Abstract
CD4+ CCR6+ T cells are highly susceptible to HIV infection, and a high cytokine producing CCR6+ T cell subset is selectively lost during HIV infection. The CCR6 chemokine MIP-3α (CCL20) is produced at sites of infection in SIV animal models. Recently, we have shown that MIP-3α inhibits HIV replication. This inhibition of HIV infection is mediated by CCR6 signaling and eventuates in increased APOBEC3G expression. Since there are few existing reports on the role of MIP-3α in health or disease, we studied its production by PBMCs from HIV-seronegative and HIV+ subjects. We evaluated the ability of PBMCs to produce MIP-3α in response to antigen stimulation using cells obtained from two groups: one composed of HIV-seronegative subjects (n = 16) and the other composed of HIV+ subjects (n = 58), some asymptomatic and some with clinically defined AIDS. Antigens included fragment C of the tetanus toxin, Candida albicans, whole-inactivated HIV, and HIV p24. MIP-3α was detected by ELISA in tissue culture supernatants of antigen-stimulated PBMCs. MIP-3α production by antigen-stimulated PBMCs was readily measured for HIV-negative subjects and for HIV-seropositive asymptomatic subjects, but not for patients with AIDS. These results suggest that subversion of the MIP-3α-CCR6 axis by HIV during the course of infection contributes to the loss of immune function that eventually leads to AIDS.
1. Introduction
Chemokine receptors play an important role in HIV immunopathogenesis and immune antiviral response. [1,2]. We demonstrated that CCR5 ligands are potent and specific inhibitors of certain isolates of HIV-1 [3]. Subsequently, we [4,5] and others [6,7,8,9,10,11,12,13] showed that high levels of chemokine production by antigen-activated cells are associated with protection from HIV-1 infection in high-risk subjects. More recently, studies on HIV and SIV immunopathogenesis have highlighted the role played by the MIP-3α-CCR6 axis [14,15,16,17,18,19,20,21,22,23,24,25,26].
We reported that CCR6 mediates the HIV inhibitory activity of its ligands, MIP-3α and hBD2 [26]. CCR6 is expressed on immature dendritic cells [27,28,29,30,31,32], which are among the first cells to come in contact with HIV [33,34,35,36]. Further, CCR6 is expressed on activated and memory CD4+ T cells, especially on lymphocytes that infiltrate inflamed mucosa [37]. Studies of CD4+ CCR6+ and CCR6− cells have shown that the former are highly susceptible to HIV infection, so that protection of CCR6+ cells has become a goal for new approaches to HIV prevention and therapy [18,19,20,21,23,24,26,38,39,40,41,42]. Although CCR6 is at best a very marginal HIV coreceptor, it is expressed on CD4+ T cells that express HIV coreceptors CCR5 and CXCR4 [43,44]. CCR6 is expressed on subsets of both central and effector memory T cells that secrete high levels of IL-2 and TNF-α in response to polyclonal and antigen-specific stimulation [45]. Notably, it has been shown that CCR6+ T lymphocytes are lost from the peripheral blood of HIV-infected patients as disease progresses [45]. Furthermore, the capacity of CD4+ CCR6+ cells to secrete multiple cytokines remained intact among HIV-infected long-term non-progressors but was partially lost from subjects with progressive disease [45]. In the same study, CCR6+ T cells accumulated in the spleen of HIV-infected patients, then died by apoptosis. Perhaps as a consequence of migration, production of MIP-3α from splenocytes of HIV-infected individuals was increased, compared with HIV-seronegative controls [45]. In animal models, the expression of both MIP-3α and CCR6 are increased in acutely infected macaques and decreased in macaques with AIDS [15,46].
CCR6 is also expressed on all IL-17 producing (Th17) T cells [47,48,49]. CCR6+ Th17 cells are depleted in HIV-infected individuals and in pathogenic SIV rhesus macaque models of infection [19,22,38,41,50,51]. Further, rhesus macaques with larger pre-existing Th17 compartments have lower viral loads [52]. However, in SIV sooty mangabey non-pathogenic models of infection, Th17 cells are depleted during the acute phase of infection but are subsequently restored, suggesting that sustained loss of Th17 cells plays a role in disease progression [22]. In humans, the presence of mucosal Th17 cells inversely correlates with viremia, [53] and restoration of gut CD4+ T cells is associated with accumulation of Th17 cells [51]. Further, Th17 cells are preserved in long-term non-progressor and elite controller subjects, but not in progressor subjects [54,55,56,57].
We have shown that MIP-3α possesses antiviral activity when added post-infection and that the CCR6 ligands MIP-3α and human β-defensin 2 (hBD2) induce the expression of the host restriction factor APOBEC3G in both unstimulated and PHA-stimulated PBMCs and CD4+ T cells [26]. Consequently, production of CCR6 ligands could contribute to protection of CD4+ CCR6+ cells. We therefore investigated whether the CCR6 ligand MIP-3α is produced by PBMCs in response to recall antigen stimulation in HIV-negative subjects using fragment C of the tetanus toxin (TTC) and C. albicans. To investigate the relevance of MIP-3α in HIV infection, we measured its production in response to HIV antigens and to C. albicans by PBMCs from HIV-infected subjects. We detected MIP-3α production in PBMCs after antigen stimulation and found that its production was significantly greater in PBMCs of healthy subjects than in PBMCs of subjects with AIDS. Our data suggest that the CCR6-MIP-3α axis is negatively affected in the course of HIV infection.
2. Results
2.1. Antigen Stimulation-Induced Expression of MIP-3α Is Elevated in PBMCs
Data previously published by our group showed that antigen stimulation with either TTC or C. albicans induced production of CCR5 chemokines [58]. Since CCR5 and CCR6 are often coexpressed in CD4+ T memory cells [59], we investigated whether MIP-3α is also produced upon antigen stimulation. We analyzed tissue culture supernatants from fresh PBMCs obtained from healthy volunteers (who reported having been vaccinated against tetanus) and cultured them in media without stimulation or activated with TTC or C. albicans or PHA. Our results show that as little as 3 µg/mL of TTC induced significant MIP-3α production as early as 3 days post-stimulation (Figure 1). At day 3, low levels of MIP-3α (69 ± 23 pg/mL, mean ± SEM) were detected in control cultures, while significantly higher levels (301 ± 79 pg/mL; p = 0.018) were detected in cells treated with 3 µg/mL of TTC. This effect was dose-dependent, with maximal accumulation of MIP-3α occurring in PBMCs treated with TTC at 20 µg/mL, where production was induced approximately nine-fold over control levels (induced level 633 ± 213 pg/mL, p = 0.011). MIP-3α production in supernatants from cells stimulated with C. albicans was even higher (807 ± 329 pg/mL, p = 0.03) than that measured in TTC-stimulated cells at any concentration, perhaps reflecting a higher number of cells that responded to this antigen or, alternatively, different potencies of each antigen. PHA was also a potent inducer of production of MIP-3α, which reached a concentration of 1426 ± 257 pg/mL (p = 0.0002) at day 3 after stimulation and decreased at days 6 and 9 (not shown). Production of MIP-3α was highest at day 3 for all antigens, decreasing at days 6 and 9 (Figure 1).
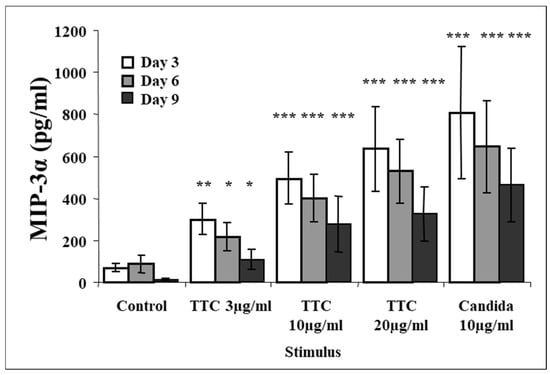
Figure 1.
Production of MIP-3α following antigen stimulation of PBMCs at day 3, day 6, and day 9 with TTC or Candida albicans. PBMCs (1 × 106 for each activation group) were obtained from healthy subjects and stimulated with antigens as described in the Methods section. MIP-3α was quantified by ELISA. Data are presented as mean ± SEM (n = 12). Antigen-stimulated levels significantly different from media control value at * p < 0.05, ** p ≤ 0.01, *** p ≤ 0.005.
2.2. Kinetics of Release of MIP-3α
As a result of the decline in production of MIP-3α after day 3, we further evaluated the kinetics of its release post-stimulation in shorter time-course experiments. Initial experiments revealed that this chemokine is released as early as one day after activation (not shown). Next, we analyzed supernatants of PBMCs after 2, 4, 8, 20, and 24 h of antigen stimulation. MIP-3α was detectable above background levels as early as 8 h after stimulation with antigens and as early as 2 h post stimulation with PHA (Figure 2). Peak expression of MIP-3α occurred at 20 h after stimulation (Figure 2). These results suggest that chemokine release occurs relatively rapidly as a consequence of stimulation by recall antigens.
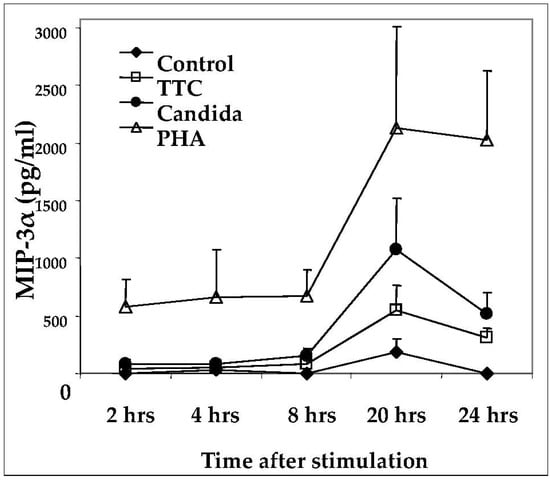
Figure 2.
Time-course of MIP-3α production in response to antigen activation. PBMCs (1 × 106 for each activation group) were obtained from healthy subjects and stimulated with antigens indicated as described in Methods. MIP-3α was quantified by ELISA. Data are presented as mean ± SEM (n = 4).
2.3. MIP-3α Release as a Function of Time from Vaccination
To address the question of whether the MIP-3α response to recall antigens would diminish in magnitude with time from vaccination, we analyzed MIP-3α production at 3, 6, and 9 days after stimulation using antigens in four subjects who had not received a tetanus booster at least 10 years prior to the blood donation. All of these subjects responded to activation by producing MIP-3α, but the average increase in MIP-3α induced by TTC was not statistically significant compared to control levels (Figure 3).
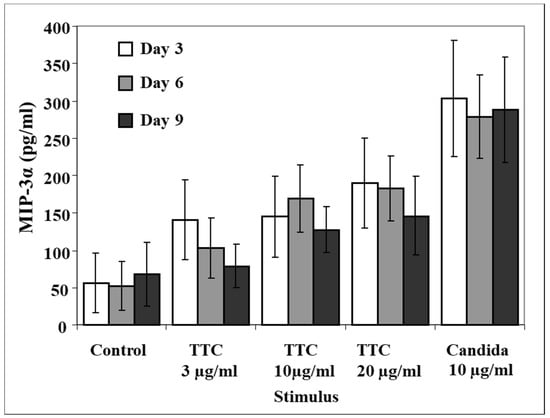
Figure 3.
Production of MIP-3α at day 3, day 6, and day 9 following antigen stimulation of PBMCs from subjects vaccinated more than 10 years prior to testing. PBMCs (1 × 106 for each activation group) were obtained from healthy subjects and stimulated with TTC or C. albicans as described in Methods. Production of MIP-3α was measured by ELISA. Data are presented as mean ± SEM (n = 4).
2.4. Antigen-Induced CCR6 Ligand Release in Cryopreserved Samples
Since freshly obtained samples are not always available for study, it was important to investigate whether our assay was effective using cryopreserved specimens. An aliquot of the same PBMCs from healthy donors (n = 12) that were used to obtain the data shown in Figure 1 was cryopreserved in liquid nitrogen for four months. The cells were then thawed and stimulated using the same protocol represented in Figure 1.
The supernatants were harvested at days 3, 6, and 9. At day 3, the levels of MIP-3α in all antigen-stimulated groups were higher than in the control group (i.e., 207 ± 60 pg/mL; Figure 4), particularly the production in response to TTC 10 µg/mL (462 ± 99 pg/mL, p = 0.011) and TTC 20 µg/mL (502 ± 107 pg/mL, p = 0.017; Figure 4). Significantly higher levels of MIP-3α were also observed in the C. albicans (Figure 4) and PHA groups (not shown) than in the TTC groups. Unlike the fresh PBMCs, the cryopreserved PBMCs did not exhibit a decline in MIP-3α levels at days 6 and 9. In summary, antigen-induced levels of MIP-3α are detectable also in cryopreserved samples.
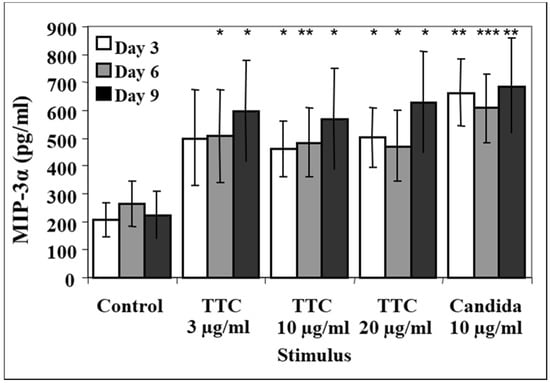
Figure 4.
Release of MIP-3α from cryopreserved cells. PBMCs (1 × 106 for each activation group) were obtained from 12 healthy subjects. Aliquots of the cells used in Figure 1 were cryopreserved in liquid nitrogen for 4 months, then thawed and stimulated. Cryopreserved cells were stimulated with 3, 10 or 20 µg of TTC or 10 µg/mL C. albicans or culture with media only as a control. Aliquots of supernatants were harvested at days 3, 6 and 9. MIP-3α was quantified by ELISA. Data are presented as mean ± SEM (n = 12). Antigen-stimulated values are significantly different from media control values at * p < 0.05, ** p ≤ 0.01, *** p ≤ 0.005.
2.5. Antigen-Induced MIP-3α Production in PBMCs of HIV-Infected and AIDS Patients
Since MIP-3α production was associated with the memory response to recall antigens (Figure 1, Figure 2, Figure 3 and Figure 4) that is progressively lost in the course of HIV infection, and since we have shown that MIP-3α has HIV-inhibitory activity [26], we evaluated the production of this chemokine in supernatants from antigen-activated PBMCs that we had previously prepared in the course of a cross-sectional study of an HIV cohort [4]. In that study, cells had been stimulated for 3 days with HIV or purified p24 protein, C. albicans, PHA, or media alone [4]. MIP-3α levels were quantified by ELISA, and values measured in media from the stimulated group were adjusted by subtracting the background production measured with control media. We analyzed supernatants from 58 HIV-seropositive subjects, 10 of whom had progressed to AIDS and 48 of whom were asymptomatic. MIP-3α production in response to C. albicans did not differ significantly between these groups (Figure 5, top panel), and the same was true for PHA (Figure 5, lower panel) and p24 (not shown). However, the response to HIV was significantly lower in the male subjects with AIDS than in the asymptomatic male subjects (Figure 5, middle panel). Our data therefore suggest that the loss of immune function over the course of HIV infection includes a decreased ability to produce MIP-3α in response to the pathogen.
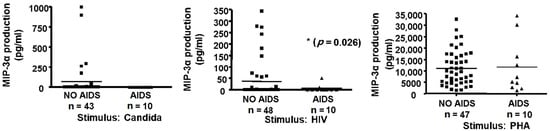
Figure 5.
Antigen-induced MIP-3α production and AIDS status. Fresh PBMCs (1.6 × 106) were cultured for 3 days as described in Methods in medium alone or with 10 µg/mL C. albicans (Candida, left panel) or with 10 µg/mL gp120-depleted, inactivated HIV-1 (HIV, middle panel), or 5 µg/mL PHA (right panel). Supernatants were collected, frozen, stored for nine years, and assayed for MIP-3α by ELISA. Control (medium alone) values were subtracted from antigen-activated values to correct for background (horizontal bars represent mean values).
3. Discussion
In this study, we evaluated the expression of MIP-3α in the context of the immune response to protein antigens and inactivated pathogens, including TTC, C. albicans, HIV, and HIV p24, in both HIV-seronegative and HIV-positive subjects. MIP-3α displays expression patterns characteristic of both constitutive and inflammatory chemokines, and its production is inducible in different cell types, including many types of leukocytes [27,28,31,44,59,60,61,62,63,64,65,66,67,68].
Our results show that MIP-3α release was induced by antigen stimulation of PBMC. MIP-3α release was dose- and antigen-dependent, and the maximal response was to TTC 20 µg/mL group at day 3, followed by a decline at days 6 and 9. These findings are consistent with our previous findings on the release of CCR5 ligands in the course of the immune response to the same antigens used here [58].
In four subjects who had not received a tetanus booster in the past 10 years, we did not observe high enough levels of MIP-3α in response to TTC stimulation to reach statistical significance. This finding differs from our observations on CCR5 ligands MIP-1α and MIP-1ß, which were significantly induced by TTC treatment in the same samples [58]. Some caution in interpreting these data is necessary, however, since these data were obtained from a small number of subjects.
Our kinetics data showed that the production of MIP-3α occurred relatively soon after antigen stimulation, i.e., beginning at about 8 h and peaking at about 20 h. However, this is still slower than the release of CCR5 ligands, which we have shown are detectable by ELISA within 2 h of activation [58].
This study is the first to analyze the expression of MIP-3α protein in response to antigen stimulation in PBMCs obtained from HIV-positive subjects. One limitation of our study was that we did not identify the cells that produced MIP-3α by flow cytometry, leaving unanswered the question of which cells produced the chemokine in the conditions we tested. We observed variability in the response to all antigens, which is to be expected in a human HIV study, and probably reflects differences in immune status between patients. Our data indicate that antigen-induced production of MIP-3α is decreased in response to HIV antigens in HIV+ men with AIDS relative to HIV+ men without AIDS, suggesting that cells producing this chemokine, or their ability to produce it, are lost as disease progresses.
Our data shows that production of MIP-3α in response to HIV is more informative than in response to C. albicans, p24, or PHA. The HIV+ men with AIDS in our study were viremic, and an association between viremia and decreased ability to respond to HIV antigens has been reported [69]). Our data differ from data on MIP-3α levels in serum and plasma, which previous reports showed to be consistently higher than those measured in HIV-seronegative donors [70,71]. This discrepancy may be due to MIP-3α levels in plasma and serum likely being the result of chemokine production by a variety of sources, including neutrophils, and it is not clear whether these high levels would be relevant in the tissues, where HIV replication and immune response are critical. Indeed, levels of MIP-3α were found to be lower in the small intestine mucosa of HIV-infected individuals as compared to HIV- seronegative controls, a finding that correlated with impaired restoration of Th17 following antiretroviral therapy. Further, a study of expression of cytokines from ectocervix samples in the context of HIV infection found lower levels of production of MIP-3α in HIV-infected women as compared to HIV-seronegative controls [72]. In addition, a study of mucosal tissues from HIV-2-infected individuals suggested that levels of MIP-3α in the lamina propria correlated with markers of mucosal integrity [73]. Thus, the decrease in production of MIP-3α by antigen-activated cells in HIV-infected subjects with AIDS may contribute to a reduced mucosal integrity and ability to control HIV in vivo. In one study, a CD90+ subpopulation of cells producing high levels of MIP-3α was selectively depleted in the course of HIV infection, providing a potential explanation for our findings [41]. Decreased production of MIP-3α in response to antigen activation is also likely to reflect the loss of HIV-specific cells observed in the course of HIV infection, so that CCR6+ cell numbers and MIP-3α production in response to HIV antigens may be useful as markers of disease progression [74]. Alternatively, it is possible that the loss of MIP-3α production might impede interactions between MIP-3α-secreting cells and CCR6-expressing cells, which include DC and B and T cells, and thus might contribute to the loss of cellular immunity observed in the course of HIV infection.
4. Materials and Methods
4.1. Study Subjects
We used samples (supernatants from PBMCs) that we had obtained from two previous studies:
- (a)
- A study of antigen stimulation on PBMCs from healthy donors. PBMCs from 16 healthy (by self-assessment), HIV-negative donors were obtained by venipuncture and Histopaque isolation as described [59]. Of these, 4 donors reported not having been vaccinated against tetanus for more than 10 years, while the other subjects had been vaccinated within the previous 10 years. All subjects signed informed consent forms approved by the Institutional Review Board.
- (b)
- Studies on subjects from an HIV cohort. Subjects were obtained from the Baltimore–Washington DC center of the Multicenter AIDS Cohort Study (MACS), a longitudinal study of the natural history of HIV-1 infection in men who have sex with men [4]. Briefly, 1253 men were recruited in 1984–85 and in 1987–90 and followed at 6-month intervals with clinical and laboratory testing as well as storage of repository specimens. For this study, blood was obtained from 58 HIV-1 seropositive men at visit 27 (April–October 1997). HIV-positive subjects were categorized into two groups: 10 with AIDS and 48 who were asymptomatic and did not have AIDS according to the 1993 definition by the Centers for Disease Control and Prevention. Thirteen control, non-MACS HIV-seronegative subjects with no history of exposure to HIV were recruited from the laboratory staff at the Institute of Human Virology.
4.2. Cells and Laboratory Studies
- (a)
- HIV-seronegative subjects. PBMCs were incubated with 3–20 µg/mL Fragment C of tetanus toxin (TTC–Calbiochem, La Jolla, CA, USA), 10 µg/mL Candida albicans (Greer Laboratories, Lenoir, NC, USA), or 2.5 µg/mL phytohemoagglutinin (PHA; Sigma-Aldrich, St Louis, MO, USA). Supernatants from cells incubated with media alone were used as controls. Supernatants were collected on days 3, 6, and 9 after activation and frozen at −80 °C. Supernatants had been stored for about 4 years prior to testing for MIP-3α.
- (b)
- PBMC obtained from HIV-1 seropositive subjects in the MACS were collected in CPT tubes (Falcon-BD, Franklin Lakes, NJ, USA), following the instructions of the manufacturer. Fresh PBMCs were cultured in round-bottom 96-well plates (Falcon-BD) in RPMI medium (GIBCO-Invitrogen, Carlsbad, CA, USA) supplemented with 10% human AB serum and antibiotics (100 U/mL penicillin, 100 U/mL streptomycin) (GIBCO-Invitrogen) at a density of 2 × 105 cells/100 µL. Cells were incubated for 3 days with 10 µg/mL gp120-depleted, inactivated HIV-1, 10 µg/mL purified p24 antigen (see below), 10 µg/mL C. albicans, or 2.5 µg/mL PHA, or with media alone as a control. Supernatants were collected on day 3 (for PHA) or day 6 (for antigen stimulation) and frozen at −80 °C [4]. HIV-1 HZ321 immunogen was obtained by concentration and purification from the supernatant fluid of HZ321-infected HUT-78 cells. In the preparation of the immunogen, envelope gp120 was depleted during freezing and thawing and during the purification process [75].
4.3. MIP-3α Measurement
Human MIP-3α was measured using commercial ELISA plates pre-coated with an anti-hMIP-3α monoclonal antibody from R&D Systems (Minneapolis, MN, USA), following the manufacturer’s instructions.
4.4. Statistical Analysis
Data of patients were abstracted from clinical records and maintained in an Excel database. Analysis of data distribution and of statistical significance was performed with GraphPad InStat, Version 4.00, for Windows XP (GraphPad Software, San Diego, CA, USA) using a two-tailed, parametric (paired t-test, for normally-distributed data) or non-parametric (Wilcoxon signed rank test or Mann–Whitney test for non-normally distributed data) statistical tests. The level of statistical significance attained is indicated in the text and in figure legends. Intervention studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
Author Contributions
Conceptualization, A.G.-D.; methodology, F.Z. and L.S.; validation, M.K.L.; formal analysis, A.G.-D.; writing—original draft preparation, F.Z.; writing—review and editing, A.G.-D., M.K.L. and J.B.M.; supervision, A.G.-D.; funding acquisition, A.G.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by awards R01NS066842 from the National Institute of Neurological Disorders and Stroke (A.G.-D.) and U01-AI35042 from the National Institute of Allergy and Infectious Diseases (J.B.M.) and by the Johns Hopkins Clinical and Translational Research Unit: UL1-RR025005. M.K. Lafferty was a trainee under an Institutional Training Grant T32AI007540 from the National Institute of Allergy and Infectious Diseases.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of MACS/WIHS Combined Cohort Study (MWCCS) (protocol code X21064, approved 7 September 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Garzino-Demo, A.; DeVico, A.L.; Gallo, R.C. Chemokine Receptors and Chemokines in HIV Infection. J. Clin. Immunol. 1998, 18, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.A.; Murphy, P.M.; Farber, J.M. Chemokine Receptors as HIV-1 Coreceptors: Roles in Viral Entry, Tropism, and Disease. Annu. Rev. Immunol. 1999, 17, 657–700. [Google Scholar] [CrossRef]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1 Alpha, and MIP-1 Beta as the Major HIV-Suppressive Factors Produced by CD8+ T Cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef] [Green Version]
- Garzino-Demo, A.; Moss, R.B.; Margolick, J.B.; Cleghorn, F.; Sill, A.; Blattner, W.A.; Cocchi, F.; Carlo, D.J.; DeVico, A.L.; Gallo, R.C. Spontaneous and Antigen-Induced Production of HIV-Inhibitory Beta-Chemokines Are Associated with AIDS-Free Status. Proc. Natl. Acad. Sci. USA 1999, 96, 11986–11991. [Google Scholar] [CrossRef] [Green Version]
- Cocchi, F.; DeVico, A.L.; Yarchoan, R.; Redfield, R.; Cleghorn, F.; Blattner, W.A.; Garzino-Demo, A.; Colombini-Hatch, S.; Margolis, D.; Gallo, R.C. Higher Macrophage Inflammatory Protein (MIP)-1alpha and MIP-1beta Levels from CD8+ T Cells Are Associated with Asymptomatic HIV-1 Infection. Proc. Natl. Acad. Sci. USA 2000, 97, 13812–13817. [Google Scholar] [CrossRef] [Green Version]
- Zagury, D.; Lachgar, A.; Chams, V.; Fall, L.S.; Bernard, J.; Zagury, J.F.; Bizzini, B.; Gringeri, A.; Santagostino, E.; Rappaport, J.; et al. C-C Chemokines, Pivotal in Protection against HIV type 1 Infection. Proc. Natl. Acad. Sci. USA 1998, 95, 3857–3861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullum, H.; Lepri, A.C.; Victor, J.; Aladdin, H.; Phillips, A.N.; Gerstoft, J.; Skinhøj, P.; Pedersen, B.K. Production of Beta-Chemokines in Human Immunodeficiency Virus (HIV) Infection: Evidence that High Levels of Macrophage Inflammatory Protein-1beta Are Associated with a Decreased Risk of HIV Disease Progression. J. Infect. Dis. 1998, 177, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.A.; Martin, S.R.; Tse, D.; O’Brien, T.R.; Skurnick, J.; VanDevanter, N.L.; Padian, N.; Braun, J.F.; Kotler, D.P.; Wolinsky, S.M.; et al. Relative Resistance to HIV-1 Infection of CD4 Lymphocytes from Persons Who Remain Uninfected Despite Multiple High-Risk Sexual Exposure. Nat. Med. 1996, 2, 412–417. [Google Scholar] [CrossRef]
- Paxton, W.A.; Liu, R.; Kang, S.; Wu, L.; Gingeras, T.R.; Landau, N.R.; Mackay, C.R.; Koup, R.A. Reduced HIV-1 Infectability of CD4+ Lymphocytes from Exposed-Uninfected Individuals: Association with Low Expression of CCR5 and High Production of Beta-Chemokines. Virology 1998, 244, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Tartakovsky, B.; Turner, D.; Vardinon, N.; Burke, M.; Yust, I. Increased Intracellular Accumulation of Macrophage Inflammatory Protein 1beta and Its Decreased Secretion Correlate with Advanced HIV Disease. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1999, 20, 420–422. [Google Scholar] [CrossRef]
- Wasik, T.J.; Bratosiewicz, J.; Wierzbicki, A.; Whiteman, V.E.; Rutstein, R.R.; Starr, S.E.; Douglas, S.D.; Kaufman, D.; Sison, A.V.; Polansky, M.; et al. Protective Role of Beta-Chemokines Associated with HIV-Specific Th Responses against Perinatal HIV Transmission. J. Immunol. 1999, 162, 4355–4364. [Google Scholar] [PubMed]
- Furci, L.; Scarlatti, G.; Burastero, S.; Tambussi, G.; Colognesi, C.; Quillent, C.; Longhi, R.; Loverro, P.; Borgonovo, B.; Gaffi, D.; et al. Antigen-Driven C-C Chemokine-Mediated HIV-1 Suppression by CD4(+) T Cells from Exposed Uninfected Individuals Expressing the Wild-Type CCR-5 Allele. J. Exp. Med. 1997, 186, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ferbas, J.; Giorgi, J.V.; Amini, S.; Grovit-Ferbas, K.; Wiley, D.J.; Detels, R.; Plaeger, S. Antigen-Specific Production of RANTES, Macrophage Inflammatory Protein (MIP)-1alpha, and MIP-1beta in vitro Is a Correlate of Reduced Human Immunodeficiency Virus Burden in vivo. J. Infect. Dis. 2000, 182, 1247–1250. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Fahey, J.V.; Shen, Z.; Lahey, T.; Cu-Uvin, S.; Wu, Z.; Mayer, K.; Wright, P.F.; Kappes, J.C.; Ochsenbauer, C.; et al. Anti-HIV Activity in Cervical-Vaginal Secretions from HIV-Positive and-Negative Women Correlate with Innate Antimicrobial Levels and IgG Antibodies. PLoS ONE 2010, 5, e11366. [Google Scholar] [CrossRef]
- Choi, Y.K.; Fallert, B.A.; Murphey-Corb, M.A.; Reinhart, T.A. Simian Immunodeficiency Virus Dramatically Alters Expression of Homeostatic Chemokines and Dendritic cell Markers during Infection in vivo. Blood 2003, 101, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Whelton, K.M.; Mlechick, B.; Murphey-Corb, M.A.; Reinhart, T.A. Productive Infection of Dendritic Cells by Simian Immunodeficiency Virus in Macaque Intestinal Tissues. J. Pathol. 2003, 201, 616–628. [Google Scholar] [CrossRef]
- Demberg, T.; Ettinger, A.C.; Aladi, S.; McKinnon, K.; Kuddo, T.; Venzon, D.; Patterson, L.J.; Phillips, T.M.; Robert-Guroff, M. Strong Viremia Control in Vaccinated Macaques Does Not Prevent Gradual Th17 Cell Loss from Central Memory. Vaccine 2011, 29, 6017–6028. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, P.; Gosselin, A.; Wacleche, V.S.; El-Far, M.; Said, E.A.; Kared, H.; Grandvaux, N.; Boulassel, M.R.; Routy, J.P.; Ancuta, P. Memory CCR6+ CD4+ T Cells Are Preferential Targets for Productive HIV Type 1 Infection Regardless of Their Expression of Integrin Beta7. J. Immunol. 2011, 186, 4618–4630. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, A.; Monteiro, P.; Chomont, N.; Diaz-Griffero, F.; Said, E.A.; Fonseca, S.; Wacleche, V.; El-Far, M.; Boulassel, M.R.; Routy, J.P.; et al. Peripheral Blood CCR4+ CCR6+ and CXCR3+ CCR6+ CD4+ T Cells Are Highly Permissive to HIV-1 Infection. J. Immunol. 2010, 184, 1604–1616. [Google Scholar] [CrossRef] [Green Version]
- Lafferty, M.K.; Sun, L.; Christensen-Quick, A.; Lu, W.; Garzino-Demo, A. Human Beta Defensin 2 Selectively Inhibits HIV-1 in Highly Permissive CCR6(+)CD4(+) T Cells. Viruses 2017, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Christensen-Quick, A.; Lafferty, M.; Sun, L.; Marchionni, L.; DeVico, A.; Garzino-Demo, A. Human Th17 Cells Lack HIV-Inhibitory RNases and Are Highly Permissive to Productive HIV Infection. J. Virol. 2016, 90, 7833–7847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenchley, J.M.; Paiardini, M.; Knox, K.S.; Asher, A.I.; Cervasi, B.; Asher, T.E.; Scheinberg, P.; Price, D.A.; Hage, C.A.; Kholi, L.M.; et al. Differential Th17 CD4 T-Cell Depletion in Pathogenic and Nonpathogenic Lentiviral Infections. Blood 2008, 112, 2826–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharucha, J.P.; Sun, L.; Lu, W.; Gartner, S.; Garzino-Demo, A. Human Beta-Defensin 2 and 3 Inhibit HIV-1 Replication in Macrophages Frontiers in Cellular and Infection Microbiology. Front. Cell. Infect. Microbiol. 2021, 11, 535352. [Google Scholar] [CrossRef]
- Anderson, J.L.; Khoury, G.; Fromentin, R.; Solomon, A.; Chomont, N.; Sinclair, E.; Milush, J.M.; Hartogensis, W.; Bacchetti, P.; Roche, M.; et al. Human Immunodeficiency Virus (HIV)-Infected CCR6+ Rectal CD4+ T Cells and HIV Persistence On Antiretroviral Therapy. J. Infect. Dis. 2020, 221, 744–755. [Google Scholar] [CrossRef]
- Loiseau, C.; Requena, M.; Mavigner, M.; Cazabat, M.; Carrere, N.; Suc, B.; Barange, K.; Alric, L.; Marchou, B.; Massip, P.; et al. CCR6(−) Regulatory T Cells Blunt the Restoration of Gut Th17 Cells along the CCR6-CCL20 Axis in Treated HIV-1-Infected Individuals. Mucosal. Immunol. 2016, 9, 1137–1150. [Google Scholar] [CrossRef]
- Lafferty, M.K.; Sun, L.; DeMasi, L.; Lu, W.; Garzino-Demo, A. CCR6 Ligands Inhibit HIV by Inducing APOBEC3G. Blood 2010, 115, 1564–1571. [Google Scholar] [CrossRef] [Green Version]
- Power, C.A.; Church, D.J.; Meyer, A.; Alouani, S.; Proudfoot, A.E.; Clark-Lewis, I.; Sozzani, S.; Mantovani, A.; Wells, T.N. Cloning and Characterization of a Specific Receptor for the Novel CC Chemokine MIP-3alpha from Lung Dendritic Cells. J. Exp. Med. 1997, 186, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Greaves, D.R.; Wang, W.; Dairaghi, D.J.; Dieu, M.C.; Saint-Vis, B.D.; Franz-Bacon, K.; Rossi, D.; Caux, C.; McClanahan, T.; Gordon, S.; et al. CCR6, a CC Chemokine Receptor that Interacts with Macrophage Inflammatory Protein 3alpha and Is Highly Expressed in Human Dendritic Cells. J. Exp. Med. 1997, 186, 837–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varona, R.; Zaballos, A.; Gutiérrez, J.; Martín, P.; Roncal, F.; Albar, J.P.; Ardavín, C.; Márquez, G. Molecular Cloning, Functional Characterization and mRNA Expression Analysis of the Murine Chemokine Receptor CCR6 and Its Specific Ligand MIP-3alpha. FEBS Lett. 1998, 440, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Dieu, M.C.; Vanbervliet, B.; Vicari, A.; Bridon, J.M.; Oldham, E.; Aït-Yahia, S.; Brière, F.; Zlotnik, A.; Lebecque, S.; Caux, C. Selective Recruitment of Immature and Mature Dendritic Cells by Distinct Chemokines Expressed in Different Anatomic Sites. J. Exp. Med. 1998, 188, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.N.; Pross, D.M.; Forster, R.; Zhang, J.; Kuklin, N.A.; Abbondanzo, S.J.; Niu, X.D.; Chen, S.C.; Manfra, D.J.; Wiekowski, M.T.; et al. CCR6 Mediates Dendritic Cell Localization, Lymphocyte Homeostasis, and Immune Responses in Mucosal Tissue. Immunity 2000, 12, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Howard, O.Z.; Chen, Q.; Oppenheim, J.J. Cutting Edge: Immature Dendritic Cells Generated from Monocytes in the Presence of TGF-Beta 1 Express Functional C-C Chemokine Receptor 6. J. Immunol. 1999, 163, 1737–1741. [Google Scholar]
- Geijtenbeek, T.B.; van Kooyk, Y. DC-SIGN: A Novel HIV Receptor on DCs that Mediates HIV-1 Transmission. Curr. Top. Microbiol. Immunol. 2003, 276, 31–54. [Google Scholar]
- Steinman, R.M.; Granelli-Piperno, A.; Pope, M.; Trumpfheller, C.; Ignatius, R.; Arrode, G.; Racz, P.; Tenner-Racz, K. The Interaction of Immunodeficiency Viruses with Dendritic Cells. Dendritic Cells Virus Infect. 2003, 276, 1–30. [Google Scholar]
- Teleshova, N.; Frank, I.; Pope, M. Immunodeficiency Virus Exploitation of Dendritic Cells in the Early Steps of Infection. J. Leukoc. Biol. 2003, 74, 683–690. [Google Scholar] [CrossRef]
- Turville, S.; Wilkinson, J.; Cameron, P.; Dable, J.; Cunningham, A.L. The Role of Dendritic Cell C-Type Lectin Receptors in HIV Pathogenesis. J. Leukoc. Biol. 2003, 74, 710–718. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Nakanishi, T.; Yamaguchi, D.; Takahashi, K.; Yumoto, H.; Ozaki, K.; Matsuo, T. Macrophage Inflammatory Protein 3alpha-CC Chemokine Receptor 6 Interactions Play an Important Role in CD4+ T-Cell Accumulation in Periodontal Diseased Tissue. Clin. Exp. Immunol. 2002, 128, 548–554. [Google Scholar] [CrossRef]
- El Hed, A.; Khaitan, A.; Kozhaya, L.; Manel, N.; Daskalakis, D.; Borkowsky, W.; Valentine, F.; Littman, D.R.; Unutmaz, D. Susceptibility of Human Th17 Cells to Human Immunodeficiency Virus and Their Perturbation during Infection. J. Infect. Dis. 2010, 201, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Y.; Tuen, M.; Nàdas, A.; Hioe, C.E. In Vitro Restoration of Th17 Response During HIV Infection with an Antiretroviral Drug and Th17 Differentiation Cytokines. AIDS Res. Hum. Retrovir. 2011, 28, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Kader, M.; Wang, X.; Piatak, M.; Lifson, J.; Roederer, M.; Veazey, R. Alpha4(+)beta7(hi)CD4(+) Memory T Cells Harbor Most Th-17 Cells and Are Preferentially Infected during Acute SIV Infection. Mucosal. Immunol. 2009, 2, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillot-Delost, M.; Le Gouvello, S.; Mesel-Lemoine, M.; Cheraï, M.; Baillou, C.; Simon, A.; Levy, Y.; Weiss, L.; Louafi, S.; Chaput, N.; et al. Human CD90 Identifies Th17/Tc17 T Cell Subsets That Are Depleted in HIV-Infected Patients. J. Immunol. 2012, 188, 981–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Finnegan, C.M.; Kish-Catalone, T.; Blumenthal, R.; Garzino-Demo, P.; La Terra Maggiore, G.M.; Berrone, S.; Kleinman, C.; Wu, Z.; Abdelwahab, S.; et al. Human Beta-Defensins Suppress Human Immunodeficiency Virus Infection: Potential Role in Mucosal Protection. J. Virol. 2005, 79, 14318–14329. [Google Scholar] [CrossRef] [Green Version]
- Unutmaz, D.; Xiang, W.; Sunshine, M.J.; Campbell, J.; Butcher, E.; Littman, D.R. The Primate Lentiviral Receptor Bonzo/STRL33 Is Coordinately Regulated with CCR5 and Its Expression Pattern Is Conserved between Human and Mouse. J. Immunol. 2000, 165, 3284–3292. [Google Scholar] [CrossRef] [Green Version]
- Ebert, L.M.; McColl, S.R. Up-Regulation of CCR5 and CCR6 on Distinct Subpopulations of Antigen-Activated CD4+ T Lymphocytes. J. Immunol. 2002, 168, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lecureuil, C.; Combadière, B.; Mazoyer, E.; Bonduelle, O.; Samri, A.; Autran, B.; Debré, P.; Combadière, C. Trapping and Apoptosis of Novel Subsets of Memory T Lymphocytes Expressing CCR6 in the Spleen of HIV-Infected Patients. Blood 2007, 109, 3649–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol Monolaurate Prevents Mucosal SIV Transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef]
- Cosmi, L.; De Palma, R.; Santarlasci, V.; Maggi, L.; Capone, M.; Frosali, F.; Rodolico, G.; Querci, V.; Abbate, G.; Angeli, R.; et al. Human Interleukin 17-Producing Cells Originate from a CD161+ CD4+ T Cell Precursor. J. Exp. Med. 2008, 205, 1903–1916. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Rivino, L.; Geginat, J.; Jarrossay, D.; Gattorno, M.; Lanzavecchia, A.; Sallusto, F.; Napolitani, G. Surface Phenotype and Antigenic Specificity of Human Interleukin 17-Producing T Helper Memory Cells. Nat. Immunol. 2007, 8, 639–646. [Google Scholar] [CrossRef]
- Singh, S.P.; Zhang, H.H.; Foley, J.F.; Hedrick, M.N.; Farber, J.M. Human T Cells That Are Able to Produce IL-17 Express the Chemokine Receptor CCR6. J. Immunol. 2008, 180, 214–221. [Google Scholar] [CrossRef]
- Cecchinato, V.; Trindade, C.J.; Laurence, A.; Heraud, J.M.; Brenchley, J.M.; Ferrari, M.G.; Zaffiri, L.; Tryniszewska, E.; Tsai, W.P.; Vaccari, M.; et al. Altered Balance between Th17 and Th1 Cells at Mucosal Sites Predicts AIDS Progression in Simian Immunodeficiency Virus-Infected Macaques. Mucosal. Immunol. 2008, 1, 279–288. [Google Scholar] [CrossRef]
- Macal, M.; Sankaran, S.; Chun, T.W.; Reay, E.; Flamm, J.; Prindiville, T.J.; Dandekar, S. Effective CD4+ T-Cell Restoration in Gut-Associated Lymphoid Tissue of HIV-Infected Patients Is Associated with Enhanced Th17 Cells And Polyfunctional HIV-Specific T-Cell Responses. Mucosal. Immunol. 2008, 1, 475–488. [Google Scholar] [CrossRef]
- Hartigan-O’Connor, D.J.; Abel, K.; Van Rompay, K.K.; Kanwar, B.; McCune, J.M. SIV Replication in the Infected Rhesus Macaque Is Limited by the Size of the Preexisting TH17 Cell Compartment. Sci. Transl. Med. 2012, 4, 136ra69. [Google Scholar] [CrossRef] [Green Version]
- Ndhlovu, L.C.; Chapman, J.M.; Jha, A.R.; Snyder-Cappione, J.E.; Pagán, M.; Leal, F.E.; Boland, B.S.; Norris, P.J.; Rosenberg, M.G.; Nixon, D.F. Suppression of HIV-1 Plasma Viral Load below Detection Preserves IL-17 Producing T Cells in HIV-1 Infection. Aids 2008, 22, 990–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, M.; Rallón, N.I.; Rodés, B.; López, M.; Soriano, V.; Benito, J.M. Long-Term Non-Progressors Display a Greater Number of Th17 Cells Than HIV-Infected Typical Progressors. Clin. Immunol. 2011, 139, 110–114. [Google Scholar] [CrossRef]
- Ciccone, E.J.; Greenwald, J.H.; Lee, P.I.; Biancotto, A.; Read, S.W.; Yao, M.A.; Hodge, J.N.; Thompson, W.L.; Kovacs, S.B.; Chairez, C.L.; et al. CD4+ T Cells, Including Th17 and Cycling Subsets, Are Intact in the Gut Mucosa of HIV-1-Infected Long-Term Nonprogressors. J. Virol. 2011, 85, 5880–5888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, L.; Benfield, T.; Mens, H.; Clausen, L.N.; Katzenstein, T.L.; Fomsgaard, A.; Karlsson, I. Low Level of Regulatory T Cells and Maintenance of Balance between Regulatory T Cells and TH17 Cells in HIV-1-Infected Elite Controllers. J. Acquir. Immune Defic. Syndr. 2011, 57, 101–108. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Jia, M.; Hong, K.; Ruan, Y.; Liang, H.; Liu, S.; Zhang, X.; Zhao, H.; Peng, H.; et al. Loss of Balance between T Helper Type 17 and Regulatory T Cells in Chronic Human Immunodeficiency Virus Infection. Clin. Exp. Immunol. 2011, 165, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Abdelwahab, S.F.; Lewis, G.K.; Garzino-Demo, A. Recall Antigen Activation Induces Prompt Release of CCR5 Ligands from PBMC: Implication in Memory Responses and Immunization. Int. Immunol. 2004, 16, 1623–1631. [Google Scholar] [CrossRef]
- Liao, F.; Rabin, R.L.; Smith, C.S.; Sharma, G.; Nutman, T.B.; Farber, J.M. CC-Chemokine Receptor 6 Is Expressed on Diverse Memory Subsets of T Cells and Determines Responsiveness to Macrophage Inflammatory Protein 3 Alpha. J. Immunol. 1999, 162, 186–194. [Google Scholar] [PubMed]
- Liao, F.; Alderson, R.; Su, J.; Ullrich, S.J.; Kreider, B.L.; Farber, J.M. STRL22 Is a Receptor for the CC Chemokine MIP-3alpha. Biochem. Biophys. Res. Commun. 1997, 236, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Baba, M.; Imai, T.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Hieshima, K.; Nomiyama, H.; Yoshie, O. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 1997, 272, 14893–14898. [Google Scholar] [CrossRef] [Green Version]
- Krzysiek, R.; Lefevre, E.A.; Bernard, J.; Foussat, A.; Galanaud, P.; Louache, F.; Richard, Y. Regulation of CCR6 Chemokine Receptor Expression and Responsiveness to Macrophage Inflammatory Protein-3alpha/CCL20 in Human B Cells. Blood 2000, 96, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Fitzhugh, D.J.; Naik, S.; Caughman, S.W.; Hwang, S.T. Cutting Edge: C-C Chemokine Receptor 6 Is Essential for Arrest of a Subset of Memory T Cells on Activated Dermal Microvascular Endothelial Cells under Physiologic Flow Conditions in vitro. J. Immunol. 2000, 165, 6677–6681. [Google Scholar] [CrossRef] [Green Version]
- Kleinewietfeld, M.; Puentes, F.; Borsellino, G.; Battistini, L.; Rötzschke, O.; Falk, K. CCR6 Expression Defines Regulatory Effector/Memory-like Cells within the CD25(+) CD4+ T-Cell Subset. Blood 2005, 105, 2877–2886. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, R.M.; Niess, J.H.; Zammit, D.J.; Ravindran, R.; Srinivasan, A.; Maxwell, J.R.; Stoklasek, T.; Yadav, R.; Williams, I.R.; Gu, X.; et al. CCR6-Mediated Dendritic Cell Activation of Pathogen-Specific T Cells in Peyer’s Patches. Immunity 2006, 24, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Le Borgne, M.; Etchart, N.; Goubier, A.; Lira, S.A.; Sirard, J.C.; Van Rooijen, N.; Caux, C.; Aït-Yahia, S.; Vicari, A.; Kaiserlian, D.; et al. Dendritic Cells Rapidly Recruited into Epithelial Tissues Via CCR6/CCL20 Are Responsible for CD8+ T Cell Crosspriming in vivo. Immunity 2006, 24, 191–201. [Google Scholar] [CrossRef]
- Liao, F.; Shirakawa, A.K.; Foley, J.F.; Rabin, R.L.; Farber, J.M. Human B Cells Become Highly Responsive to Macrophage-Inflammatory Protein-3 Alpha/CC Chemokine Ligand-20 after Cellular Activation without Changes in CCR6 Expression or Ligand Binding. J. Immunol. 2002, 168, 4871–4880. [Google Scholar] [CrossRef] [Green Version]
- Kohler, R.E.; Caon, A.C.; Willenborg, D.O.; Clark-Lewis, I.; McColl, S.R. A Role for Macrophage Inflammatory Protein-3 Alpha/CC Chemokine Ligand 20 in Immune Priming during T Cell-Mediated Inflammation of the Central Nervous System. J. Immunol. 2003, 170, 6298–6306. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.; McMichael, A. The T Cell Response to HIV. Cold Spring Harb. Perspect. Med. 2012, 2, a007054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, N.; Detels, R.; Chang, L.C.; Butch, A.W. Macrophage Inflammatory Protein-3 Alpha (MIP-3alpha)/CCL20 in HIV-1-Infected Individuals. J. AIDS Clin. Res. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontaine, J.; Poudrier, J.; Roger, M. Short Communication: Persistence of High Blood Levels of the Chemokines CCL2, CCL19, and CCL20 during the Course of HIV Infection. AIDS Res. Hum. Retrovir. 2011, 27, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.P.; Falivene, J.; Holgado, M.P.; Zurita, D.H.; Laufer, N.; Castro, C.; Nico, Á.; Maeto, C.; Salido, J.; Pérez, H.; et al. Impact of HIV-ART on the Restoration of Th17 and Treg Cells in Blood and Female Genital Mucosa. Sci. Rep. 2019, 9, 1978. [Google Scholar] [CrossRef]
- Fernandes, S.M.; Pires, A.R.; Matoso, P.; Ferreira, C.; Nunes-Cabaco, H.; Correia, L.; Valadas, E.; Poças, J.; Pacheco, P.; Veiga-Fernandes, H.; et al. HIV-2 Infection Is Associated with Preserved GALT Homeostasis and Epithelial Integrity Despite Ongoing Mucosal Viral Replication. Mucosal. Immunol. 2018, 11, 236–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douek, D.C.; Brenchley, J.M.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Okamoto, Y.; Casazza, J.P.; Kuruppu, J.; Kunstman, K.; Wolinsky, S.; et al. HIV Preferentially Infects HIV-Specific CD4+ T Cells. Nature 2002, 417, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Richieri, S.P.; Bartholomew, R.; Aloia, R.C.; Savary, J.; Gore, R.; Holt, J.; Ferre, F.; Musil, R.; Tian, H.R.; Trauger, R.; et al. Characterization of Highly Purified, Inactivated HIV-1 Particles Isolated by Anion Exchange Chromatography. Vaccine 1998, 16, 119–129. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).