Overcoming the Negligence in Laboratory Diagnosis of Mucosal Leishmaniasis
Abstract
1. Introduction
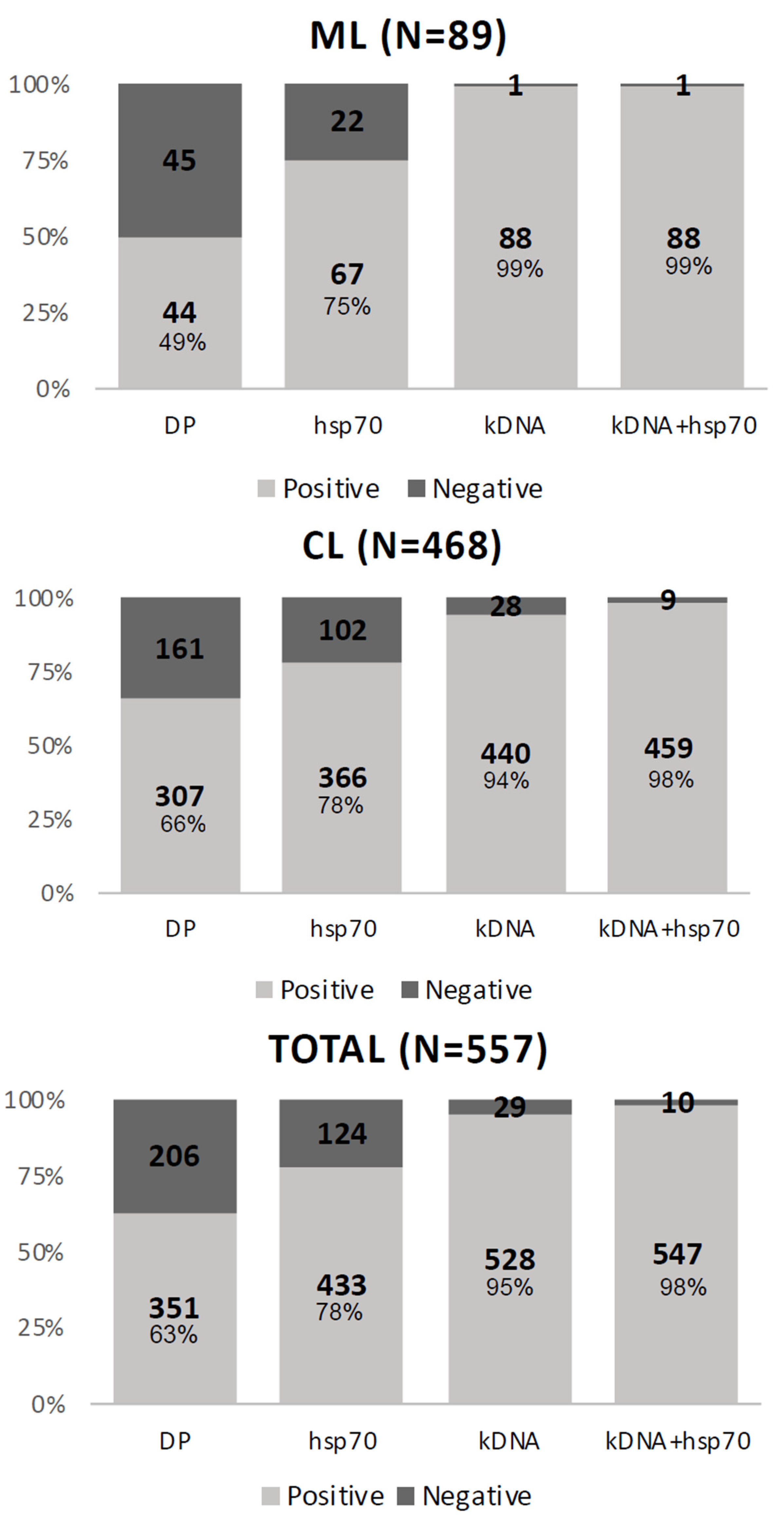
2. Results
The qPCR in Blood Samples from ML Patients
3. Discussion
4. Material and Methods
4.1. Collection of Clinical Data and Biological Samples
4.2. Examination of Samples
4.2.1. Direct Parasitological (DP) Tests
4.2.2. kDNA and hsp70 PCR
4.2.3. Real-Time PCR for Blood Mucosal Samples
4.2.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- WHO. Leishmaniasis; Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis# (accessed on 12 July 2021).
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous manifestations of human and murine leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Pace, D. Leishmaniasis. J. Infect. 2014, 69, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Marsden, P.D. Clinical presentations of Leishmania braziliensis braziliensis. Parasitol. Today 1985, 1, 129–133. [Google Scholar] [CrossRef]
- Bailey, F.; Mondragon-Shem, K.; Hotez, P.; Ruiz-Postigo, J.A.; Al-Salem, W.; Acosta-Serrano, Á.; Molyneux, D.H. A new perspective on cutaneous leishmaniasis—Implications for global prevalence and burden of disease estimates. PLoS Negl. Trop. Dis. 2017, 11, e0005739. [Google Scholar] [CrossRef] [PubMed]
- WHO. Leishmaniasis; WHO: Geneva, Switzerland, 2020; Volume 14. [Google Scholar]
- Lainson, R. The Neotropical Leishmania species: A brief historical review of their discovery, ecology and taxonomy. Rev. Pan-Amaz. Saúde 2010, 1, 13–32. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; Corbett, C.E. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: A review. Mem. Inst. Oswaldo Cruz 2004, 99, 239–251. [Google Scholar] [CrossRef]
- Departamento de Vigilancia das Doencas Transmissiveis de Brasília. Manual de Controle da Leishmaniose Tegumentar Americana, 2nd ed.; Editora do Ministério da Saude: Brasília, Brazil, 2010; ISBN 9788533412705.
- Silveira, F.T.; Ishikawa, E.A.Y.; De Souza, A.A.A.; Lainson, R. An outbreak of cutaneous leishmaniasis among soldiers in Belém, Pará State, Brazil, caused by Leishmania (Viannia) lindenbergi n. sp. Parasite 2002, 9, 43–50. [Google Scholar] [CrossRef]
- Editora do Ministério da Saude: Brasília. Brasil Manual de Vigilância e Controle da Leishmaniose Visceral; Editora do Ministério da Saude: Brasília, Brazil, 2008. [Google Scholar]
- Amato, V.S.; Tuon, F.F.; Bacha, H.A.; Neto, V.A.; Nicodemo, A.C. Mucosal leishmaniasis. Current scenario and prospects for treatment. Acta Trop. 2008, 105, 1–9. [Google Scholar] [CrossRef]
- David, C.V.; Craft, N. Cutaneous and mucocutaneous leishmaniasis. Dermatol. Ther. 2009, 22, 491–502. [Google Scholar] [CrossRef]
- Guery, R.; Walker, S.; Harms, G.; Neumayr, A.; Thiel, P.V.; Gangneux, J.-P.; Clerinx, J.; Sobirk, S.K.; Visse, L.G.; Bailey, M.; et al. Worldwide diversity of tegumentary leishmaniasis and the risk of mucosal lesions: A clinical report in 459 European travellers. In Proceedings of the 30th European Congress of Clinical Microbiology and Infectious Disease—30th ECCMID 2020, Paris, France, 18–21 April 2020. [Google Scholar]
- Ministério da Saúde. Leishmaniose Tegumentar Americana—Casos Confirmados Notificados No Sistema de Informação de Agravos de Notificação—Brasil. Available online: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinanwin/cnv/ltabr.def (accessed on 29 August 2021).
- Carvalho, E.M.; Barral, A.; Costa, J.M.L.; Bittencourt, A.; Marsden, P. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 1994, 56, 315–325. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Fasel, N. How to master the host immune system? Leishmania parasites have the solutions! Int. Immunol. 2017, 30, 103–111. [Google Scholar] [CrossRef]
- Hartley, M.-A.A.; Bourreau, E.; Rossi, M.; Castiglioni, P.; Eren, R.O.; Prevel, F.; Couppié, P.; Hickerson, S.M.; Launois, P.; Beverley, S.M.; et al. Leishmaniavirus-Dependent Metastatic Leishmaniasis Is Prevented by Blocking IL-17A. PLoS Pathog. 2016, 12, e1005852. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, M.; Kar, S.; Goldsmith-Pestana, K.; McMahon-Pratt, D. Mechanisms of pathogenesis: Differences amongst Leishmania species. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, S3–S7. [Google Scholar] [CrossRef]
- Cantanhêde, L.M.; da Silva Júnior, C.F.; Ito, M.M.; Felipin, K.P.; Nicolete, R.; Salcedo, J.M.V.; Porrozzi, R.; Cupolillo, E.; de Ferreira, R.G.M. Further Evidence of an Association between the Presence of Leishmania RNA Virus 1 and the Mucosal Manifestations in Tegumentary Leishmaniasis Patients. PLoS Negl. Trop. Dis. 2015, 9, e0004079. [Google Scholar] [CrossRef]
- García-Bustos, M.F.; González-Prieto, G.; Pániz-Mondolfi, A.E.; Parodi, C.; Beckar, J.; Monroig, S.; Ramos, F.; Mora, M.C.; Delgado-Noguera, L.A.; Hashiguchi, Y.; et al. Risk factors for antimony treatment failure in american cutaneous leishmaniasis in Northwestern-Argentina. PLoS Negl. Trop. Dis. 2021, 15, e0009003. [Google Scholar] [CrossRef]
- Blum, J.; Lockwood, D.N.J.; Visser, L.; Harms, G.; Bailey, M.S.; Caumes, E.; Clerinx, J.; van Thiel, P.P.A.M.; Morizot, G.; Hatz, C.; et al. Local or systemic treatment for New World cutaneous leishmaniasis? Re-evaluating the evidence for the risk of mucosal leishmaniasis. Int. Health 2012, 4, 153–163. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Manual de Procedimientos Para Vigilancia y Control. de Las Leishmaniasis en Las Américas; Pan American Health Organization (PAHO): Washington, DC, USA, 2019; Volume 53, ISBN 9788578110796. [Google Scholar]
- Gomes, A.H.S.; Armelin, I.M.; Menon, S.Z.; Pereira-Chioccola, V.L. Leishmania (V.) braziliensis: Detection by PCR in biopsies from patients with cutaneous leishmaniasis. Exp. Parasitol. 2008, 119, 319–324. [Google Scholar] [CrossRef]
- Cardozo, R.S.; García-Montero, P.P.; Chicharro, C.; Tardío, J.C. Cutaneous leishmaniasis: A pathological study of 360 cases with special emphasis on the contribution of immunohistochemistry and polymerase chain reaction to diagnosis. J. Cutan. Pathol. 2020, 47, 1018–1025. [Google Scholar] [CrossRef]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and treatment of leishmaniasis: Clinical practice guidelines by the infectious diseases society of America (IDSA) and the American Society of tropical medicine and hygiene (ASTMH). Am. J. Trop. Med. Hyg. 2017, 96, 24–45. [Google Scholar] [CrossRef]
- Galluzzi, L.; Ceccarelli, M.; Diotallevi, A.; Menotta, M.; Magnani, M. Real-time PCR applications for diagnosis of leishmaniasis. Parasites Vectors 2018, 11, 1–13. [Google Scholar] [CrossRef]
- León, C.M.; Muñoz, M.; Hernández, C.; Ayala, M.S.; Flórez, C.; Teherán, A.; Cubides, J.R.; Ramírez, J.D. Analytical performance of Four Polymerase Chain Reaction (PCR) and real time PCR (qPCR) assays for the detection of six Leishmania species DNA in Colombia. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, A.M.; Alba, A.; Fraga, J.; Marzoa, A.; Torres, C.; Muskus, C. Improving the sensitivity of an hsp20-based PCR for genus detection of Leishmania parasites in cutaneous clinical samples: A proof of concept. Parasitol. Res. 2020, 119, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.E.; Joya, C.A. Cutaneous Leishmaniasis: Updates in Diagnosis and Management. Infect. Dis. Clin. N. Am. 2019, 33, 101–117. [Google Scholar] [CrossRef]
- Conter, C.C.; Lonardoni, M.V.C.; Aristides, S.M.A.; Cardoso, R.F.; Silveira, T.G.V. New primers for the detection Leishmania species by multiplex polymerase chain reaction. Parasitol. Res. 2017, 117, 501–511. [Google Scholar] [CrossRef]
- Filgueira, C.P.B.; Moreira, O.C.; Cantanhêde, L.M.; de Farias, H.M.T.; Porrozzi, R.; Britto, C.; Boité, M.C.; Cupolillo, E. Comparison and clinical validation of QPCR assays targeting leishmania 18s rDNA and HSP70 genes in patients with american tegumentary leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, e0008750. [Google Scholar] [CrossRef] [PubMed]
- Da Graça, G.C.; Volpini, A.C.; Romero, G.A.S.; de Oliveira Neto, M.P.; Hueb, M.; Porrozzi, R.; Boité, M.C.; Cupolillo, E. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Mem. Inst. Oswaldo Cruz 2012, 107, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, A.M.; Fraga, J.; Tirado, D.; Blandón, G.; Alba, A.; Van der Auwera, G.; Vélez, I.D.; Muskus, C. Detection and identification of Leishmania spp.: Application of two hsp70-based PCR-RFLP protocols to clinical samples from the New World. Parasitol. Res. 2017, 116, 1843–1848. [Google Scholar] [CrossRef]
- Neitzke-Abreu, H.C.; Venazzi, M.S.; Bernal, M.V.Z.; Reinhold-Castro, K.R.; Vagetti, F.; Mota, C.A.; Silva, N.R.; Aristides, S.M.A.; Silveira, T.G.V.; Lonardoni, M.V.C. Detection of DNA from Leishmania (Viannia): Accuracy of polymerase chain reaction for the diagnosis of cutaneous leishmaniasis. PLoS ONE 2013, 8, e62473. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, E.; Nasereddin, A.; Schnur, L.F.; Jaffe, C.L.; Jonas, F. Comparison of PCR Assays for Diagnosis of Cutaneous Leishmaniasis Comparison of PCR Assays for Diagnosis of Cutaneous Leishmaniasis. J. Clin. Microbiol. 2006, 44, 1435–1439. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef]
- Oliart-Guzmán, H.; Camargo Martins, A.; Silva Mantovani, S.A.; Braña, A.M.; Delfino, B.M.; Moraes Pereira, T.; Santos, A.P.; Filgueira Júnior, J.A.; Cunha Castelo Branco, F.L.; Gardinali Campos, R.; et al. Características Epidemiológicas Da Leishmaniose Tegumentar Americana Na Fronteira Amazônica: Estudo Retrospectivo Em Assis Brasil, Acre. Rev. Patol. Trop. 2013, 42, 187–200. [Google Scholar] [CrossRef]
- Aparecida, E.; Galati, B. Epidemiologia da Leishmaniose Tegumentar e descrição das populações de flebotomíneos no município de Acrelândia, Acre, Brasil Epidemiology of Cutaneous Leishmaniasis and description of in the city of Acrelandia, Acre, Brazil. Rev. Bras. Epidemiol. 2008, 11, 241–251. [Google Scholar]
- Teles, C.B.G.; Medeiros, J.F.; Dos Santos, A.P.A.; De Freitas, L.A.R.; Katsuragawa, T.H.; Cantanhêde, L.M.; Ferreira, R.G.M.; Camargo, L.M.A. Molecular characterization of American cutaneous leishmaniasis in the tri-border area of Assis Brasil, acre state, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 343–347. [Google Scholar] [CrossRef]
- Llanos-Cuentas, A.; Tulliano, G.; Araujo-Castillo, R.; Miranda-Verastegui, C.; Santamaria-Castrellon, G.; Ramirez, L.; Lazo, M.; De Doncker, S.; Boelaert, M.; Robays, J.; et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 46, 223–231. [Google Scholar] [CrossRef]
- Arevalo, J.; Ramirez, L.; Adaui, V.; Zimic, M.; Tulliano, G.; Miranda-Verástegui, C.; Lazo, M.; Loayza-Muro, R.; De Doncker, S.; Maurer, A.; et al. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J. Infect. Dis. 2007, 195, 1846–1851. [Google Scholar] [CrossRef]
- Adaui, V.; Lye, L.-F.; Akopyants, N.S.; Zimic, M.; Llanos-Cuentas, A.; Garcia, L.; Maes, I.; De Doncker, S.; De Doncker, S.; Dobson, D.E.; et al. Association of the Endobiont Double-Stranded RNA Virus LRV1 With Treatment Failure for Human Leishmaniasis Caused by Leishmania braziliensis in Peru and Bolivia. J. Infect. Dis. 2015, 213, 1–32. [Google Scholar] [CrossRef]
- Bourreau, E.; Ginouves, M.; Prévot, G.; Hartley, M.-A.; Gangneux, J.-P.; Robert-Gangneux, F.; Dufour, J.; Sainte Marie, D.; Bertolotti, A.; Pratlong, F.; et al. Leishmania-RNA virus presence in L. guyanensis parasites increases the risk of first-line treatment failure and symptomatic relapse. J. Infect. Dis. 2015, 213, 1–28. [Google Scholar] [CrossRef]
- Tuon, F.F.; Gomes-Silva, A.; Da-Cruz, A.M.; Duarte, M.I.S.; Neto, V.A.; Amato, V.S. Local immunological factors associated with recurrence of mucosal leishmaniasis. Clin. Immunol. 2008, 128, 442–446. [Google Scholar] [CrossRef]
- Reveiz, L.; Maia-Elkhoury, A.N.S.; Nicholls, R.S.; Sierra Romero, G.A.; Yadon, Z.E. Interventions for American Cutaneous and Mucocutaneous Leishmaniasis: A Systematic Review Update. PLoS ONE 2013, 8, e61843. [Google Scholar] [CrossRef]
- Lessa, M.M.; Lessa, H.A.; Castro, T.W.N.; Oliveira, A.; Scherifer, A.; Machado, P.; Carvalho, E.M. Mucosal leishmaniasis: Epidemiological and clinical aspects. Braz. J. Otorhinolaryngol. 2007, 73, 843–847. [Google Scholar] [CrossRef]
- Tedesqui, V.L.; Calleja, G.N.C.; Parra, R.; Pabón, J.P.; Bóia, M.N.; Carvalho-Costa, F.A. Active surveillance of American tegumentary leishmaniasis in endemic areas in rural Bolivia. Rev. Soc. Bras. Med. Trop. 2012, 45, 30–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tirera, S.; Ginouves, M.; Donato, D.; Caballero, I.S.; Bouchier, C.; Lavergne, A.; Bourreau, E.; Mosnier, E.; Vantilcke, V.; Couppié, P.; et al. Unraveling the genetic diversity and phylogeny of Leishmania RNA virus 1 strains of infected Leishmania isolates circulating in French Guiana. PLoS Negl. Trop. Dis. 2017, 11, e0005764. [Google Scholar] [CrossRef]
- Salinas, G.; Zamora, M.; Stuart, K.; Saravia, N. Leishmania RNA viruses in Leishmania of the Viannia subgenus. Am. J. Trop. Med. Hyg. 1996, 54, 425–429. [Google Scholar] [CrossRef]
- Pereira, L.D.O.R.; Maretti-Mira, A.C.; Rodrigues, K.M.; Lima, R.B.; de Oliveira-Neto, M.P.; Cupolillo, E.; Pirmez, C.; de Oliveira, M.P. Severity of tegumentary leishmaniasis is not exclusively associated with Leishmania RNA virus 1 infection in Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 665–667. [Google Scholar] [CrossRef]
- Macedo, D.H.; Menezes-Neto, A.; Rugani, J.M.; Rocha, A.C.; Silva, S.O.; Melo, M.N.; Lye, L.F.; Beverley, S.M.; Gontijo, C.M.; Soares, R.P. Low frequency of LRV1 in Leishmania braziliensis strains isolated from typical and atypical lesions in the State of Minas Gerais, Brazil. Mol. Biochem. Parasitol. 2016, 210, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Hueb, M.; Rodrigues Dos Santos, T.A.R.; Fernandes Fontes, C.J. Factors associated with treatmet failure of cutaneous leishmaniasis with meglumine antimoniate. Rev. Soc. Bras. Med. Trop. 2006, 39, 139–145. [Google Scholar] [CrossRef]
- Nassif, P.W.; Castilho-Peres, M.; Rosa, A.P.Z.; Da Silva, A.L.; Aristides, S.M.A.; Lonardoni, M.V.C.; Teixeira, J.J.V.; Silveira, T.G.V. Clinical, laboratory, and therapeutic characteristics of american tegumentary leishmaniasis in the 15th state health division, northwest Paraná state, southern Brazil. Rev. Soc. Bras. Med. Trop. 2016, 49, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Del Castro, M.M.; Cossio, A.; Velasco, C.; Osorio, L. Risk factors for therapeutic failure to meglumine antimoniate and miltefosine in adults and children with cutaneous leishmaniasis in Colombia: A cohort study. PLoS Negl. Trop. Dis. 2017, 11, e0005515. [Google Scholar] [CrossRef] [PubMed]
- Guerra, J.A.D.O.; Prestes, S.R.; Silveira, H.; Coelho, L.I.D.A.R.C.; Gama, P.; Moura, A.; Amato, V.; Barbosa, M.D.G.V.; Ferreira, L.C.D.L. Mucosal Leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2011, 5, e980. [Google Scholar] [CrossRef] [PubMed]
- Camara Coelho, L.I.; Paes, M.; Guerra, J.A.; Barbosa, M.D.G.; Coelho, C.; Lima, B.; Brito, M.E.; Brandão Filho, S.P. Characterization of Leishmania spp. causing cutaneous leishmaniasis in Manaus, Amazonas, Brazil. Parasitol. Res. 2011, 108, 671–677. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.V.; de Souza, C.F.; Fuzari, A.A.; Joya, C.A.; Valdivia, H.O.; Bartholomeu, D.C.; Brazil, R.P. Diagnosis and identification of Leishmania species in patients with cutaneous leishmaniasis in the state of Roraima, Brazil’s Amazon Region. Parasites Vectors 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cantanhêde, L.M.; Mattos, C.B.; De Souza Ronconi, C.; Filgueira, C.P.B.; Da Silva Júnior, C.F.; Limeira, C.; De Jesus Silva, H.P.; Ferreira, G.E.M.; Porrozzi, R.; De Godoi Mattos Ferreira, R.; et al. First report of Leishmania (Viannia) lindenbergi causing tegumentary leishmaniasis in the Brazilian western Amazon region. Parasite 2019, 26, 30. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.D.L.R.; de Andrade, A.S.R.; Fontes, C.J.F.; Hueb, M.; de Oliveira Silva, S.; Melo, M.N. Leishmania (Viannia) braziliensis is the prevalent species infecting patients with tegumentary leishmaniasis from Mato Grosso State, Brazil. Acta Trop. 2006, 98, 277–285. [Google Scholar] [CrossRef]
- Martinez, E.; Pont, F.L.; Mollinedo, S.; Cupolillo, E. A first case of cutaneous leishmaniasis due to Leishmania (Viannia) lainsoni in Bolivia. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 375–377. [Google Scholar] [CrossRef]
- Szargiki, R.; Alcântara de Castro, E.; Luz, E.; Kowalthuk, W.; Machado, A.M.; Thomaz-Soccol, V. Comparison of serological and parasitological methods for cutaneous leishmaniasis diagnosis in the state of Paraná, Brazil. BJID 2009, 12, 47–52. [Google Scholar] [CrossRef]
- Goto, H.; Lindoso, J.A.L. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev. Anti-Infect. Ther. 2010, 8, 419–433. [Google Scholar] [CrossRef]
- Lemrani, M.; Hamdi, S.; Laamrani, A.; Hassar, M. PCR detection of Leishmania in skin biopsies. J. Infect. Dev. Ctries. 2009, 3, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, E.B.; Santander, S.; Rojas-Silva, P.; Cardenas, P.A.; Fornasini, M.; Cifuentes, S.C.; Salvador, D.; Baldeón, M.E. Diagnostic Efficacy of Molecular Techniques for Detection and Identification of Leishmania Species in Human Whole Blood and Skin Samples from Ecuador. Am. J. Trop. Med. Hyg. 2016, 95, 803–805. [Google Scholar] [CrossRef][Green Version]
- Reithinger, R.; Dujardin, J.-C. Molecular diagnosis of leishmaniasis: Current status and future applications. J. Clin. Microbiol. 2007, 45, 21–25. [Google Scholar] [CrossRef]
- Disch, J.; Pedras, M.J.; Orsini, M.; Pirmez, C.; de Oliveira, M.C.; Castro, M.; Rabello, A. Leishmania (Viannia) subgenus kDNA amplification for the diagnosis of mucosal leishmaniasis. Diagn. Microbiol. Infect. Dis. 2005, 51, 185–190. [Google Scholar] [CrossRef]
- Suárez, M.; Valencia, B.M.; Jara, M.; Alba, M.; Boggild, A.K.; Dujardin, J.C.; Llanos-Cuentas, A.; Arevalo, J.; Adaui, V. Quantification of leishmania (Viannia) kinetoplast DNA in ulcers of cutaneous leishmaniasis reveals inter-site and intersampling variability in parasite load. PLoS Negl. Trop. Dis. 2015, 9, e0003936. [Google Scholar] [CrossRef]
- Jara, M.; Adaui, V.; Valencia, B.M.; Martinez, D.; Alba, M.; Castrillon, C.; Cruz, M.; Cruz, I.; Van Der Auwera, G.; Llanos-Cuentas, A.; et al. Real-time PCR assay for detection and quantification of Leishmania (Viannia) organisms in skin and mucosal lesions: Exploratory study of parasite load and clinical parameters. J. Clin. Microbiol. 2013, 51, 1826–1833. [Google Scholar] [CrossRef]
- Garcia, A.L.; Parrado, R.; De Doncker, S.; Bermudez, H.; Dujardin, J.-C. American tegumentary leishmaniasis: Direct species identification of Leishmania in non-invasive clinical samples. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 368–371. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Fraga, J.; Monzote, L.; Montano, I.; De Doncker, S.; Dujardin, J.C.; Van Der Auwera, G. Heat-shock protein 70 PCR-RFLP: A universal simple tool for Leishmania species discrimination in the New and Old World. Parasitology 2010, 137, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- De Pereira, L.O.R.; Moreira, R.B.; de Oliveira, M.P.; de Reis, S.O.; de Oliveira Neto, M.P.; Pirmez, C. Is Leishmania (Viannia) braziliensis parasite load associated with disease pathogenesis? Int. J. Infect. Dis. 2017, 57, 132–137. [Google Scholar] [CrossRef]
- Ovalle-Bracho, C.; Díaz-Toro, Y.R.; Muvdi-Arenas, S. Polymerase chain reaction-miniexon: A promising diagnostic 7ethod for mucocutaneous leishmaniasis. Int. J. Dermatol. 2016, 55, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Amir, G.; Salant, H.; Resnick, I.B.; Karplus, R. Cutaneous toxoplasmosis after bone marrow transplantation with molecular confirmation. J. Am. Acad. Dermatol. 2008, 59, 781–784. [Google Scholar] [CrossRef]
- Taxy, J.B.; Goldin, H.M.; Dickie, S.; Cibull, T. Cutaneous Leishmaniasis: Contribution of Routine Histopathology in Unexpected Encounters. Am. J. Surg. Pathol. 2019, 43, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Stetsenko, G.Y.; Pottinger, P.S.; George, E. Cutaneous presentation of disseminated histoplasmosis as a solitary peri-anal ulcer. Case report and discussion. J. Cutan. Pathol. 2016, 43, 438–443. [Google Scholar] [CrossRef]
- Fraga, T.L.; Brustoloni, Y.M.; Lima, R.B.; Dorval, M.E.C.; Oshiro, E.T.; Oliveira, J.; de Oliveira, A.L.L.; Pirmez, C. Polymerase chain reaction of peripheral blood as a tool for the diagnosis of visceral leishmaniasis in children. Mem. Inst. Oswaldo Cruz 2010, 105, 310–313. [Google Scholar] [CrossRef][Green Version]
- De Avelar, D.M.; Carvalho, D.M.; Rabello, A. Development and Clinical Evaluation of Loop-Mediated Isothermal Amplification (LAMP) Assay for the Diagnosis of Human Visceral Leishmaniasis in Brazil. BioMed Res. Int. 2019, 2019, 8240784. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Chilama, M.; Gongora, R.E.; Valderrama, L.; Jojoa, J.; Alexander, N.; Rubiano, L.C.; Cossio, A.; Adams, E.R.; Saravia, N.G.; Gomez, M.A. Parasitological Confirmation and Analysis of Leishmania Diversity in Asymptomatic and Subclinical Infection following Resolution of Cutaneous Leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0004273. [Google Scholar] [CrossRef]
- De Camera, P.O.; Junger, J.; do Pires, F.E.S.S.; Mattos, M.; Oliveira-Neto, M.P.; Fernandes, O.; Pirmez, C. Haematogenous dissemination of Leishmania (Viannia) braziliensis in human American tegumentary leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Vergel, C.; Palacios, R.; Cadena, H.; Posso, C.J.; Valderrama, L.; Perez, M.; Walker, J.; Travi, B.L.; Saravia, N.G. Evidence for Leishmania (Viannia) Parasites in the Skin and Blood of Patients Before and After Treatment. J. Infect. Dis. 2006, 194, 503–511. [Google Scholar] [CrossRef]
- Martinez, J.E.; Arias, A.L.; Escobar, M.A.; Saravia, N.G. MEDICINE Haemoculture leishmaniasis: Of Leishmania of haematogenous from two cases of mucosal dissemination. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 392–394. [Google Scholar] [CrossRef]
- Oliveira, E.G.; Vasconcelos, A.W.; Vasconcelos, I.A.B.; Lima, J.W.O.; Pompeu, M.M.L.; Filho, J.M.C.; David, J.R.; de Sousa, A.Q.; Maguire, J.H.; Parise, M.E. Bubonic Leishmaniasis: A Common Manifestation of Leishmania (Viannia) braziliensis Infection in Ceara, Brazil. Am. J. Trop. Med. Hyg. 1995, 53, 380–385. [Google Scholar] [CrossRef]
- Bowdre, J.H.; Campbell, J.L.; Walker, D.H.; Tart, D.E. American Mucocutaneous Leishmaniasis: Culture of a Leishmania Species from Peripheral Blood Leukocytes. Am. J. Clin. Pathol. 1981, 75, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Passos, V.M.A.; Falcão, A.L.; Marzochi, M.C.A.; Gontijo, C.M.F.; Dias, E.S.; Barbosa-Santos, E.G.O.; Guerra, H.L.; Katz, N.; Passos, V.M.A.; Falcão, A.L.; et al. Epidemiological aspects of American Cutaneous Leishmaniasis in a periurban area of the metropolitan region of Belo Horizonte, Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 1993, 88, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Randolph, J.J. Online Kappa Calculator. 2008. Available online: http://justusrandolph.net/kappa/ (accessed on 29 August 2021).
- Adams, E.R.; Gomez, M.A.; Scheske, L.; Rios, R.; Marquez, R.; Cossio, A.; Albertini, A.; Schallig, H.; Saravia, N.G. Sensitive diagnosis of cutaneous leishmaniasis by lesion swab sampling coupled to qPCR. Parasitology 2014, 141, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.M.; Cesetti, M.V.; Paula, N.A.; Vernal, S.; Gupta, G.; Sampaio, R.N.R.; Roselino, A.M. Field Validation of SYBR Green- and TaqMan-Based Real-Time PCR Using Biopsy and Swab Samples To Diagnose American Tegumentary Leishmaniasis in an Area Where Leishmania (Viannia) braziliensis Is Endemic. J. Clin. Microbiol. 2017, 55, 526–534. [Google Scholar] [CrossRef]
| Test | Result | CL + ML (%) | CL (%) | ML (%) |
|---|---|---|---|---|
| DP | Positive | 351 (48.4) | 307 (51.2) | 44 (36.0) |
| Negative | 372 (51.6) | 293 (48.8) | 79 (64.0) | |
| kDNA | Positive | 528 (73.0) | 440 (73.3) | 88 (71.5) |
| Negative | 195 (27.0) | 160 (26.7) | 35 (28.5) | |
| hsp70 | Positive | 433 (59.9) | 366 (61.0) | 67 (54.5) |
| Negative | 290 (40.1) | 234 (39.0) | 56 (45.5) | |
| Positive for one of the molecular tests | Yes | 547 (75.7) | 459 (76.5) | 88 (71.5) |
| No | 176 (24.3) | 141 (23.5) | 35 (28.5) | |
| Positive for one of parasitological tests | Yes | 557 (77.0) | 468 (78.0) | 89 (72.4) |
| No | 166 (23.0) | 132 (22.0) | 34 (27.6) |
| Variable | Categories | N | % |
|---|---|---|---|
| Gender | Male | 438 | 78.6 |
| Female | 119 | 21.4 | |
| Type of lesion | Cutaneous | 468 | 84.0 |
| Mucosal | 89 | 16.0 | |
| Putative local of infection | Work | 470 | 84.4 |
| Leisure | 87 | 15.6 | |
| Previous leishmaniasis | Yes | 130 | 23.3 |
| No | 427 | 76.7 | |
| Treatment in the first infection | Yes | 5 | 4.5 |
| No | 125 | 95.5 | |
| Not applicable * | 427 | ||
| DP | Positive | 351 | 63.0 |
| Negative | 206 | 37.0 | |
| kDNA | Positive | 528 | 94.8 |
| Negative | 29 | 5.2 | |
| hsp70 | Positive | 433 | 77.7 |
| Negative | 124 | 22.3 | |
| Positive for one of the molecular tests | Yes | 547 | 98.2 |
| No | 10 | 1.8 |
| Test | CL + ML | CL | ML | |||||
|---|---|---|---|---|---|---|---|---|
| DP | kDNA | hsp70 | N | %(+) | N | %(+) | N | %(+) |
| + | + | + | 298 | 53.5 | 263 | 56.2 | 35 | 39.3 |
| + | + | - | 40 | 7.2 | 32 | 6.8 | 8 | 9.0 |
| + | - | + | 3 | 0.5 | 3 | 0.6 | 0 | 0 |
| + | - | - | 10 | 1.8 | 9 | 1.9 | 1 | 1.1 |
| - | + | + | 116 | 20.8 | 84 | 17.9 | 32 | 36.0 |
| - | + | - | 74 | 13.3 | 62 | 13.2 | 13 | 14.6 |
| - | - | + | 16 | 2.9 | 16 | 3.4 | 0 | 0 |
| Total | 557 | 468 | 89 | |||||
| CL + ML | CL | ML | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| “Clinical reference” | Test | “Clinical reference” | Sensibility | “Clinical reference” | Sensibility | “Clinical reference” | Sensibility | ||||
| Result | + | - | + | - | + | - | |||||
| Set of tests | DP | + | 351 | 0 | 0.63 | 307 | 0 | 0.66 | 44 | 0 | 0.49 |
| - | 206 | 166 | 161 | 132 | 45 | 34 | |||||
| kDNA | + | 528 | 0 | 0.95 | 440 | 0 | 0.99 | 83 | 5 | 0.94 | |
| - | 29 | 166 | 28 | 132 | 1 | 34 | |||||
| hsp70 | + | 433 | 0 | 0.78 | 366 | 0 | 0.78 | 67 | 0 | 0.75 | |
| - | 124 | 166 | 102 | 132 | 22 | 34 | |||||
| Combined molecular | + | 547 | 0 | 0.98 | 459 | 0 | 0.98 | 88 | 0 | 0.99 | |
| - | 10 | 166 | 9 | 132 | 1 | 34 | |||||
| Combined molecular tests | DP | + | 341 | 10 | 0.62 | 298 | 9 | 0.65 | 43 | 1 | 0.49 |
| - | 206 | 166 | 161 | 132 | 45 | 34 | |||||
| kDNA | + | 528 | 0 | 0.97 | 440 | 0 | 1 | 83 | 5 | 0.96 | |
| - | 19 | 176 | 19 | 141 | 0 | 35 | |||||
| hsp70 | + | 433 | 0 | 0.79 | 366 | 0 | 0.8 | 67 | 0 | 0.76 | |
| - | 114 | 176 | 93 | 141 | 21 | 35 | |||||
| Species | N | % (hsp70+) | % (ID+) |
|---|---|---|---|
| L. braziliensis | 340 | 78.52 | 84.80 |
| L. guyanensis | 43 | 9.93 | 10.70 |
| L. lainsoni | 8 | 1.85 | 2.00 |
| L. shawi | 7 | 1.62 | 1.75 |
| L. amazonensis | 2 | 0.46 | 0.50 |
| L. lindenbergi | 1 | 0.23 | 0.25 |
| Unidentified fragment pattern | 32 | 7.39 | - |
| Patient Information | Parasitological Test | ||||||
|---|---|---|---|---|---|---|---|
| Lesion | Blood | ||||||
| Patient | Time of ML (months) | Previous leishmaniasis/Year | Treatment in the first infection | PCR kDNA | DP | qPCR hsp70 | qPCR hsp70 |
| 1 | 72 | Yes/1979 | Glu/60 doses | + | - | - | + |
| 2 | 7 | Yes/1994 | Incomplete treatment | + | + | + | + |
| 3 | 6 | No | -- | + | - | + | + |
| 4 | 24 | Yes/1974 | Glu/50 doses | + | - | + | - |
| 5 | 6 | Yes/2014 | Glu/109 doses | + | + | + | - |
| 6 | 60 | Yes/ND | Incomplete treatment | + | - | - | - |
| 7 | 24 | Yes/2016 | Glu/32 doses; Anfo/09 doses | + | + | - | + |
| 8 | 0.25 | Yes/2015 | Glu/60 doses | + | - | + | + |
| 9 | 2 | No | -- | + | - | + | - |
| 10 | 36 | Yes/1980 | Glu/20 doses | + | - | + | - |
| 11 | 12 | No | -- | + | - | + | - |
| 12 | 50 | Yes/1988 | Glu/60 doses | + | - | + | - |
| 13 | 0.25 | Yes/2004 | Glu/60 doses | + | - | + | + |
| 14 | 36 | Yes/2017 | Glu/60 doses | + | - | - | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantanhêde, L.M.; Mattos, C.B.; Cruz, A.K.; Ikenohuchi, Y.J.; Fernandes, F.G.; Medeiros, E.H.R.T.; da Silva-Júnior, C.F.; Cupolillo, E.; Ferreira, G.E.M.; Ferreira, R.d.G.M. Overcoming the Negligence in Laboratory Diagnosis of Mucosal Leishmaniasis. Pathogens 2021, 10, 1116. https://doi.org/10.3390/pathogens10091116
Cantanhêde LM, Mattos CB, Cruz AK, Ikenohuchi YJ, Fernandes FG, Medeiros EHRT, da Silva-Júnior CF, Cupolillo E, Ferreira GEM, Ferreira RdGM. Overcoming the Negligence in Laboratory Diagnosis of Mucosal Leishmaniasis. Pathogens. 2021; 10(9):1116. https://doi.org/10.3390/pathogens10091116
Chicago/Turabian StyleCantanhêde, Lilian Motta, Cristiane Batista Mattos, Ana Karoline Cruz, Yoda Janaina Ikenohuchi, Flavia Gonçalves Fernandes, Enmanuella Helga Ratier Terceiro Medeiros, Cipriano Ferreira da Silva-Júnior, Elisa Cupolillo, Gabriel Eduardo Melim Ferreira, and Ricardo de Godoi Mattos Ferreira. 2021. "Overcoming the Negligence in Laboratory Diagnosis of Mucosal Leishmaniasis" Pathogens 10, no. 9: 1116. https://doi.org/10.3390/pathogens10091116
APA StyleCantanhêde, L. M., Mattos, C. B., Cruz, A. K., Ikenohuchi, Y. J., Fernandes, F. G., Medeiros, E. H. R. T., da Silva-Júnior, C. F., Cupolillo, E., Ferreira, G. E. M., & Ferreira, R. d. G. M. (2021). Overcoming the Negligence in Laboratory Diagnosis of Mucosal Leishmaniasis. Pathogens, 10(9), 1116. https://doi.org/10.3390/pathogens10091116







