Molecular Detection of Tick-Borne Agents in Cats from Southeastern and Northern Brazil
Abstract
:1. Introduction
2. Results
2.1. PCR Assays for Gapdh mammalian Endogenous gene
2.2. PCR Assays for Anaplasma spp. and Ehrlichia spp.
2.3. PCR Assays for Piroplasmida
2.3.1. Babesia spp. and Theileria spp.
2.3.2. Cytauxzoon spp.
2.3.3. Hepatozoon spp.
2.4. Co-positivity for Anaplasmataceae and Piroplasmida Agents
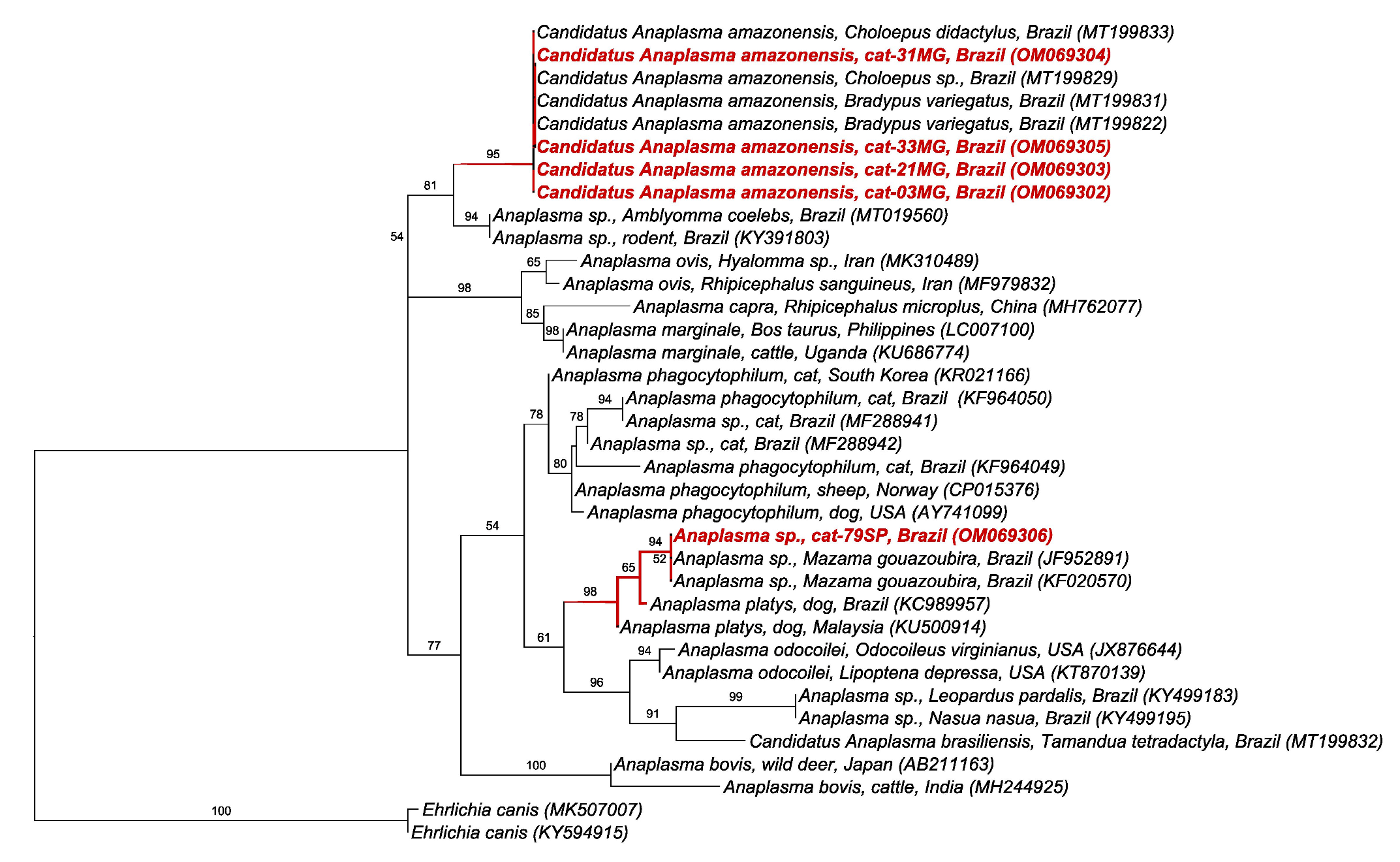
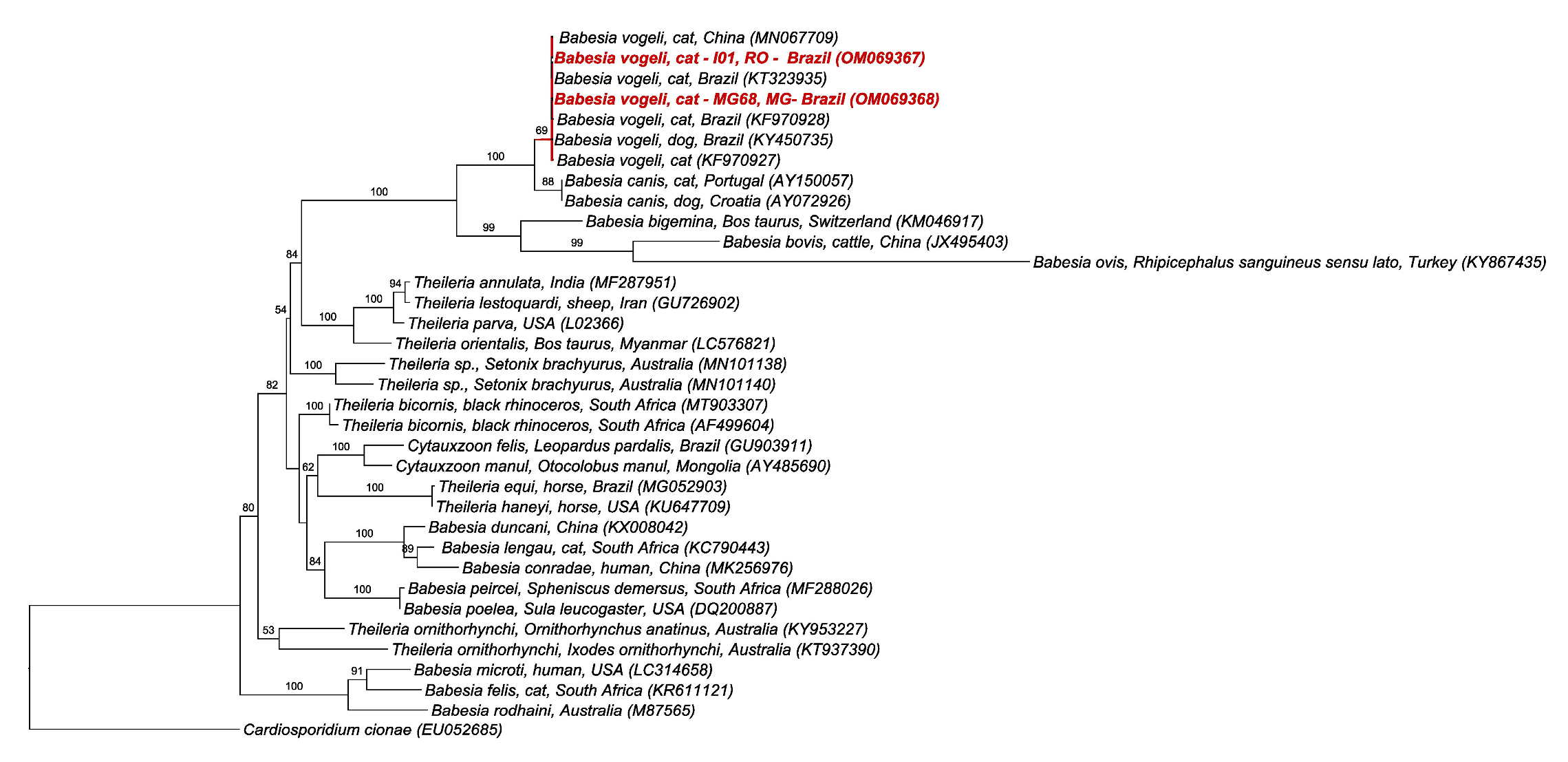
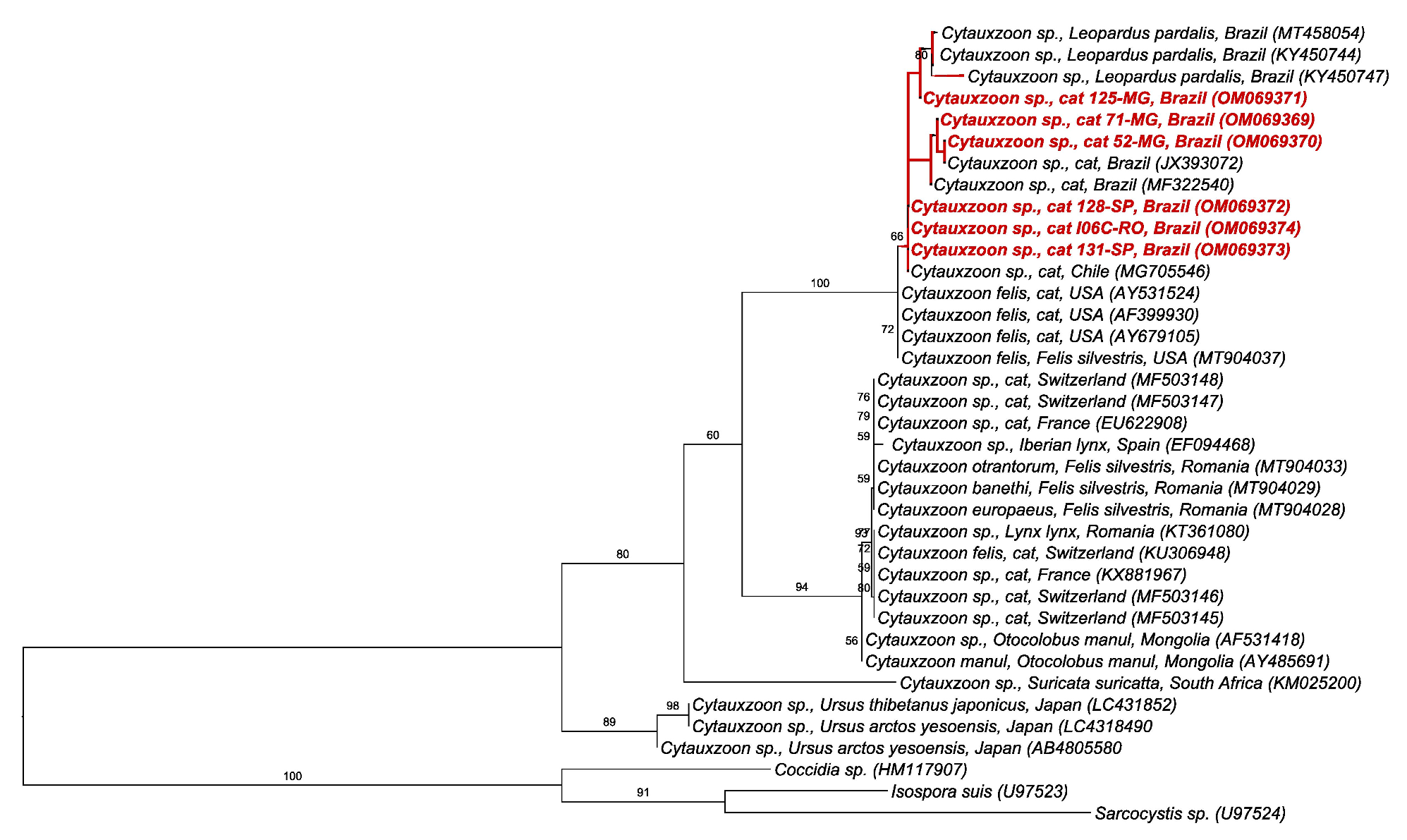
2.5. BLAST and Phylogenetic Analyses
3. Discussion
4. Materials and Methods
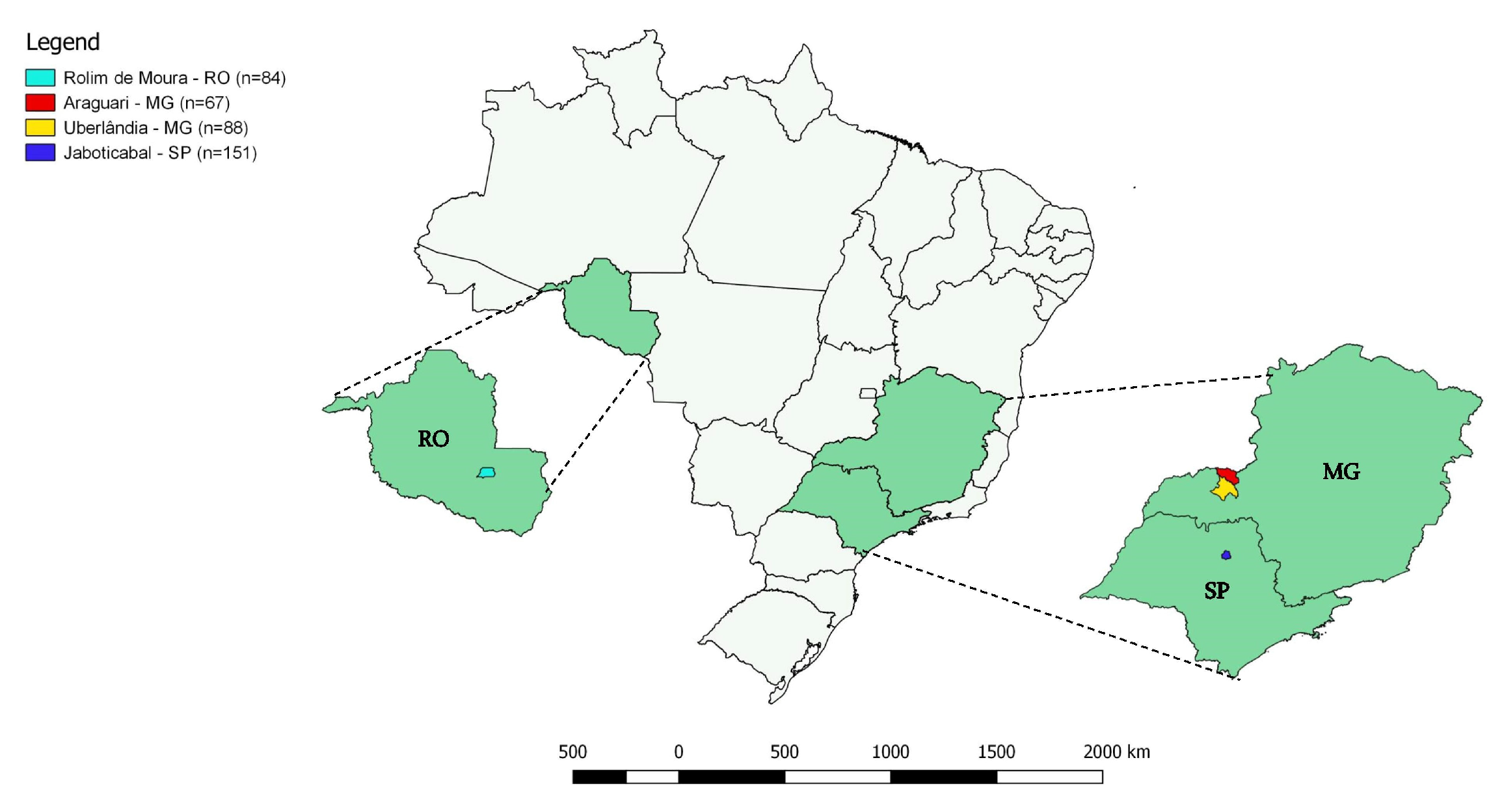
4.1. Cats’ Blood Sampling
4.2. DNA Extraction from Cats’ Blood Samples and PCR Assays for Mammalian endogenous gene
4.3. PCR Assays for Anaplasma spp. and Ehrlichia spp.
4.4. PCR Assays for Piroplasmida
4.4.1. Babesia spp. and Theileria spp.
4.4.2. Cytauxzoon spp.
4.5. PCR assay for Hepatozoon spp.
4.6. Agarose Gel Electrophoresis
4.7. Purification of PCR Amplified Products and Sequencing
4.8. Phylogenetic Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.J.; Dasch, G.A.; Palmer, G.H.; Stuart, C.R.; Rikihisa, Y.; Rrurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the Order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six news species combinations and designation of Ehrlichia equi and ‘HGE agent’as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar]
- Pennisi, M.G.; Hofmann-Lehmann, R.; Radford, A.D.; Tasker, S.; Belák, S.; Addie, D.D.; Boucraut-Baralon, C.; Egnerink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2017, 19, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, I.; Kohn, B. Anaplasma phagocytophilum infection in cats: A literature review to raise clinical awareness. J. Feline Med. Surg. 2020, 22, 428–441. [Google Scholar] [CrossRef] [Green Version]
- Moraes-Filho, J.; Krawczak, F.S.; Costa, F.B.; Soares, J.F.; Labruna, M.B. Comparative evaluation of the vector competence of four South American populations of the Rhipicephalus sanguineus group for the bacterium Ehrlichia canis, the agent of canine monocytic ehrlichiosis. PLoS ONE 2015, 10, e0139386. [Google Scholar] [CrossRef]
- Snellgrove, A.N.; Krapiunaya, I.; Ford, S.L.; Stanley, H.M.; Wickson, A.G.; Hartzer, K.L.; Levin, M.L. Vector competence of Rhipicephalus sanguineus sensu stricto for Anaplasma platys. Ticks Tick Borne Dis. 2020, 11, 101517. [Google Scholar] [CrossRef]
- Penzhorn, B.L.; Oosthuizen, M.C. Babesia Species of Domestic Cats: Molecular Characterization Has Opened Pandora’s Box. Front. Vet. Sci. 2020, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- André, M.R.; Denardi, N.C.B.; de Sousa, K.C.M.; Gonçalves, L.R.; Henrique, P.C.; Ontivero, C.R.G.R.; Ontivero, C.R.G.R.; Gonzalez, I.H.L.; Nery, C.V.C.; Chagas, C.R.F.; et al. Arthropod-borne pathogens circulating in free-roaming domestic cats in a zoo environment in Brazil. Ticks Tick Borne Dis. 2014, 5, 545–551. [Google Scholar] [CrossRef] [PubMed]
- André, M.R.; Herrera, H.M.; Fernandes, S.J.; de Sousa, K.C.M.; Gonçalves, L.R.; Domingos, I.H.; Domingos, I.H.; Macedo, G.C.; Machado, R.Z. Tick-borne agents in domesticated and stray cats from the city of Campo Grande, state of Mato Grosso do Sul, midwestern Brazil. Ticks Tick Borne Dis. 2015, 6, 779–786. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, T.T.; Liu, G.H.; Zhu, X.Q.; Yao, C. Two tales of Cytauxzoon felis infections in domestic cats. Clin. Microbiol. Rev. 2017, 30, 861–885. [Google Scholar] [CrossRef] [Green Version]
- Lloret, A.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Horzinek, M.C.; Hosie, M.J.; Lutz, H.; et al. Cytauxzoonosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2015, 17, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G. The genus hepatozoon (apicomplexa: Adeleina). J. Parasitol. 1996, 82, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Jones-Gruffydd, T.; Hartmann, K.; Horzinek, M.C.; Hosie, M.J.; Lutz, H.; et al. Hepatozoonosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2015, 17, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M. A survey of ixodid tick species and molecular identification of tick-borne pathogens. Vet. Parasitol. 2014, 200, 276–283. [Google Scholar] [CrossRef]
- Maia, C.; Ferreira, A.; Nunes, M.; Vieira, M.L.; Campino, L.; Cardoso, L. Molecular detection of bacterial and parasitic pathogens in hard ticks from Portugal. Ticks Tick Borne Dis. 2014, 5, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Kegler, K.; Nufer, U.; Alic, A.; Posthaus, H.; Olias, P.; Basso, W. Fatal infection with emerging apicomplexan parasite Hepatozoon silvestris in a domestic cat. Parasit. Vectors 2018, 11, 428. [Google Scholar] [CrossRef] [Green Version]
- Pedrassani, D.; Biolchi, J.; Gonçalves, L.R.; Mendes, N.S.; Zanatto, D.C.D.S.; Calchi, A.C.; Machado, R.Z.; André, M.R. Molecular detection of vector-borne agents in cats in Southern Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Raimundo, J.M.; Rodrigues, R.B.; Peixoto, M.P.; Santos, H.A.; André, M.R.; Machado, R.Z.; Baldani, C.D. Ehrlichia spp. infection in domestic cats from Rio de Janeiro State, southeast Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 180–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calchi, A.C.; Vultão, J.G.; Alves, M.H.; Yogui, D.R.; Desbiez, A.L.J.; De Santi, M.; Santana, M.S.; da Silva, T.M.V.; Werther, K.; Teixeira, M.M.G.; et al. Ehrlichia spp. and Anaplasma spp. in Xenarthra mammals from Brazil, with evidence of novel ‘Candidatus Anaplasma spp.’. Sci. Rep. 2020, 10, 126115. [Google Scholar]
- Lima, M.L.F.; Soares, P.T.; Ramos, C.A.N.; Araújo, F.R.; Ramos, R.A.N.; Souza, I.I.F.; Faustino, M.A.G.; Alves, L.C.A. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz. J. Microbiol. 2010, 41, 381–385. [Google Scholar] [CrossRef] [Green Version]
- André, M.R.; Filgueira, K.D.; Calchi, A.C.; Sousa, K.C.M.D.; Gonçalves, L.R.; Medeiros, V.B.; Ximenes, P.A.; Lelis, I.C.N.G.; Meireles, M.V.N.; Machado, R.Z. Co-infection with arthropod-borne pathogens in domestic cats. Rev. Bras. Parasitol. Vet. 2017, 26, 525–531. [Google Scholar] [CrossRef]
- Braga, M.D.S.C.D.O.; André, M.R.; Freschi, C.R.; Teixeira, M.C.A.; Machado, R.Z. Molecular and serological detection of Ehrlichia spp. in cats on São Luís Island, Maranhão, Brazil. Rev. Bras. Parasitol. Vet. 2012, 21, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga, Í.A.; Santos, L.G.F.D.; Melo, A.L.T.; Jaune, F.W.; Ziliani, T.F.; Girardi, Â.F.; Aguiar, D.M.D. Hematological values associated to the serological and molecular diagnostic in cats suspected of Ehrlichia canis infection. Rev. Bras. Parasitol. Vet. 2013, 22, 470–474. [Google Scholar] [CrossRef]
- De Oliveira, L.S.; Mourão, L.C.; Oliveira, K.A.; Agostini, M.M.; De Oliveira, A.C.; De Almeida, J.L.; Fietto, J.L.R.; Conceição, L.G.; Filho, J.D.R.; Galvão, M.A.M.; et al. Molecular detection of Ehrlichia canis in cats in Brazil. Clin. Microbiol. Infect. 2009, 15, 53–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitschwerdt, E.B.; Abrams-Ogg, A.C.; Lappin, M.R.; Bienzle, D.; Hancock, S.I.; Cowan, S.M.; Hawkins, E.C. Molecular evidence supporting Ehrlichia canis-like infection in cats. J. Vet. Int. Med. 2002, 16, 642–649. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Luz, M.F.; Granada, S.; Vilhena, H.; Nachum-Biala, Y.; Lopes, A.P.; Cardoso, L.; Baneth, G. Molecular detection of Anaplasma bovis, Ehrlichia canis and Hepatozoon felis in cats from Luanda, Angola. Parasit. Vectors 2018, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Malheiros, J.; Costa, M.M.; Do Amaral, R.B.; De Sousa, K.C.M.; André, M.R.; Machado, R.Z.; Vieira, M.I.B. Identification of vector-borne pathogens in dogs and cats from Southern Brazil. Ticks Tick Borne Dis. 2016, 7, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Simking, P.; Wongnakphet, S.; Stich, R.W.; Jittapalapong, S. Detection of Babesia vogeli in stray cats of metropolitan Bangkok, Thailand. Vet. Parasitol. 2010, 173, 70–75. [Google Scholar] [CrossRef]
- Vilhena, H.; Martinez-Díaz, V.L.; Cardoso, L.; Vieira, L.; Altet, L.; Francino, O.; Pastor, J.; Silvestre-Ferreira, A.C. Feline vector-borne pathogens in the north and centre of Portugal. Par Vect. 2013, 6, 99. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.J.; Köster, L.; Li, J.; Zhang, J.; Huang, K.; Branford, G.M.; March, S.; Vandenplas, M.; Wang, C. Survey of vector-borne agents in feral cats and first report of Babesia gibsoni in cats on St Kitts, West Indies. BMC Vet. Res. 2017, 13, 331. [Google Scholar] [CrossRef]
- Alho, A.M.; Lima, C.; Latrofa, M.S.; Colella, V.; Ravagnan, S.; Capelli, G.; Otranto, D. Molecular detection of vector-borne pathogens in dogs and cats from Qatar. Par Vect. 2017, 10, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furquim, M.E.C.; do Amaral, R.; Dias, C.M.; Gonçalves, L.R.; Perles, L.; de Paula Lima, C.A.; Barros-Battesti, D.M.; Machado, R.Z.; André, M.R. Genetic diversity and Multilocus Sequence Typing Analysis of Bartonella henselae in domestic cats from Southeastern Brazil. Acta Trop. 2021, 222, 106037. [Google Scholar] [CrossRef] [PubMed]
- André, M.R.; Adania, C.H.; Machado, R.Z.; Allegretti, S.M.; Felippe, P.A.; Silva, K.F.; Nakagui, A.C.H.; Dagnone, A.S. Molecular detection of Cytauxzoon spp. in asymptomatic Brazilian wild captive felids. J. Wildl. Dis. 2009, 45, 234–237. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, K.C.M.; Fernandes, M.P.; Herrera, H.M.; Freschi, C.R.; Machado, R.Z.; André, M.R. Diversity of piroplasmids among wild and domestic mammals and ectoparasites in Pantanal wetland, Brazil. Ticks Tick Borne Dis. 2018, 9, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, P.V.; Soares, C.O.; Scofield, A.; Santiago, C.D.; Franca, T.N.; Barros, S.S. Fatal cytauxzoonosis in captive-reared lions in Brazil. Vet. Parasitol. 2007, 145, 383–387. [Google Scholar] [CrossRef]
- Guizelini, C.C.; Nascimento, C.A.; Echeverria, J.T.; Soares, R.L.; Pimenta, M.M.; de Deco-Souza, T.; Gomes, D.C. Fatal infection caused by Cytauxzoon felis in a captive-reared jaguar (Panthera onca). Int. J. Parasitol. Parasites Wildl. 2021, 16, 187–190. [Google Scholar] [CrossRef]
- Raimundo, J.M.; Guimarães, A.; André, M.R.; Baldani, C.D. Cytauxzoon felis DNA detection in healthy cats from Rio de Janeiro, Brazil. J. Parasitol. 2021, 107, 676–678. [Google Scholar] [CrossRef]
- Panait, L.C.; Mihalca, A.D.; Modrý, D.; Juránková, J.; Ionică, A.M.; Deak, G.; Gherman, C.M.; Heddergott, M.; Hodzic, A.; Veronesi, F.; et al. Three new species of Cytauxzoon in European wild felids. Vet. Parasitol. 2021, 290, 109344. [Google Scholar] [CrossRef] [PubMed]
- de Bortoli, C.P.; André, M.R.; Braga, M.S.C.; Machado, R.Z. Molecular characterization of Hepatozoon sp. in cats from São Luís Island, Maranhão, northeastern Brazil. Parasitol. Res. 2011, 109, 1189–1192. [Google Scholar] [CrossRef]
- Braga, Í.A.; Ramos, D.G.S.; Marcili, A.; Melo, A.L.T.; Taques, I.I.G.G.; Amude, A.M.; Chitarra, C.S.; Nakazato, L.; Dutra, V.; Pacheco, R.C.; et al. Molecular detection of tick-borne protozoan parasites in a population of domestic cats in midwestern Brazil. Ticks Tick Borne Dis. 2016, 7, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Rubini, A.S.; Paduan, K.S.; Perez, R.R.; Ribolla, P.E.M.; O’Dwyer, L.H. Molecular characterization of feline Hepatozoon species from Brazil. Vet. Parasitol 2006, 137, 168–171. [Google Scholar] [CrossRef]
- Díaz-Regañón, D.; Villaescusa, A.; Ayllón, T.; Rodríguez-Franco, F.; Baneth, G.; Calleja-Bueno, L.; Garcia-Sancho, M.; Agulla, B.; Sainz, Á. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid, Spain. Parasit. Vectors 2017, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, A.; Latrofa, M.S.; Nachum-Biala, Y.; Hodžić, A.; Greco, G.; Attanasi, A.; Annoscia, G.; Otranto, D.; Baneth, G. Three different Hepatozoon species in domestic cats from southern Italy. Ticks Tick Borne Dis. 2017, 8, 721–724. [Google Scholar] [CrossRef]
- Grillini, M.; Simonato, G.; Tessarin, C.; Dotto, G.; Traversa, D.; Cassini, R.; Marchiori, E.; Frangipane di Regalbono, A. Cytauxzoon sp. and Hepatozoon spp. in domestic cats: A preliminary study in North-Eastern Italy. Pathogens 2021, 10, 1214. [Google Scholar] [CrossRef]
- Harris, D.J.; Santos, J.; Rampedi, K.M.; Halajian, A.; Xavier, R. Genetic diversity of Hepatozoon (Apicomplexa) from domestic cats in South Africa, with a global reassessment of Hepatozoon felis diversity. J. S. Afr. Vet. Assoc. 2019, 90, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, C.; Maia, J.P.; Marcos, R.; Luzzago, C.; Puente-Payo, P.; Dall’Ara, P.; Faustino, A.; Lauzi, S. Molecular detection of Hepatozoon felis in cats from Maio Island, Republic of Cape Verde and global distribution of feline hepatozoonosis. Parasit. Vectors 2019, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Basso, W.; Görner, D.; Globokar, M.; Keidel, A.; Pantchev, N. First autochthonous case of clinical Hepatozoon felis infection in a domestic cat in Central Europe. Parasitol Int. 2019, 72, 101945. [Google Scholar] [CrossRef]
- Perez, R.R.; Rubini, A.S.; O’Dwyer, L.H. The first report of Hepatozoon spp. (Apicomplexa, Hepatozoidae) in domestic cats from São Paulo state, Brazil. Parasitol. Res. 2004, 94, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Mascarelli, E.P.; Havenga, L.N.; Naidoo, V.; Breitschwerdt, E.B. Co-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarian. Parasites Vectors 2013, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Kuramae-Izioka, E.E. A rapid, easy and high yield protocol for total genomic DNA isolation of Colletotrichum gloeosporioides and Fusarium oxysporum. Rev. Unimar 1997, 19, 683–689. [Google Scholar]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in Canine Blood Samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Miller, M.A.; Holder, M.T.; Vos, R.; Midford, P.E.; Liebowitz, T.; et al. Nested PCR assay for detection of granulocytic Ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef] [Green Version]
- Gofton, A.W.; Doggett, S.; Ratchford, A.; Ryan, U.; Irwin, P. Phylogenetic characterization of two Novel Anaplasmataceae from Australian Ixodes holocyclus ticks: “Candidatus Neoehrlichia australis” and “Candidatus Neoehrlichia arcana”. Int. J. Syst. Evol. Microbiol. 2016, 66, 4256–4261. [Google Scholar] [CrossRef] [PubMed]
- Rejmanek, D.; Bradburd, G.; Foley, J. Molecular characterization reveals distinct genospecies of Anaplasma phagocytophilum from diverse North American hosts. J. Med. Microbiol. 2012, 61, 204–212. [Google Scholar] [CrossRef]
- Doyle, C.K.; Labruna, M.B.; Breitschwerdt, E.B.; Tang, Y.; Corstvet, R.E.; Hegarty, B.C.; Block, K.C.; Li, P.; Walker, D.C.; Mcbride, J.W. Detection of medically important Ehrlichia by quantitative multicolor Taq-Man Real Time PCR of the dsb gene. J. Mol. Diagn. 2005, 7, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Monti, G.; Otth, C.; Sepúlveda, P.; Bittencourt, P.; Nachum-Bala, Y.; Gutiérresz, R.; Harrus, S. “Candidatus Neoehrlichia chilensis” sp. nov.: Molecular detection and characterization of a novel Anaplasmataceae in wild rodents from Valdivia, Southern Chile. Transbound. Emerg. Dis. 2018, 65, 357–362. [Google Scholar] [CrossRef] [PubMed]
- O’Nion, V.L.; Montilla, H.J.; Qurollo, B.A.; Maggui, R.G.; Hegarty, B.C.; Tornquist, S.J.; Breitschwerdt, E.B. Potentially novel Ehrlichia species in horses, Nicaragua. Emerg. Infect. Dis. 2015, 21, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Inayoshi, M.; Naitou, H.; Kawamori, F.; Masuzawa, T.; Ohashi, N. Characterization of Ehrlichia species from Ixodes ovatus ticks at the foot of Mt.Fuji, Japan. Microbiol. Immunol. 2004, 48, 737–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, D.M.; Saito, T.B.; Hagiwara, M.K.; Machado, R.Z.; Labruna, M.B. Serological diagnosis of canine monocytic ehrlichiosis with Brazilian antigen of Ehrlichia canis. Cienc. Rural 2007, 37, 796–802. [Google Scholar] [CrossRef]
- Jefferies, R.; Ryan, U.M.; Irwin, P.J. PCR–RFLP for the detection and differentiation of the canine piroplasm species and its use with filter paper-based technologies. Vet. Parasitol. 2007, 144, 20–27. [Google Scholar] [CrossRef]
- Corduneanu, A.; Hrazdilová, K.; Sándor, A.D.; Matei, I.A.; Ionică, A.M.; Barti, J.L.; Ciocanau, M.A.; Mantoiu, D.S.; Coroiu, I.; Hornok, S.; et al. Babesia vesperuginis, a neglected piroplasmid: New host and geographical records, and phylogenetic relations. Parasit. Vectors 2017, 10, 598. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.F.; Girotto, A.; Brandão, P.E.; Da Silva, A.S.; França, R.T.; Lopes, S.T.; Labruna, M.B. Detection and molecular characterization of a canine piroplasm from Brazil. Vet. Parasitol. 2011, 180, 203–208. [Google Scholar] [CrossRef]
- Zamoto, A.; Tsuji, M.; Wei, Q.; Cho, S.H.; Shin, E.H.; Kim, T.S.; Leonova, G.N.; Hagiwara, K.; Asakawa, M.; Kariwa, H.; et al. Epizootiologic survey for Babesia microti among small wild mammals in northeastern Eurasia and a geographic deversity in the β-tubulin gene sequences. J. Vet. Med. Sci. 2004, 66, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Hrazdilová, K.; Myśliwy, I.; Hildebrand, J.; Buńkowska-Gawlik, K.; Janaczyk, B.; Perec-Matysiak, A.; Modrý, D. Paralogs vs. genotypes? Variability of Babesia canis assessed by 18S rDNA and two mitochondrial markers. Vet. Parasit. 2019, 266, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shock, B.C.; Birkenheuer, A.J.; Patton, L.L.; Olfenbuttel, C.; Beringer, J.; Grove, D.M.; Peek, M.; Butfiloski, J.W.; Hughes, D.W.; Lockhart, J.M.; et al. Variation in the ITS-1 and ITS-2 rRNA genomic regions of Cytauxzoon felis from bobcats and pumas in the eastern United States and comparison with sequences from domestic cats. Vet. Parasitol. 2012, 190, 29–35. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Marr, H.; Alleman, A.R.; Levy, M.G.; Breitschwerdt, E.B. Development and evaluation of a PCR assay for the detection of Cytauxzoon felis DNA in feline blood samples. Vet. Parasitol. 2006, 137, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Criado-Fornelio, A.; Ruas, J.L.; Casado, N.; Farias, N.A.R.; Soares, M.P.; Müller, G.; Brumt, J.G.W.; Berne, M.E.A.; Bulingsaraña, A.; Barba-Carretero, J.C. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J. Parasitol. 2006, 92, 93–99. [Google Scholar] [CrossRef]
- Mathew, J.S.; Van Den Bussche, R.A.; Ewing, S.A.; Malayer, J.R.; Latha, B.R.; Panciera, R.J. Phylogenetic relationships of Hepatozoon (Apicomplexa: Adeleorina) based on molecular, morphologic, and life-cycle characters. J. Parasitol. 2000, 86, 366–372. [Google Scholar] [CrossRef]
- da Silva, M.R.L.; Fornazari, F.; Martins, T.F.; Hippólito, A.G.; Rolim, L.S.; Bisca, J.M.; O’Dwyer, L.H. A survey of hemoparasites and ectoparasites in Nasua nasua Linnaeus, 1766 with a redescription of Hepatozoon procyonis Richards, 1961 based on morphological and molecular data. Parasitol. Res. 2018, 117, 2159–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, P.I.; Oliveira, T.M.F.D.S.; Teixeira, M.C.A.; Rocha, A.G.; Machado, R.Z.; Tinucci-Costa, M. Comparison between a soluble antigen-based ELISA and IFAT in detecting antibodies against Babesia canis in dogs. Rev. Bras. Parasitol. Vet. 2009, 18, 41–45. [Google Scholar] [CrossRef]
- Perles, L.; Barranco, G.H.F.; Soriano, I.M.; Cruz, N.D.R.N.; Bueno, P.J.; Santana, Á.E.; Machado, R.Z.; Werther, K.; André, M.R. Hepatozoon sp. gamonts as an accidental finding in synovial liquid from an injured maned wolf (Chrysocyon brachyurus) in southeastern Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 779–785. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, B.; Hillier, L.; Wendl, M.C.; Green, P. Base-calling of automated sequencer traces using Phred. I. Acuracy Assessement. Genome Res. 1998, 8, 175–1985. [Google Scholar] [CrossRef] [Green Version]
- Ewing, B.; Green, P. Basecalling of automated sequencer traces using Phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2002, 30, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Trifinopoulos, J.; Nguyen, L.T.; Von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Stover, B.C.; Muller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Co-Positivity | Positivity for Only One Agent | ||||||
|---|---|---|---|---|---|---|---|
| State | Cytauxzoon + Anaplasma | Cytauxzoon + Ehrlichia | Cytauxzoon + Babesia/ Theileria | Anaplasma | Ehrlichia | Babesia/ Theileria | Cytauxzoon |
| São Paulo | 2 | 0 | 0 | 8 | 1 | 1 | 35 |
| Minas Gerais | 6 | 1 | 1 | 13 | 6 | 0 | 57 |
| Rondônia | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| Total | 8 | 1 | 1 | 21 | 7 | 2 | 96 |
| Cat ID | Target Gene | Query Length (bp) | Query-Coverage (%) | E-Value | Identity (%) | GenBank Acession Numbers |
|---|---|---|---|---|---|---|
| MG03 | rrs | 281 | 100 | 4 × 10−143 | 100 | ‘Candidatus Anaplasma amazonensis’—Bradypus variegatus from Brazil (MT199833) |
| MG21 | rrs | 372 | 100 | 0 | 100 | ‘Candidatus Anaplasma amazonensis’—Bradypus variegatus from Brazil (MT199831) |
| MG31 | rrs | 543 | 100 | 0 | 97.61 | Anaplasma phagocytophilum—Hydropotes inermis from Korea (KR611598) |
| MG33 | rrs | 398 | 99 | 0 | 99.75 | Anaplasma spp.—Nasua nasua from Brazil (MT019560) |
| SP79 | rrs | 522 | 99 | 0 | 100 | Anaplasma sp.—Mazama gouazoubira from Brazil (JF952891) |
| ROI01 | 18S rRNA | 766 | 100 | 0 | 100 | Babesia vogeli—cat from China (MN067709) |
| MG68 | 18S rRNA | 763 | 100 | 0 | 100 | Babesia vogeli—cat from China (MN067709) |
| MG71 | 18S rRNA | 218 | 100 | 3 × 10−108 | 100 | Cytauxzoon sp.—Leopardus pardalis from Brazil (MT458054) |
| MG52 | 18S rRNA | 248 | 99 | 3 × 10−124 | 100 | Cytauxzoon sp.—Leopardus pardalis from Brazil (MT458054) |
| MG125 | 18S rRNA | 118 | 100 | 6 × 10−53 | 100 | Cytauxzoonsp.—Leopardus pardalis from Brazil (MT458054) |
| SP121 | 18S rRNA | 140 | 100 | 5 × 10−65 | 100 | Cytauxzoonsp.—Leopardus pardalis from Brazil (MT458054) |
| SP131 | 18S rRNA | 140 | 100 | 5 × 10−65 | 100 | Cytauxzoonsp.—Leopardus pardalis from Brazil (MT458054) |
| ROI06C | 18S rRNA | 273 | 100 | 1 × 10−138 | 100 | Cytauxzoonsp.—Leopardus pardalis from Brazil (MT458054) |
| Agents | Primer Sequences | Size (bp) | Thermal Sequences | References |
|---|---|---|---|---|
| Anaplasma spp. (rrs gene)—Screening External primers - gE3a - gE10R Internal primers - gE2 - gE9f | 5′-CACATGCAAGTCGAACGGATTATTC-3′ 5′-TTCCGTTAAGAAGGATCTAATCTCC-3′ 5′-GGCAGTATTAAAAGCAGCTCCAGG-3′ 5′-AACGGATTATTCTTTATAGCTTGCT-3′ | 932 546 | 94 °C for 5 min 40 cycles: 94 °C for 30 sec, 55 °C for 30 sec and 72 °C for 1 min 72 °C for 5 min | [52] |
| Anaplasma spp. (ITS—23S-5S) - ITS2F - ITS2R | 5′-AGGATCTGACTCTAGTAACGAG-3′ 5′-CTCCCATGTCTTAAGACAAAG-3′ | 300 | 94 °C for 2 min, 35 cycles; 94 °C for 30 sec, 58 °C for 30 sec, 72 °C for 1 min 72 °C for 5 min. | [54] |
| Anaplasma spp. (gltA gene) External primers - F4b - Rb1 Internal primers - EHR-CS136F - EHR-778R | 5′-CCGGGTTTTATGTCTACTGC-3′ 5′-CGATGACCAAAACCCAT-3′ 5′-TTYATGTCYACTGCTGCKTG-3′ 5′-GCNCCMCCATGMGCTGG-3′ | 800 600 | 95 °C for 5 min, 40 cycles: 95 °C for 30 sec, 55 °C for 30 sec and 72 °C for 1 min 72 °C for 5 min. | [53] |
| Ehrlichia spp. (dsb gene)—Screening - dsb-330 (F) - dsb-728 (R) | 5′-GATGATGTCTGAAGATATGAAACAAAT-3′ 5′-CTGCTCGTCTATTTTACTTCTTAAAGT-3′ | 409 | 95 °C for 2 min; 50 cycles: 95 °C for 15 sec, 58 °C for 30 sec and 72 °C for 30 sec 72 °C for 5 min | [55] |
| Anaplasma spp. /Ehrlichia spp. (groEL gene) - groEL124-F1 - groEL808-R1 | 5′-ATTAAGCCAGAAGAACCATTAGC-3′ 5′-TACTGCAATATCACCAAGCATATC-3′ | 680 | 95 °C for 5 min, 40 cycles: 95 °C for 30 sec, 54 °C for 30 sec and 72 °C for 30 sec 72 °C for 5 min | [56] |
| Ehrlichia spp. (sodB gene) - sodbEhr1600-F - sodbEhrl600-R | 5′-ATGTTTACTTTACCTGAACTTCCATATC-3′ 5′-ATCTTTGAGCTGCAAAATCCCAATT-3′ | 600 | 94 °C for 3 min; 55 cycles: 94 °C for 10 sec; 58 °C for 10 sec; 72 °C for 15 sec 72 °C for 30 sec 72 °C for 5 min | [57] |
| Ehrlichia spp. (omp-1 gene) External primers - conP28-F1 - conP28-R1 Internal primers - conP28-F2 - conP29-R2 | 5′-AT(C/T)AGT(G/C)AAA(A/G)TA(T/C)(A/G)T(G/A)CCAA-3′ 5′-TTA(G/A)AA(A/G)G(C/T)AAA(C/T)CT(T/G)CCTCC-3′ 5′-CAATGG(A/G)(T/A)GG(T/C)CC(A/C)AGA(AG)TAG-3′ 5′-TTCC(T/C)TG(A/G)TA(A/G)G(A/C)AA(T/G)TTTAGG-3′ | 300 | 94 °C for 3 min, 35 cycles: 94 °C for 1 min, 50 °C for 1 min and 72 °C for 2 min 72 °C for 5 min | [58] |
| Piroplasmida (18S rRNA)- Screening External primers - BTF1 - BTR1 Internal primers - BTF2 - BTR2 | 5′-GGCTCATTACAACAGTTATAG-3′ 5′-CCCAAAGACTTTGATTTCTCTC-3′ 5′-CCGTGCTAATTGTAGGGCTAATAC-3′ 5′-GGACTACGACGGTATCTGATCG-3′ | 800 | 94 °C for 3 min, 58 °C for 1 min, 72 °C for 2 min 45 cycles: 94 °C for 30 sec, 58 °C for 20 sec and 72 °C por 30 sec 72 °C por 7 min Annealing temperature of the 2nd reaction = 62 °C | [60] |
| Babesia sp./Theileria sp. (cox-1 gene) External primers - Bab_for1 - Bab_Rev1 Internal primers - Bab_for2 - Bab_rev2 | 5′-ATWGGATTYTATATGAGTAT-3′ 5′-ATAATCWGGWATYCTCCTTGG-3′ 5′-TCTCTWCATGGWTTAATTATGATAT-3′ 5′-TAGCTCCAATTGAHARWACAAAGTG-3′ | 924 | 95 °C for 1 min, 35 cycles; 95 °C for 15 sec, 45 °C for 30 sec and 72 °C for 1 min 72 °C por 10 min Annealing temperature of the 2nd reaction = 49 °C | [61] |
| Babesia sp./Theileria sp. (hsp70 gene) - Hsp70F1 - Hsp70R1 | 5′-CATGAAGCACTGGCCHTTCAA-3′ 5′-GCNCKGCTGATGGTGGTGTTGTA-3′ | 740 | 95 °C for 5 min 35 cycles: 95 °C for 15 sec, 60 °C for 30 sec and 72 °C for 30 sec 72 °C for 5 min | [62] |
| Babesia sp./Theileria sp. (β-tubulin gene) - Tubo3 - Tubo63F | 5′-CAAATWGGYGCMAARTTYTGGGA-3′ 5′-TCGTCCATACCTTCWCCSGTRTACCAGTG-3′ | 600 | 94 °C for 5 minues 30 cycles: 94 °C for 40 sec, 55 °C for 1 min and 72 °C for 90 sec; 72 °C for 5 min | [63] |
| Babesia sp./Theileria sp. (cytB gene) External primers - Bc_cytB_F1 - Bc_cytB_R1 Internal primers - Bc_cytB_F2 - Bc_cytB_R2 | 5′-TGGTCWTGGTATTCWGGAATG-3′ 5′-AAGMYARTCTYCCTAAACATCC-3′ 5′-RATKAGYTAYTGGGGAGC-3′ 5′-GCTGGWATCATWGGTATAC-3′ | 580 | 95 °C for 5 min 40 cycles: 95 °C for 45 sec, 55 °C for 45 sec and 72 °C for 45 sec. 72 °C por 10 min; Temp. Annealing temperature of the 2nd reaction = 52 °C | [64] |
| Babesia sp./Theileria sp./Cytauxzoon sp. (intergenic region ITS-1) External primers - ITS15C - ITS13B Internal primers - ITS15D - ITS13C | 5′-CGATCGAGTGATCCGGTGAATTA-3′ 5′-GCTGCGTCCTTCATCGTTGTG-3′ 5′-AAGGAAGGAGAAGTCGTAACAAGG-3′ 5′-TTGTGTGAGCCAAGACATCCA-3′ | 500 | 94 °C for 1 min 35 cycles: 94 °C for 30 sec, 52 °C for 30 sec and 72 °C for 1 min. 72 °C for 5 min; Annealing temperature of the 2nd reaction = 49 °C | [65] |
| Cytauxzoon sp. (18S rRNA)—Screening - CytF - CytR | 5′-GCGAATCGCATTGCTTTATGCT-3′ 5′-CCAAATGATACTCCGGAAAGAG-3′ | 300 | 95 °C for 5 min 40 cycles: 95 °C for 45 sec, 59 °C for 45 sec and 72 °C for 1 min. 72 °C for 5 min | [66] |
| Hepatozoon sp. (18S rRNA)—Screening External Primers - HAM1 - HPF2 Internal primers - 4558 - 2733 | 5′-GCCAGTAGTCATATGCTTGTC-3′ 5′-GACTTCTCCTTCGTCTAAG-3′ 5′-GCTAATACATGAGCAAAATCTCAA-3′ 5′-CGGAATTAACCAGACAAAT-3′ | 1120 | 1st reaction: 95 °C for 3 min 40 cycles: 95 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min. 72 °C for 7 min 2nd reaction: 94 °C for 3 min 40 cycles: 94 °C for 1 min, 55 °C for 2 min and 72 °C for 2 min. 72 °C for 10 min | [67,68,69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, M.R.; Calchi, A.C.; Furquim, M.E.C.; de Andrade, I.; Arantes, P.V.C.; de Melo Lopes, L.C.; Demarchi, I.K.L.d.N.; Figueiredo, M.A.P.; de Paula Lima, C.A.; Machado, R.Z. Molecular Detection of Tick-Borne Agents in Cats from Southeastern and Northern Brazil. Pathogens 2022, 11, 106. https://doi.org/10.3390/pathogens11010106
André MR, Calchi AC, Furquim MEC, de Andrade I, Arantes PVC, de Melo Lopes LC, Demarchi IKLdN, Figueiredo MAP, de Paula Lima CA, Machado RZ. Molecular Detection of Tick-Borne Agents in Cats from Southeastern and Northern Brazil. Pathogens. 2022; 11(1):106. https://doi.org/10.3390/pathogens11010106
Chicago/Turabian StyleAndré, Marcos Rogério, Ana Cláudia Calchi, Maria Eduarda Chiaradia Furquim, Isabela de Andrade, Paulo Vitor Cadina Arantes, Lara Cristina de Melo Lopes, Iuri Kauan Lins do Nascimento Demarchi, Mayra Araguaia Pereira Figueiredo, Cirilo Antonio de Paula Lima, and Rosangela Zacarias Machado. 2022. "Molecular Detection of Tick-Borne Agents in Cats from Southeastern and Northern Brazil" Pathogens 11, no. 1: 106. https://doi.org/10.3390/pathogens11010106
APA StyleAndré, M. R., Calchi, A. C., Furquim, M. E. C., de Andrade, I., Arantes, P. V. C., de Melo Lopes, L. C., Demarchi, I. K. L. d. N., Figueiredo, M. A. P., de Paula Lima, C. A., & Machado, R. Z. (2022). Molecular Detection of Tick-Borne Agents in Cats from Southeastern and Northern Brazil. Pathogens, 11(1), 106. https://doi.org/10.3390/pathogens11010106







