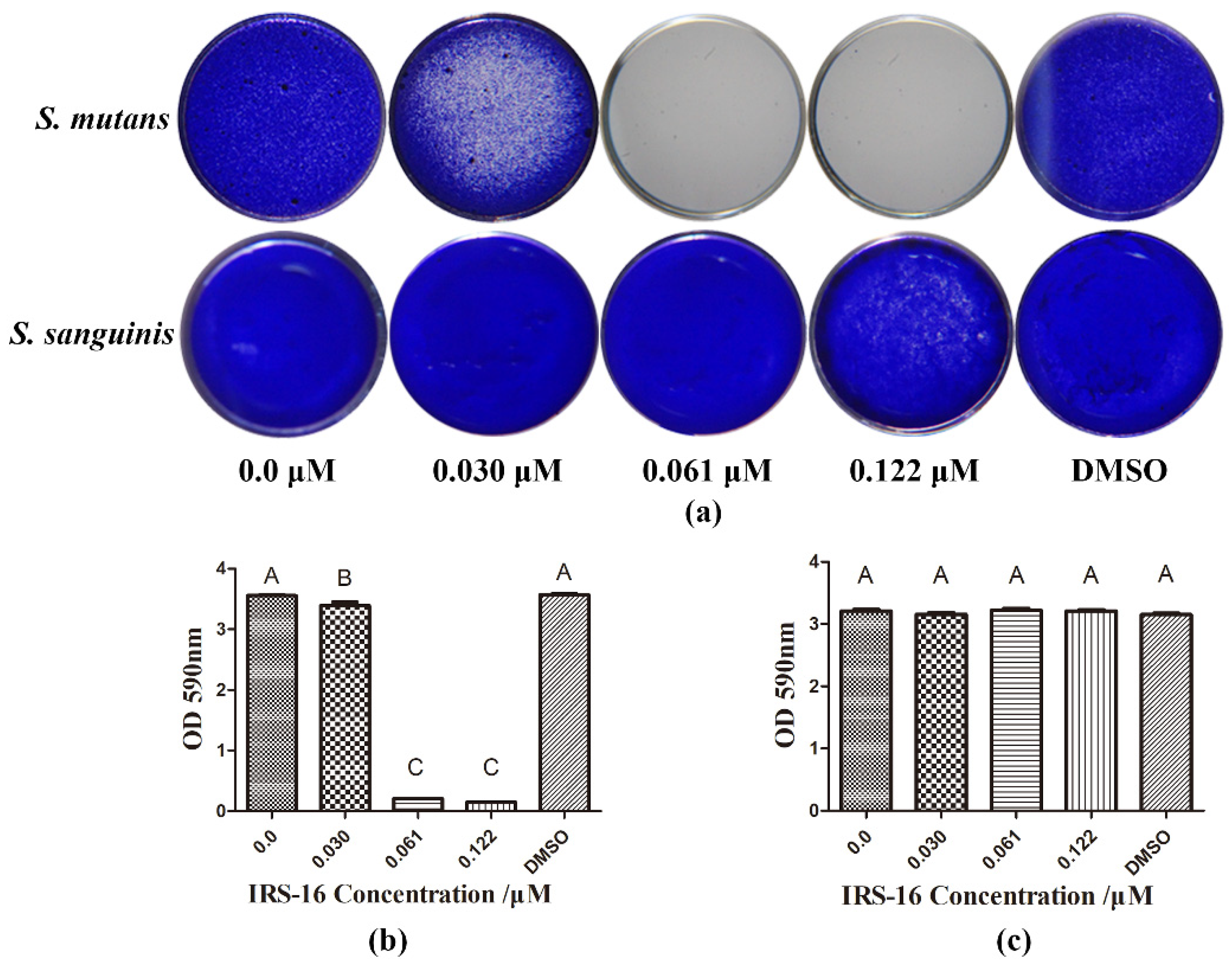
2.1. IRS-16 Showed Disparate Efficiency on Two Types of Single-Species Biofilms
2.2. IRS-16 Modulated the Microbial Constitution of Dual-Species Biofilm
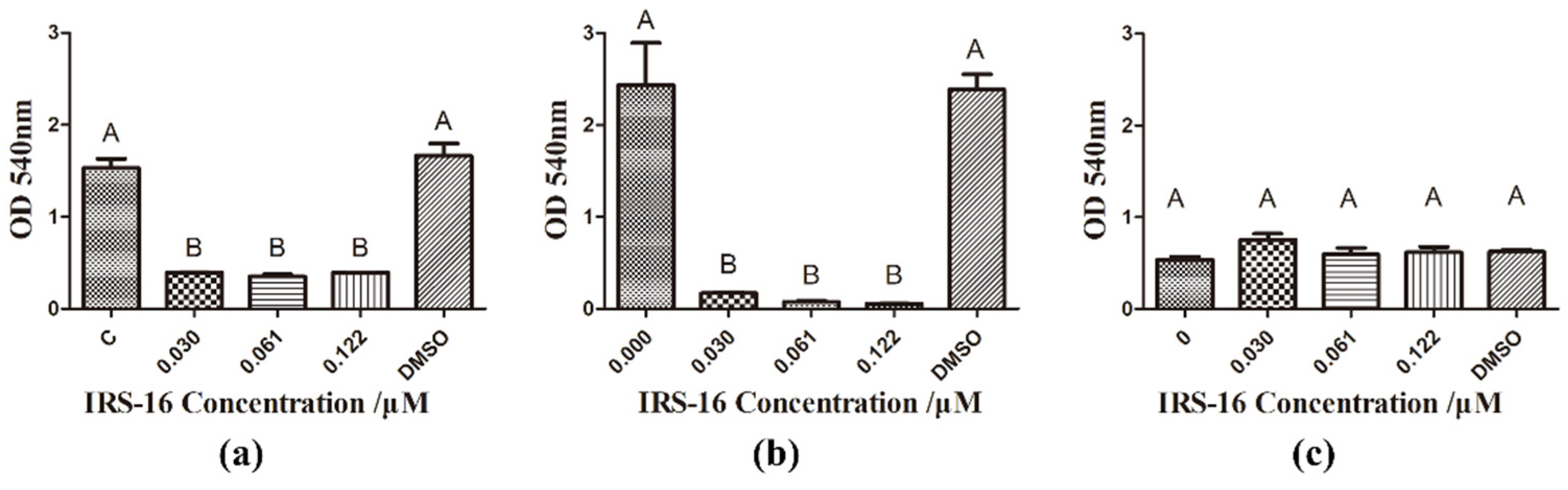
2.3. IRS-16 Decreased Metabolic Activity of Dual-Species Biofilms
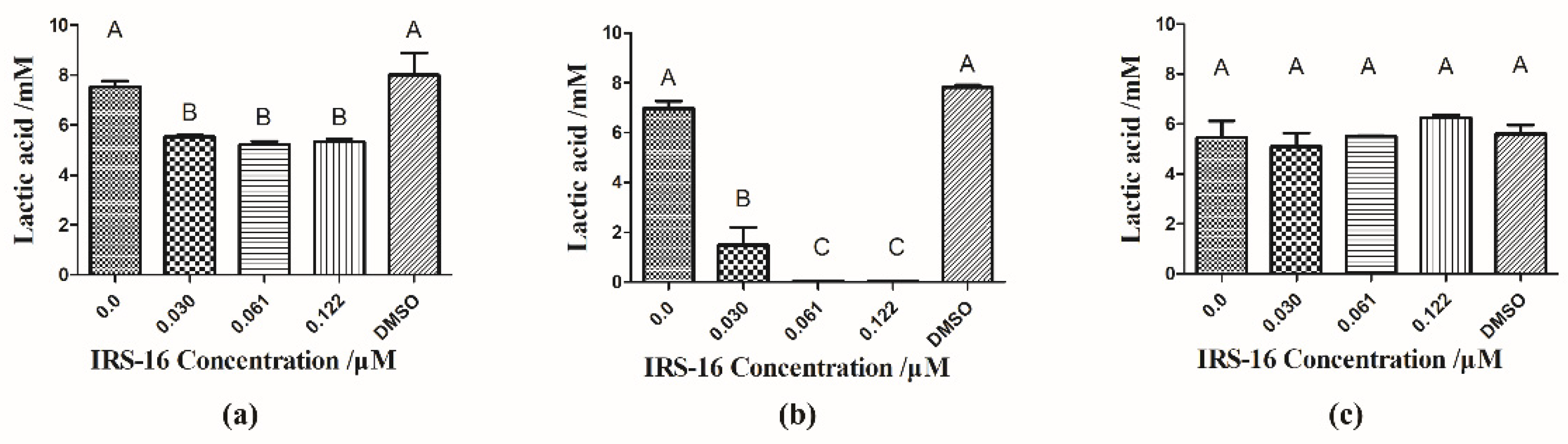
2.4. IRS-16 Inhibited Lactic Acid Generation of Dual-Species Biofilms
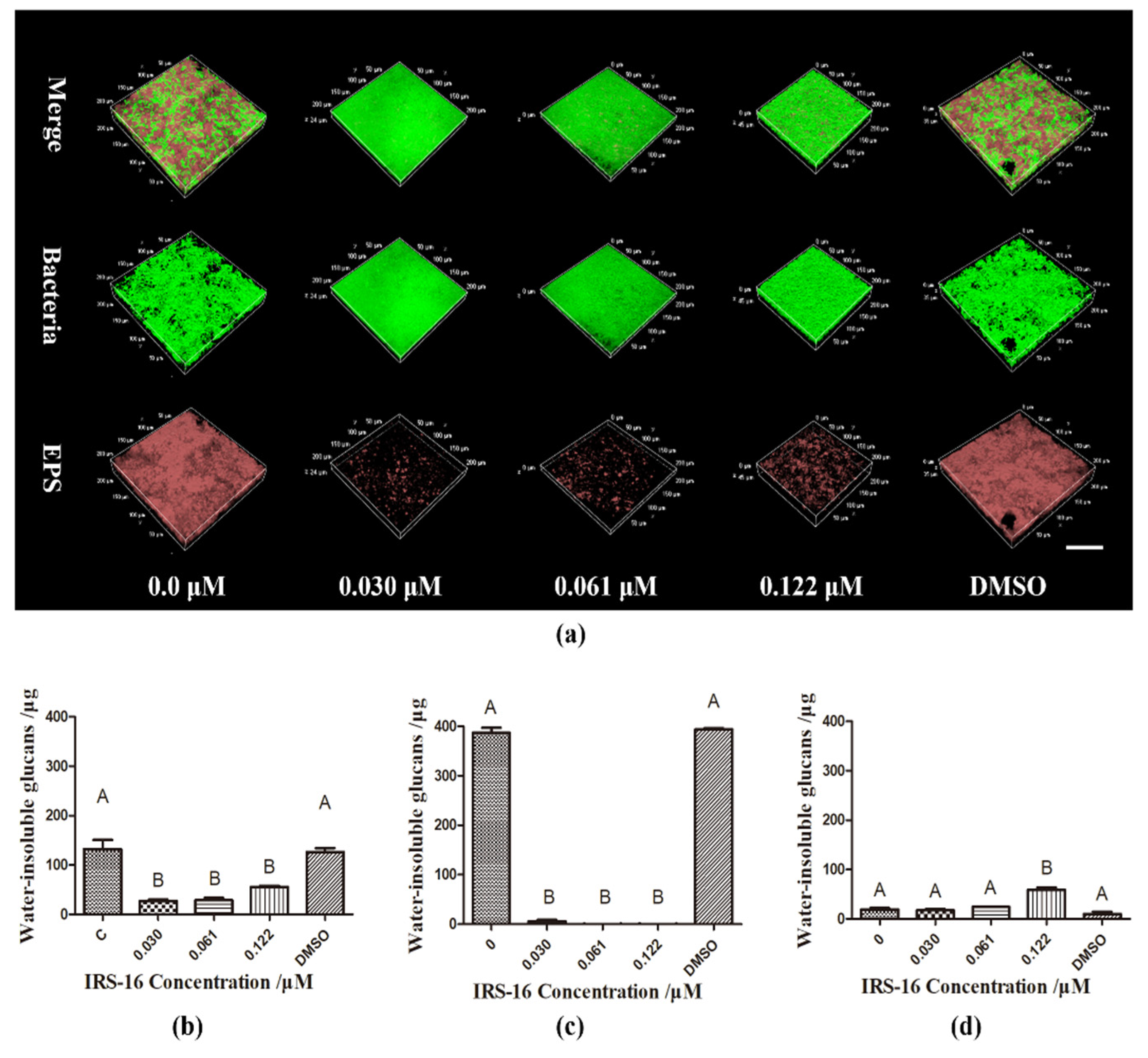
2.5. IRS-16 Reduced EPS Synthesis in Dual-Species Biofilms
2.6. IRS-16 Influenced the Bacterial Growth of the Two Species
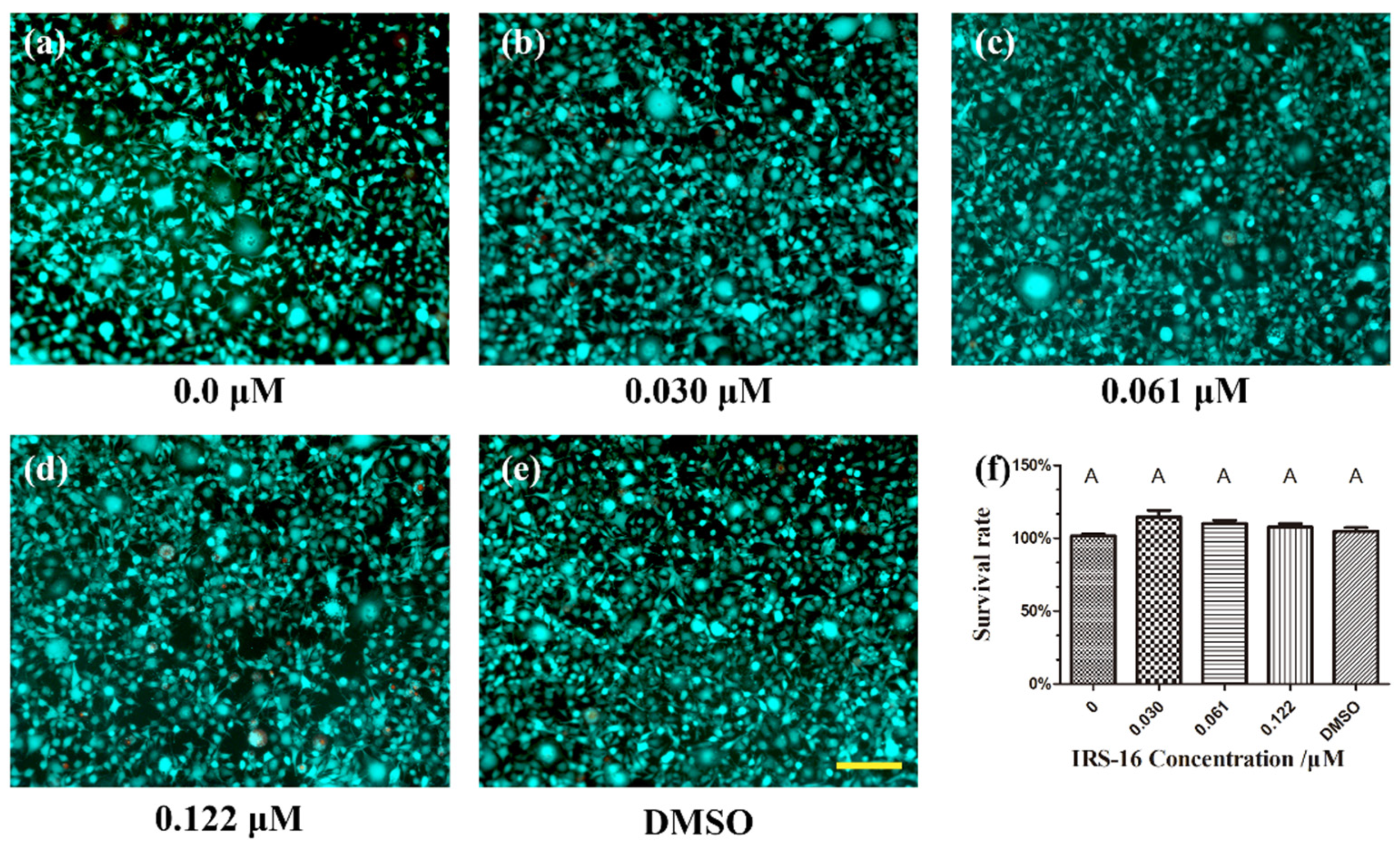
2.7. IRS-16 Exhibited No Remarkable Toxicity to L929 Cells
3. Discussion
4. Materials and Methods
4.1. Microbial Strains and Culture Conditions
4.2. Crystal Violet Test
4.3. Fluorescence In Situ Hybridization (FISH)
4.4. MTT Assay
4.5. Lactic Acid Detection
4.6. Bacterial/Extracellular Polysaccharide (EPS) Dye
4.7. Water-Insoluble Exopolysaccharide Determination
4.8. Growth Curve
4.9. Live/Dead Cellular Viability Assay
4.10. CCK-8 Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Listl, S.; Galloway, J.; Mossey, P.A.; Marcenes, W. Global Economic Impact of Dental Diseases. J. Dent. Res. 2015, 94, 1355–1361. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990-2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Hamada, S.; Slade, H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980, 44, 331–384. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Merritt, J.; Shi, W.; Qi, F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 2005, 187, 7193–7203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreth, J.; Zhang, Y.; Herzberg, M.C. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J. Bacteriol. 2008, 190, 4632–4640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Q.; Ren, B.; He, J.; Peng, X.; Guo, Q.; Zheng, L.; Li, J.; Dai, H.; Chen, V.; Zhang, L.; et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. Isme J. 2021, 15, 894–908. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Chlorhexidine—Pharmaco-biological activity and application. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1321–1326. [Google Scholar]
- Lu, J.; Cheng, L.; Huang, Y.; Jiang, Y.; Chu, C.H.; Peng, X.; Li, M.; Xu, H.H.K.; Zhou, X.; Ren, B. Resumptive Streptococcus mutans persisters induced from dimethylaminododecyl methacrylate elevated the cariogenic virulence by up-regulating the quorum-sensing and VicRK pathway Genes. Front. Microbiol. 2019, 10, 3102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kuang, X.; Zhou, Y.; Yang, R.; Zhou, X.; Peng, X.; Luo, Y.; Xu, X. Antimicrobial activities of a small molecule compound II-6s against oral streptococci. J. Oral Microbiol. 2021, 13, 1909917. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J. Dent. 2013, 41, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y.; et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Cheng, L.; Wu, E.J.; Weir, M.D.; Bai, Y.; Xu, H.H. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhou, C.; Ren, B.; Li, X.; Weir, M.D.; Masri, R.M.; Oates, T.W.; Cheng, L.; Xu, H.K.H. Formation of persisters in Streptococcus mutans biofilms induced by antibacterial dental monomer. J. Mater. Sci. Mater. Med. 2017, 28, 178. [Google Scholar] [CrossRef]
- Martin, J.K., 2nd; Sheehan, J.P.; Bratton, B.P.; Moore, G.M.; Mateus, A.; Li, S.H.; Kim, H.; Rabinowitz, J.D.; Typas, A.; Savitski, M.M.; et al. A dual-mechanism antibiotic kills Gram-negative bacteria and avoids drug resistance. Cell 2020, 181, 1518–1532.e1514. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cheng, X.; Wang, L.; Qiu, W.; Wang, S.; Zhou, Y.; Li, M.; Li, Y.; Cheng, L.; Li, J.; et al. Combinatorial effects of arginine and fluoride on oral bacteria. J. Dent. Res. 2015, 94, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of antibiotics/antimicrobial agents on dental caries. Biomed. Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Ecological approaches to oral biofilms: Control without killing. Caries Res. 2015, 49 (Suppl. 1), 46–54. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, N.; Suneja, B.; Walsh, L.J. Ecological approaches to dental caries prevention: Paradigm shift or shibboleth? Caries Res. 2018, 52, 153–165. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Falsetta, M.L.; Klein, M.I. The exopolysaccharide matrix: A virulence determinant of cariogenic biofilm. J. Dent. Res. 2013, 92, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Kuang, X.; Zhou, Y.; Peng, X.; Guo, Q.; Yang, T.; Zhou, X.; Luo, Y.; Xu, X. A novel small molecule, ZY354, inhibits dental caries-associated oral biofilms. Antimicrob. Agents Chemother. 2019, 63, e02414-18. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, M.; Hense, B.A.; Eberl, H.J.; Kuttler, C. Simulation-based exploration of quorum sensing triggered resistance of biofilms to antibiotics. Bull. Math. Biol. 2018, 80, 1736–1775. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, Y.; Sun, Y.; Li, M.; Huang, S.; Qiu, W.; Tu, H.; Zhang, K. Effects of Norspermidine on dual-species biofilms composed of Streptococcus mutans and Streptococcus sanguinis. Biomed. Res. Int. 2019, 2019, 1950790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying adhesive materials to improve the longevity of resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashper, S.G.; Reynolds, E.C. Effects of organic acid anions on growth, glycolysis, and intracellular pH of oral streptococci. J. Dent. Res. 2000, 79, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Iwami, Y.; Yamada, T. Rate-limiting steps of the glycolytic pathway in the oral bacteria Streptococcus mutans and Streptococcus sanguis and the influence of acidic pH on the glucose metabolism. Arch. Oral Biol. 1980, 25, 163–169. [Google Scholar] [CrossRef]
- Deng, D.M.; Hoogenkamp, M.A.; Ten Cate, J.M.; Crielaard, W. Novel metabolic activity indicator in Streptococcus mutans biofilms. J. Microbiol. Methods 2009, 77, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Jiang, Y.; Brandt, B.W.; Yang, J.; Chen, Y.; Buijs, M.J.; Crielaard, W.; Cheng, L.; Deng, D. Regrowth of microcosm biofilms on titanium surfaces after various antimicrobial treatments. Front. Microbiol. 2019, 10, 2693. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, T.; McMichael, M.; Velu, S.E.; Zou, J.; Zhou, X.; Wu, H. New small-molecule inhibitors of dihydrofolate reductase inhibit Streptococcus mutans. Int. J. Antimicrob. Agents 2015, 46, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, H.; Fan, Y.; Lv, X.; Han, S.; Zhou, X.; Zhang, L. Activity of synthetic antimicrobial peptide GH12 against oral streptococci. Caries Res. 2016, 50, 48–61. [Google Scholar] [CrossRef]
- Bijle, M.N.; Ekambaram, M.; Yiu, C. A scoping review on arginine in caries prevention. J. Evid. Based Dent Pract. 2020, 20, 101470. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Zhou, Z.; Tu, H.; Ren, Q.; Wang, X.; Ding, L.; Zhou, X.; Zhang, L. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch. Oral Biol. 2017, 80, 41–50. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, K.; Zhou, X.; Liu, C.; Li, M.; Li, Y.; Wang, R.; Li, Y.; Li, J.; Shi, W.; et al. Involvement of gshAB in the interspecies competition within oral biofilm. J. Dent. Res. 2013, 92, 819–824. [Google Scholar] [CrossRef]
- Wang, S.P.; Ge, Y.; Zhou, X.D.; Xu, H.H.; Weir, M.D.; Zhang, K.K.; Wang, H.H.; Hannig, M.; Rupf, S.; Li, Q.; et al. Effect of anti-biofilm glass-ionomer cement on Streptococcus mutans biofilms. Int. J. Oral Sci. 2016, 8, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent. Mater. 2012, 28, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, X.; Jiang, W.; Wang, K.; Luo, J.; Li, W.; Zhou, X.; Zhang, L. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J. Oral Microbiol. 2018, 10, 1442089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xu, Y.; Song, Q.; Wang, F.; Sun, L.; Liu, L.; Yang, X.; Yi, J.; Bao, Y.; Ma, H.; et al. Anti-biofilm activities from bergenia crassifolia Leaves against Streptococcus mutans. Front. Microbiol. 2017, 8, 1738. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Liu, S.; Zhou, X.; Hu, H.; Zhang, K.; Du, X.; Peng, X.; Ren, B.; Cheng, L.; Li, M. Effect of D-cysteine on dual-species biofilms of Streptococcus mutans and Streptococcus sanguinis. Sci. Rep. 2019, 9, 6689. [Google Scholar] [CrossRef] [PubMed]
- Hayat, U.; Raza, A.; Wang, H.J.; Wang, J.Y. Preparation of ciprofloxacin loaded zein conduits with good mechanical properties and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110766. [Google Scholar] [CrossRef] [PubMed]


| Probes | Nucleotide Sequence (5′–3′) | Reference |
|---|---|---|
| S. mutans S. sanguinis | Alexa Fluor 488-5′-ACTCCAGACTTTCCTGAC-3′ Alex Fluor 594-5′-GCATACTATGGTTAAGCCAC AGCC-3′ | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Wang, M.; Shen, Y.; Zhang, L.; Pan, Y.; Sun, Y.; Zhang, K. Regulatory Effect of Irresistin-16 on Competitive Dual-Species Biofilms Composed of Streptococcus mutans and Streptococcus sanguinis. Pathogens 2022, 11, 70. https://doi.org/10.3390/pathogens11010070
Hu X, Wang M, Shen Y, Zhang L, Pan Y, Sun Y, Zhang K. Regulatory Effect of Irresistin-16 on Competitive Dual-Species Biofilms Composed of Streptococcus mutans and Streptococcus sanguinis. Pathogens. 2022; 11(1):70. https://doi.org/10.3390/pathogens11010070
Chicago/Turabian StyleHu, Xiangyu, Min Wang, Yan Shen, Lingjun Zhang, Yihuai Pan, Yan Sun, and Keke Zhang. 2022. "Regulatory Effect of Irresistin-16 on Competitive Dual-Species Biofilms Composed of Streptococcus mutans and Streptococcus sanguinis" Pathogens 11, no. 1: 70. https://doi.org/10.3390/pathogens11010070
APA StyleHu, X., Wang, M., Shen, Y., Zhang, L., Pan, Y., Sun, Y., & Zhang, K. (2022). Regulatory Effect of Irresistin-16 on Competitive Dual-Species Biofilms Composed of Streptococcus mutans and Streptococcus sanguinis. Pathogens, 11(1), 70. https://doi.org/10.3390/pathogens11010070






