First Molecular Evidence of Pathogens in Fleas Collected from Dogs in Northern Vietnam
Abstract
:1. Introduction
2. Results
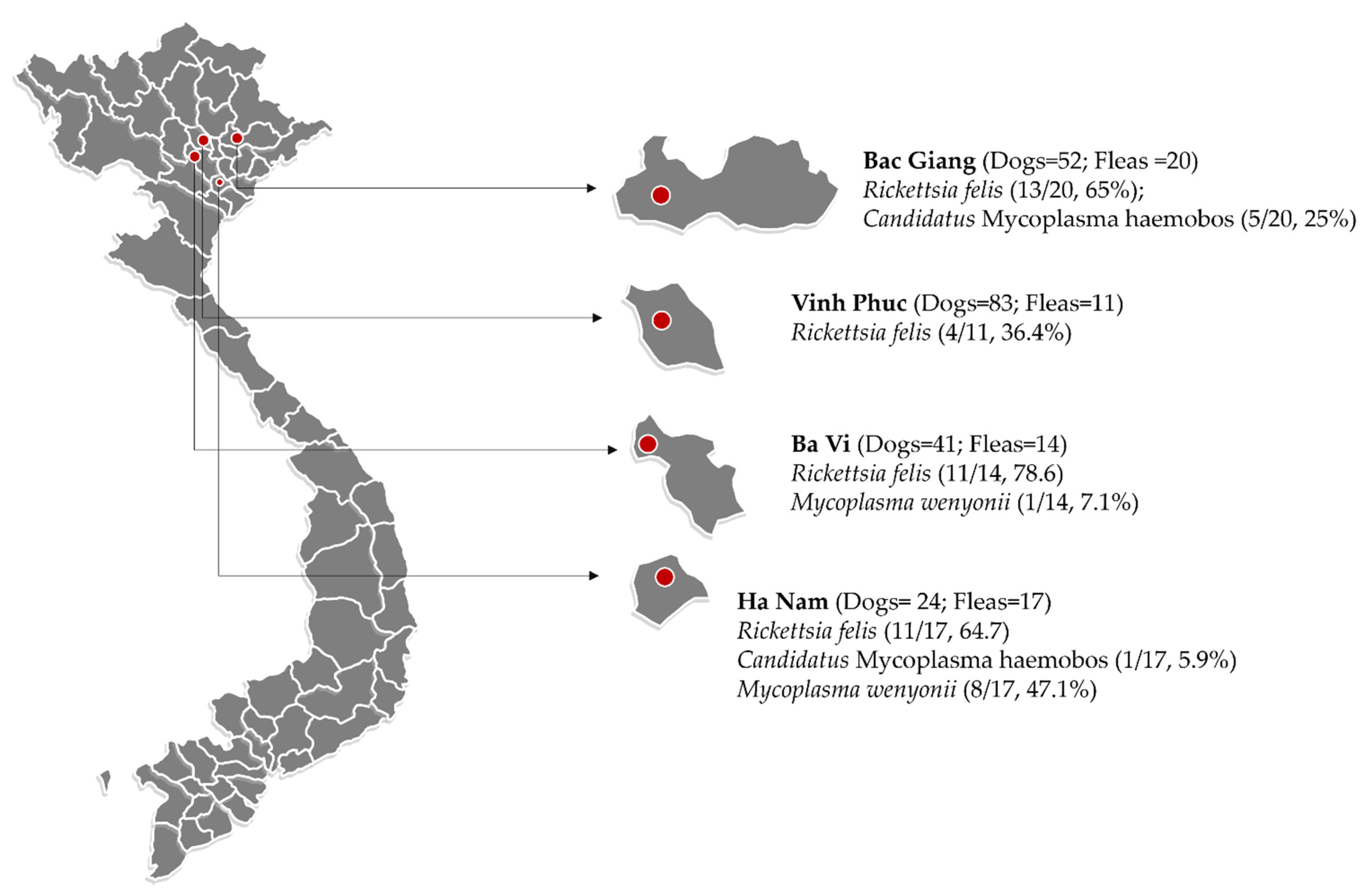
2.1. Identification of Fleas and Molecular Detection of Pathogens in Fleas
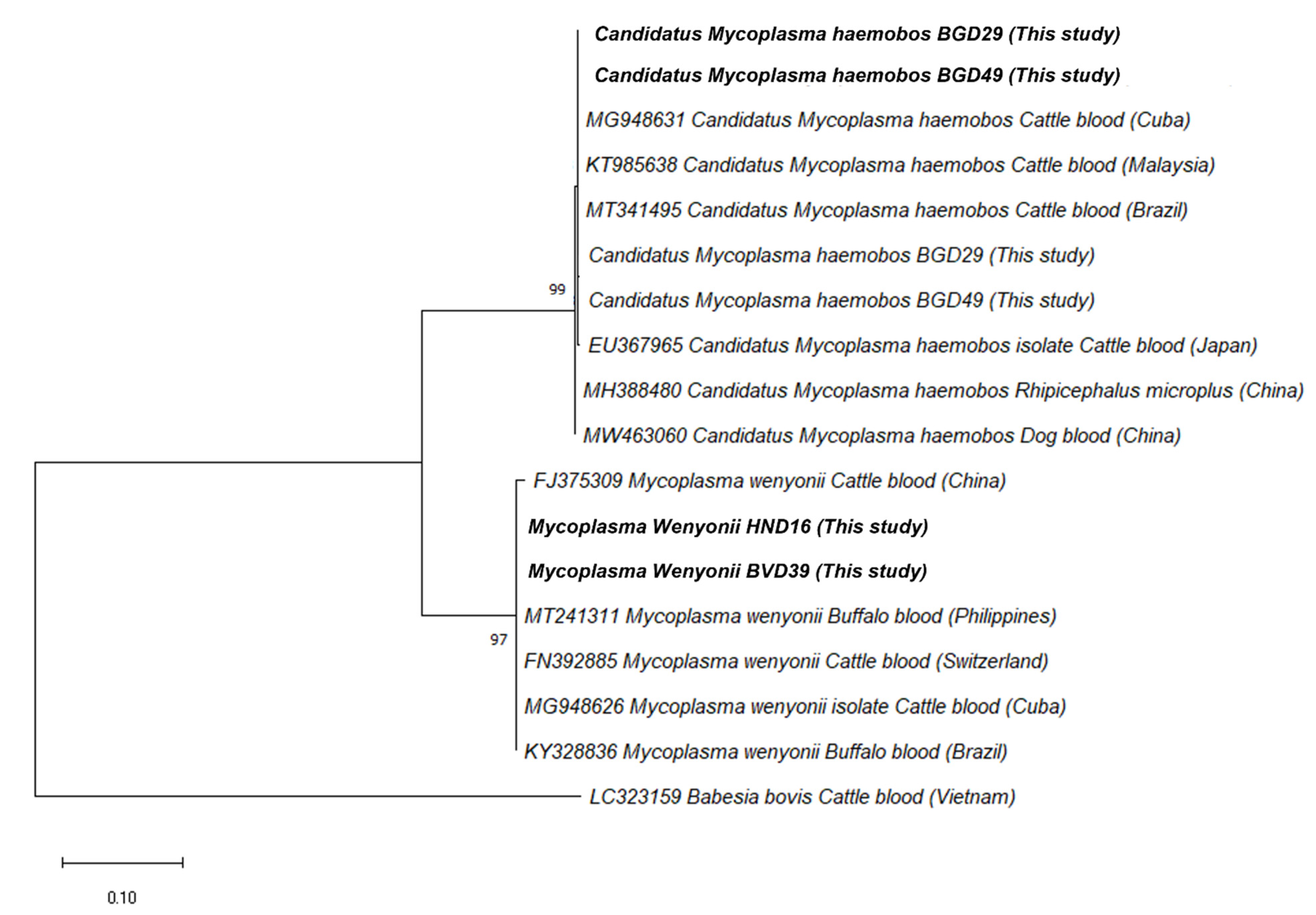
2.2. The Phylogenetic Analysis of Rickettsia felis and Hemoplasmas
3. Discussion
4. Materials and Methods
4.1. Study Area and Sample Collection
4.2. DNA Extraction and Molecular Detection
4.3. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobler, G.; Pfeffer, M. Fleas as parasites of the family Canidae. Parasites Vectors 2011, 4, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010, 14, e667–e676. [Google Scholar] [CrossRef] [Green Version]
- Beck, W.; Boch, K.; Mackensen, H.; Wiegand, B.; Pfister, K. Qualitative and quantitative observations on the flea population dynamics of dogs and cats in several areas of Germany. Veter-Parasitol. 2006, 137, 130–136. [Google Scholar] [CrossRef]
- Bond, R.; Riddle, A.; Mottram, L.; Beugnet, F.; Stevenson, R. Survey of flea infestation in dogs and cats in the United Kingdom during 2005. Vet. Rec. 2007, 160, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Beugnet, F.; Franc, M. Results of a European multicentric field efficacy study of fipronil-(S) methoprene combination on flea infestation of dogs and cats during 2009 summer. Parasite 2010, 17, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Farkas, R.; Gyurkovszky, M.; Solymosi, N.; Beugnet, F. Prevalence of flea infestation in dogs and cats in Hungary combined with a survey of owner awareness. Med. Vet. Èntomol. 2009, 23, 187–194. [Google Scholar] [CrossRef]
- Nguyen, V.-L.; Colella, V.; Greco, G.; Fang, F.; Nurcahyo, W.; Hadi, U.K.; Venturina, V.; Tong, K.B.Y.; Tsai, Y.-L.; Taweethavonsawat, P.; et al. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in East and Southeast Asia. Parasites Vectors 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Pathogens in fleas collected from cats and dogs: Distribution and prevalence in the UK. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beugnet, F.; Marie, J.L. Emerging arthropod-borne diseases of companion animals in Europe. Vet. Parasitol. 2009, 163, 298–305. [Google Scholar] [CrossRef]
- Inpankaew, T.; Hii, S.F.; Chimnoi, W.; Traub, R.J. Canine vector-borne pathogens in semi-domesticated dogs residing in northern Cambodia. Parasites Vectors 2016, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lu, G.; Kelly, P.; Zhang, Z.; Wei, L.; Yu, D.; Kayizha, S.; Wang, C. First report of Rickettsia felis in China. BMC Infect. Dis. 2014, 14, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, K.-H.; Huang, C.-G.; Fang, C.-T.; Shu, P.-Y.; Huang, J.-H.; Wu, W.-J. Prevalence of Rickettsia felis and the First Identification of Bartonella henselae Fizz/CAL-1 in Cat Fleas (Siphonaptera: Pulicidae) From Taiwan. J. Med. Èntomol. 2011, 48, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Kernif, T.; Socolovschi, C.; Wells, K.; Lakim, M.B.; Inthalad, S.; Slesak, G.; Boudebouch, N.; Beaucournu, J.-C.; Newton, P.N.; Raoult, D.; et al. Bartonella and Rickettsia in arthropods from the Lao PDR and from Borneo, Malaysia. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Compton, S.M.; Maggi, R.G.; Breitschwerdt, E.B. Candidatus Mycoplasma haematoparvum and Mycoplasma haemocanis infections in dogs from the United States. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Reif, K.E.; Macaluso, K.R. Ecology of Rickettsia felis: A Review. J. Med. Èntomol. 2009, 46, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.T.; Satjanadumrong, J.; Hughes, T.; Stenos, J.; Blacksell, S.D. Diagnosis of spotted fever group Rickettsia infections: The Asian perspective. Epidemiol. Infect. 2019, 147, 286. [Google Scholar] [CrossRef] [Green Version]
- Gracia, M.J.; Calvete, C.; Estrada, R.; Castillo, J.A.; Peribáñez, M.A.; Lucientes, J. Fleas parasitizing domestic dogs in Spain. Vet. Parasitol. 2008, 151, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Acestor, N.; Cooksey, R.; Newton, P.N.; Menard, D.; Guerin, P.J.; Nakagawa, J.; Christophel, E.; Gonzalez, I.J.; Bell, D. Mapping the aetiology of non-malarial febrile illness in Southeast Asia through a systematic review—terra incognita impairing treatment policies. PLoS ONE 2012, 7, e44269. [Google Scholar] [CrossRef] [Green Version]
- Edouard, S.; Bhengsri, S.; Dowell, S.F.; Watt, G.; Parola, P.; Raoult, D. Two Human Cases of Rickettsia felis Infection, Thailand. Emerg. Infect. Dis. 2014, 20, 1780–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.D.; Macaluso, K.R. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr. Trop. Med. Rep. 2016, 3, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le-Viet, N.; Le, V.-N.; Chung, H.; Phan, D.-T.; Phan, Q.-D.; Cao, T.-V.; Abat, C.; Raoult, D.; Parola, P. Prospective case-control analysis of the aetiologies of acute undifferentiated fever in Vietnam. Emerg. Microbes Infect. 2019, 8, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Dieme, C.; Bechah, Y.; Socolovschi, C.; Audoly, G.; Berenger, J.-M.; Faye, O.; Raoult, D.; Parola, P. Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 8088–8093. [Google Scholar] [CrossRef] [Green Version]
- Baggenstos, R.; Wenzinger, B.; Meli, M.L.; Hofmann-Lehmann, R.; Knubben-Schweizer, G. Haemoplasma infection in a dairy cow. Tierärztliche Prax. Ausg. G Großtiere Nutztiere 2012, 40, 397–400. [Google Scholar]
- Hornok, S.; Meli, M.L.; Perreten, A.; Farkas, R.; Willi, B.; Beugnet, F.; Lutz, H.; Hofmann-Lehmann, R. Molecular investigation of hard ticks (Acari: Ixodidae) and fleas (Siphonaptera: Pulicidae) as potential vectors of rickettsial and mycoplasmal agents. Vet. Microbiol. 2010, 140, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, A.L.; Hii, S.-F.; Jirsová, D.; Panáková, L.; Ionică, A.M.; Gilchrist, K.; Modrý, D.; Mihalca, A.D.; Webb, C.E.; Traub, R.J.; et al. Integrated morphological and molecular identification of cat fleas (Ctenocephalides felis) and dog fleas (Ctenocephalides canis) vectoring Rickettsia felis in central Europe. Vet. Parasitol. 2015, 210, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hii, S.-F.; Kopp, S.R.; Thompson, M.F.; O’Leary, C.A.; Rees, R.L.; Traub, R.J. Molecular evidence of Rickettsia felis infection in dogs from northern territory, Australia. Parasites Vectors 2011, 4, 198. [Google Scholar] [CrossRef] [Green Version]
- Criado-Fornelio, A.; Martinez-Marcos, A.; Buling-Saraña, A.; Barba-Carretero, J. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: A molecular study. Vet. Microbiol. 2003, 93, 307–317. [Google Scholar] [CrossRef]
- Nishimaki, T.; Sato, K. An Extension of the Kimura Two-Parameter Model to the Natural Evolutionary Process. J. Mol. Evol. 2019, 87, 60–67. [Google Scholar] [CrossRef] [Green Version]

| Factors | No. Dogs (%) n = 200 | No. Flea-Infested Dogs (%) n = 40 | Chi-Square Test |
|---|---|---|---|
| Age (year) | X-squared = 1.31, df = 2, p-value = 0.52 | ||
| <1 | 119 (59.5) | 23 (19.33) | |
| 1–3 | 52 (26) | 9 (17.31) | |
| >3 | 29 (14.5) | 8 (27.59) | |
| Gender | X-squared = 0, df = 1, p-value = 1 | ||
| Male | 85 (42.5) | 17 (20) | |
| Female | 115 (57.5) | 23 (20) | |
| Living condition | X-squared = 0.31, df = 1, p-value = 0.6 | ||
| Outdoor | 70 (35) | 16 (22.86) | |
| Semi-outdoor | 130 (65) | 24 (18.46) |
| Pathogens | No. Fleas Examined (n = 62) | Detection Rate % | 95% Confidence Interval |
|---|---|---|---|
| Single infection | |||
| Rickettsia felis | 39 | 62.90 | 55.7–70.1 |
| Candidatus Mycoplasma hemobos | 9 | 14.52 | 9.3–19.7 |
| Mycoplasma wenyonii | 6 | 9.68 | 5.3–14.1 |
| Mixed infection | |||
| Rickettsia felis and Candidatus Mycoplasma hemobos | 4 | 6.45 | 2.8–10.1 |
| Rickettsia felis and Mycoplasma wenyonii | 5 | 8.06 | 4.01–12.1 |
| Total | 54 | 87.09 | 82.1–92.1 |
| Pathogen | Oligonucleotide Sequences (5′–3′) | Product Size (bp) | PCR Protocol | Reference |
|---|---|---|---|---|
| Flea species (Cox 1) | F: TTATCTGTTTATGTTATATAAGC R: CAGTACCTATGCATATCAATCC | 601 | 94 °C for 3 min initial denaturation, followed by 35 cycles of 94 °C for 30 s, 54.3 °C for 30 s, 72 °C for 30 s, then 72 °C for 5 min for the final elongation | [25] |
| Rickettsia felis (gltA) | F1: GCAAGTATTGGTGAGGATGTAATC R1: CTGCGGCACGTGGGTCATAG F2: GCGACATCGAGGATATGACAT R2: GGAATATTCTCAGAACTACCG | 654 | 95 °C for 3 min initial denaturation, followed by 40 cycles of 95 °C for 20 s, 55 °C for 20 s, 72 °C for 20 s, then 72 °C for 5 min for the final elongation | [26] |
| Mycoplasma spp. (16S rRNA) | F: ATACGGCCCATATTCCTACG R: TGCTCCACCACTTGTTCA | 595–618 | 94 °C for 5 min initial denaturation, followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, then 72 °C for 10 min for the final elongation | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.; Inpankaew, T.; Duong, D.H.; Bui, K.L. First Molecular Evidence of Pathogens in Fleas Collected from Dogs in Northern Vietnam. Pathogens 2021, 10, 1185. https://doi.org/10.3390/pathogens10091185
Do T, Inpankaew T, Duong DH, Bui KL. First Molecular Evidence of Pathogens in Fleas Collected from Dogs in Northern Vietnam. Pathogens. 2021; 10(9):1185. https://doi.org/10.3390/pathogens10091185
Chicago/Turabian StyleDo, Thom, Tawin Inpankaew, Duc Hieu Duong, and Khanh Linh Bui. 2021. "First Molecular Evidence of Pathogens in Fleas Collected from Dogs in Northern Vietnam" Pathogens 10, no. 9: 1185. https://doi.org/10.3390/pathogens10091185
APA StyleDo, T., Inpankaew, T., Duong, D. H., & Bui, K. L. (2021). First Molecular Evidence of Pathogens in Fleas Collected from Dogs in Northern Vietnam. Pathogens, 10(9), 1185. https://doi.org/10.3390/pathogens10091185







