Screening of Dietary Ingredients against the Honey Bee Parasite Nosema ceranae
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set up
2.2. Production of N. ceranae Spores
2.3. Oral Infection with N. ceranae Spores
2.4. Cultivation of Saccharomyces sp.
2.5. Production of Plant Extracts
2.6. Treatment Administration
2.7. DNA Extraction and qPCR of N. ceranae
2.8. Statistical Analysis
3. Results
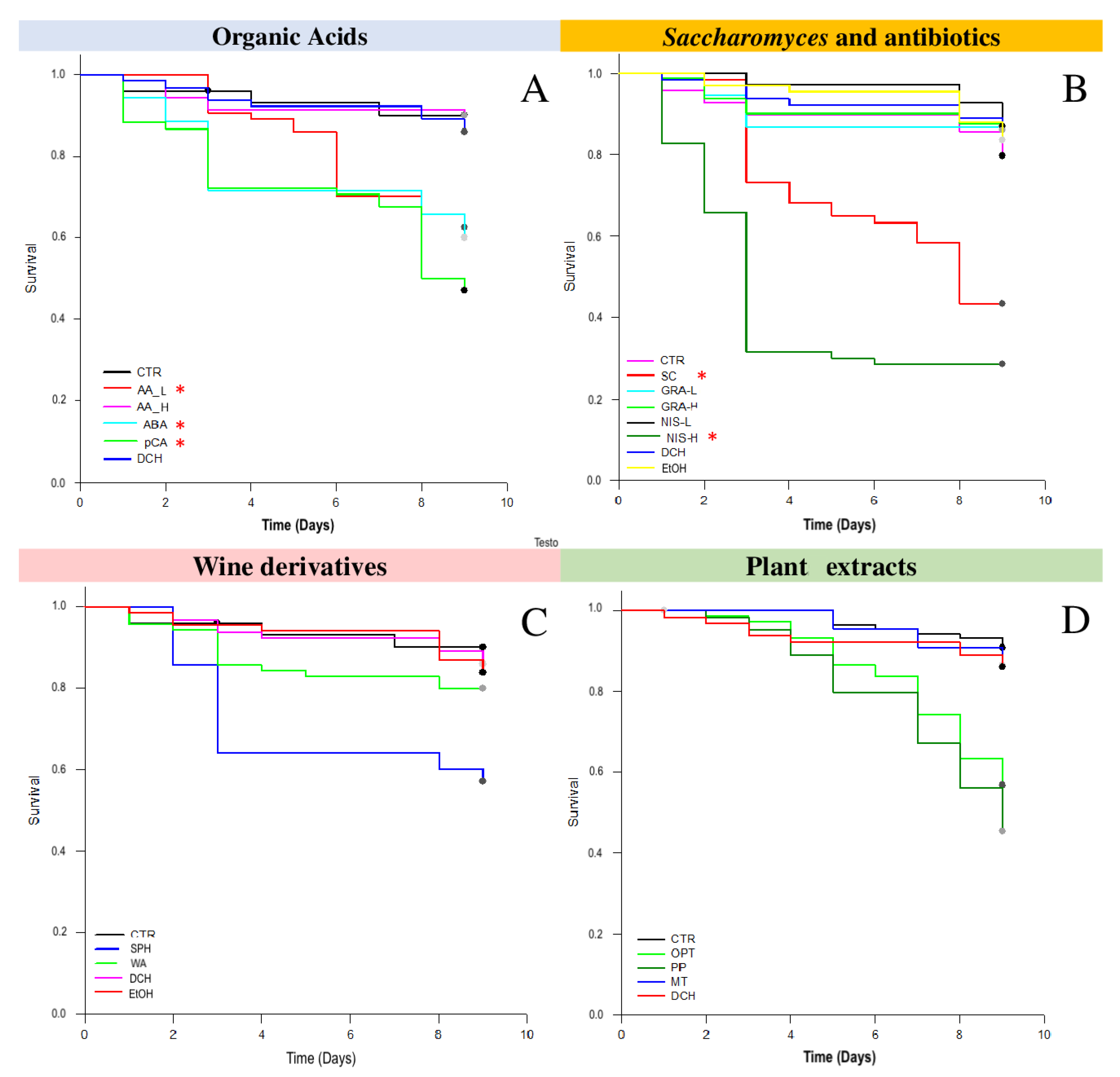
3.1. Survival Tests
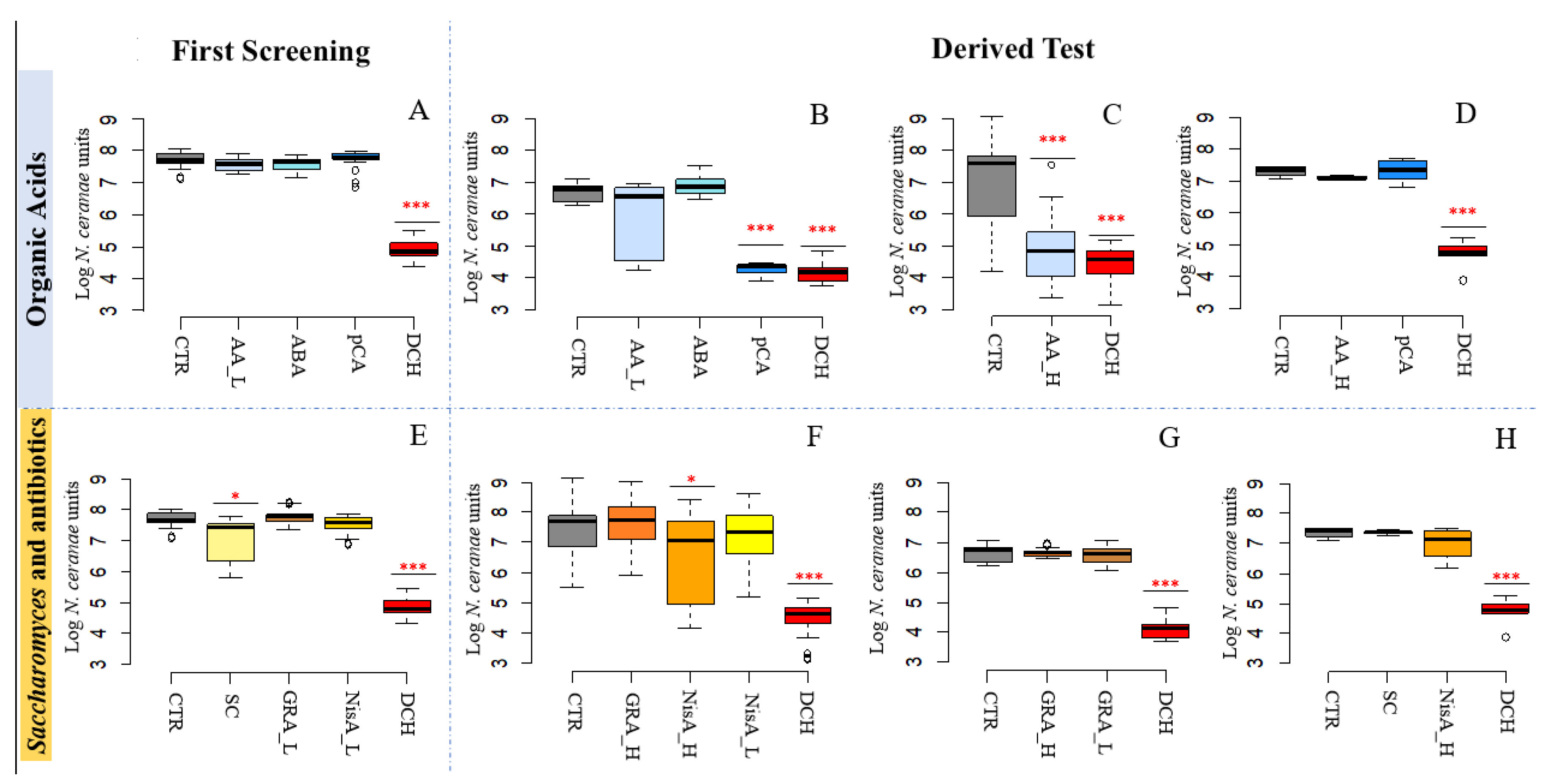
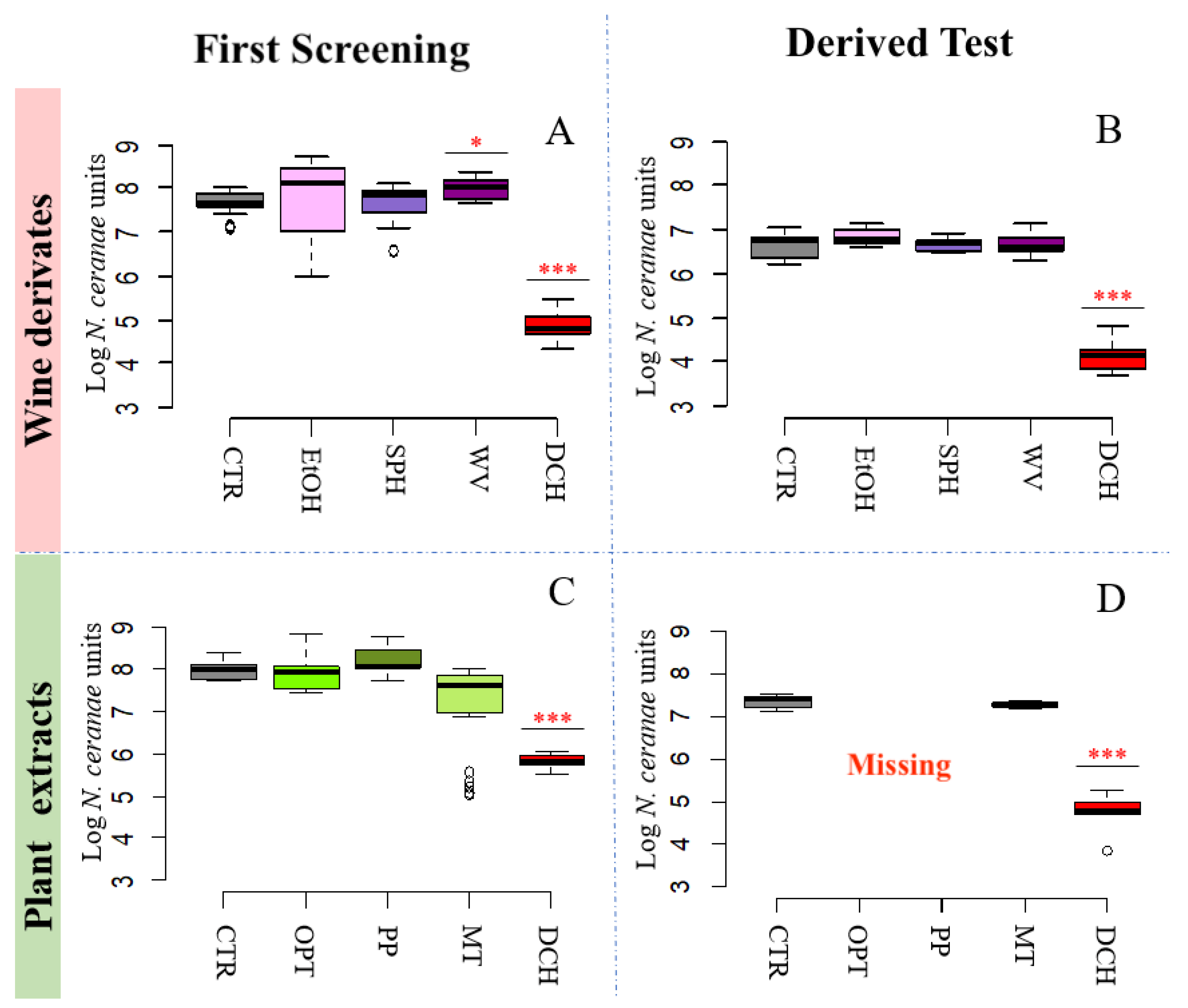
3.2. N. ceranae Quantification
3.2.1. Acetic Acid and p-Coumaric Acid Decrease N. ceranae Units Only in Winter Honeybees
3.2.2. Nisin Has a Potential Effect on N. ceranae
3.2.3. Wine Derivatives and Plant Extracts Do Not Reduce N. ceranae Parasite Development Treatments
4. Discussion
4.1. Organic Acids and Wine Derivatives
4.2. Saccharomyces and Antibiotics
4.3. Plant Extracts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| qPCR | Quantitative-polymerase chain reaction |
| MetAP2 | Methionine aminopeptidase-2 |
| AA_L | Acetic acid low concentration |
| AA_H | Acetic acid high concentration |
| ABA | Abscissic acid |
| pCA | Para-coumaric acid |
| SC | Saccharomyces sp. strain KIA1 |
| GRA_L | Mixture of gramicidin A, B, and C at low concentration |
| GRA_H | Mixture of gramicidin A, B, and C at high concentration |
| NisA_L | Nisin A at low concentration |
| NisA_H | Nisin A at high concentration |
| EtOH | Ethanol |
| SPH | Sulphites |
| WA | Wine vinegar |
| OPT | Opuntia ficus-indica extract |
| PP | Padina pavonica extract |
| MT | Manuka and tea oil mixture |
| DCH | Fumagillin |
| CTR | Control |
| PDB | Potatoes dextrose broth |
| CFU | Colony forming unit |
| RH | Relative humidity |
| PCR | Polymerase chain reaction |
| ANOVA | Analysis of variance |
| NcU | Nosema ceranae units |
References
- Higes, M.; Martín-Hernández, R.; Botías, C.; Garrido-Bailón, E.; González-Porto, A.V.; Barrios, L.; del Nozal, M.; Bernal, J.M.; Jiménez, J.J.; García-Palencia, P.; et al. How natural infection by Nosema ceranae causes honey bee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Huang, W.F.; Jiang, J.H.; Chen, Y.W.; Wang, C.H. A Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie 2007, 38, 30–37. [Google Scholar] [CrossRef]
- Higes, M.; Martin-Hernández, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honey bees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.M.; Rao, K.S.; Chaudhary, O.P. Introduction of Apis mellifera in India. Khadi Gramodyog 1993, 34, 815–819. [Google Scholar]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Tokarev, Y.S.; Huang, W.F.; Solter, L.F.; Malysh, J.M.; Becnel, J.J.; Vossbrinck, C.R. A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J. Invertebr. Pathol. 2020, 169, 107279. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; García-Palencia, P.; Meana, A. Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of foranger honey bees. J. Invert. Pathol. 2008, 97, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Meana, A. Nosema ceranae in Europe: An emergent type C nosemosis. Apidologie 2010, 41, 375–392. [Google Scholar] [CrossRef]
- Graystock, P.; Goulson, D.; Hughes, W.O.H. Parasites in bloom: Flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. 2015, 282, 20151371. [Google Scholar] [CrossRef]
- Genersch, E. Honey bee pathology: Current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–107. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; González-Porto, A.V.; García-Palencia, P.; Meana, A.; Del Nozal, M.J.; Mayo, R.; Bernal, J.L. honey bee colony collapse due to Nosema ceranae in professional apiaries. Environ. Microbiol. Rep. 2009, 1, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Nozal, M.J.; Alvaro, A.; Barrios, L.; Meana, A.; Martín-Hernández, R.; Bernal, J.L.; Bernal, J. The stability and effectiveness of fumagillin in controlling Nosema ceranae (Microsporidia) infection in honey bees (Apis mellifera) under laboratory and field conditions. Apidologie 2011, 42, 364–377. [Google Scholar] [CrossRef]
- Van den Heever, J.P.; Thompson, T.S.; Otto, S.J.G.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Evaluation of Fumagilin-B® and other potential alternative chemotherapies against Nosema ceranae -infected honey bees (Apis mellifera) in cage trial assays. Apidologie 2016, 47, 617–630. [Google Scholar] [CrossRef]
- Bailey, L. Effect of fumagillin upon Nosema apis (Zander). Nature 1953, 171, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Mutinelli, F. European legislation governing the authorization of veterinary medicinal products with particular reference to the use of drugs for the control of honey bee diseases. Apiacta 2003, 38, 156–168. [Google Scholar]
- Sin, N.; Meng, L.; Wang, M.Q.; Wen, J.J.; Bornmann, W.G.; Crews, C.M. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA 1997, 94, 6099–6103. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Jevrosima, S.; Kulić, M.; Stojic, V. Frequency of chromosomal aberrations in the evaluation of genotoxic potential of dicyclohexylamine (fumagillin) in vivo. Acta Vet. 2006, 56. [Google Scholar] [CrossRef]
- Lopez, M.I.; Pettis, J.S.; Smith, I.B.; Chu, P.S. Multiclass determination and confirmation of antibiot- ic residues in honey using LC–MS/MS. J. Agric. Food Chem. 2008, 56, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Pernal, S.F. Stability of dicyclohexylamine and fumagillin in honey. Food Chem. 2015, 179, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.C. Fumagillin Affects Nosema apis and honey bees (Hymenoptera-Apidae). J. Econ. Entomol. 1994, 87, 601–604. [Google Scholar] [CrossRef]
- Huang, W.F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef]
- Abdelkader, F.B.; Çakmak, İ.; Çakmak, S.S.; Nur, Z.; İncebıyık, E.; Aktar, A.; Erdost, H. Toxicity assessment of chronic exposure to common insecticides and bee medications on colony development and drones sperm parameters. Ecotoxicology 2021, 30, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, Y.; Diaz-Cetti, S.; Ramallo, G.; Santos, E.; Porrini, M.; Invernizzi, C. Nosema ceranae winter control: Study of the effectiveness of different fumagillin treatments and consequences on the strength of honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 2017, 110, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Fratini, F.; Tafi, E.; Turchi, B.; Mancini, S.; Sagona, S.; Nanetti, A.; Cerri, D.; Felicioli, A. Microbial profile of the ventriculum of honey bee (Apis mellifera ligustica Spinola, 1806) fed with veterinary drugs, dietary supplements and non-protein amino acids. Vet. Sci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Alberoni, D.; Baffoni, L.; Braglia, C.; Gaggìa, F.; Di Gioia, D. Honeybees Exposure to Natural Feed Additives: How Is the Gut Microbiota Affected? Microorganisms 2021, 9, 1009. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honey bees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Effects of prebiotics and probiotics on honey bees (Apis mellifera) infected with the microsporidian parasite Nosema ceranae. Microorganism 2021, 9, 481. [Google Scholar] [CrossRef]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of Api-Bioxal® and ApiHerb® treatments against Nosema ceranae infection in Apis mellifera investigated by two qPCR methods. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef]
- Porrini, M.P.; Fernández, N.J.; Garrido, P.M.; Gende, L.B.; Medici, S.K.; Eguaras, M.J. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia). Apidologie 2011, 42, 700–707. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, J.H.; Park, J.K. Evaluation of antimicrosporidian activity of plant extracts on Nosema ceranae. J. Apic. Sci. 2016, 60, 167–178. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. J. Invert Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apicult Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Xu, Y.; Gong, H.; Wu, Y.; Chen, Y.; Hu, F.; Zheng, H. Protective potential of Chinese herbal extracts against microsporidian Nosema ceranae, an emergent pathogen of western honey bees, Apis mellifera L. J. Asia Pac. Entomol. 2019, 24, 502–512. [Google Scholar] [CrossRef]
- Nanetti, A.; Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Cardaio, I.; Lazzeri, L. Seed meals from Brassica nigra and Eruca sativa control artificial Nosema ceranae infections in Apis mellifera. Microorganisms 2019, 9, 949. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggìa, F.; Alberoni, D.; Cabbri, R.; Nanetti, A.; Biavati, B.; Di Gioia, D. Effect of dietary supplementation of Bifidobacterium and Lactobacillus strains in Apis mellifera L. against Nosema ceranae. Benef. Microbes 2016, 7, 45–51. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, S.; Rousseau, A.; Lecoeur, A.; Cheaib, B.; Bouslama, S.; Mercier, P.L.; Demey, V.; Castex, M.; Giovenazzo, P.; Derome, N. Deleterious interaction between honey bees (Apis mellifera) and its microsporidian intracellular parasite Nosema ceranae was mitigated by administrating either endogenous or allochthonous gut microbiota strains. Front. Ecol. Evol. 2018, 6, 58. [Google Scholar] [CrossRef]
- Porrini, M.P.; Audisio, M.C.; Sabaté, D.C.; Ibarguren, C.; Medici, S.K.; Sarlo, E.G.; Garrido, M.P.; Eguaras, M.J. Effect of bacterial metabolites on microsporidian Nosema ceranae and on its host Apis mellifera. Parasitol. Res. 2010, 107, 381–388. [Google Scholar] [CrossRef]
- Maggi, M.; Negri, P.; Plischuk, S.; Szawarski, N.; De Piano, F.; De Feudis, L.; Eguaras, M.; Audisio, C. Effects of the organic acids produced by a lactic acid bacterium in Apis mellifera colony development, Nosema ceranae control and fumagillin efficiency. Vet. Microbiol. 2013, 167, 474–483. [Google Scholar] [CrossRef]
- De Piano, F.G.; Maggi, M.; Pellegrini, M.C.; Cugnata, N.M.; Szawarski, N.; Buffa, F.; Negri, P.; Fuselli, S.R.; Audisio, C.M.; Ruffinengo, S.R. Effects of Lactobacillus johnsonii AJ5 metabolites on nutrition, Nosema ceranae development and performance of Apis mellifera. J. Apic. Sci. 2011, 61, 93–104. [Google Scholar] [CrossRef][Green Version]
- Vaillant, J. Nourrissement au sirop de sucre acidifié. La Santé de L´abeille 1989, 110, 55–60. [Google Scholar]
- Forsgren, E.; Fries, I. Acidic-benzoic feed and nosema disease. J. Apia. Sci. 2005, 49, 81–88. [Google Scholar]
- Szawarski, N.; Saez, A.; Domínguez, E.; Dickson, R.; De Matteis, Á.; Eciolaza, C.; Justel, M.; Aliano, A.; Solar, P.; Bergara, I.; et al. Effect of abscisic acid (ABA) combined with two different beekeeping nutritional strategies to confront overwintering: Studies on honey bees’ population dynamics and nosemosis. Insects 2019, 10, 329. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Martínez-Salvador, A.; Garrido-Bailon, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Alaux, C.; Costa, C.; Csáki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; McMahon, D.P.; Medrzycki, P.; et al. Standard methods for maintaining adult Apismellifera in cages under invitro laboratory conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Porrini, M.P.; Garrido, P.M.; Eguaras, M.J. Individual feeding of honey bees: Modification of the Rinderer technique. J. Apic. Res. 2013, 52, 194–195. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.F.; Lahaye, M. Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr. Polym. 2008, 74, 344–352. [Google Scholar] [CrossRef]
- Ribeiro, R.C.D.A.; Barreto, S.M.A.G.; Ostrosky, E.A.; Rocha-Filho, P.A.D.; Veríssimo, L.M.; Ferrari, M. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) Mill extract as moisturizing agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef]
- Baffoni, L.; Alberoni, D.; Gaggìa, F.; Braglia, C.; Stanton, C.; Ross, P.R.; Di Gioia, D. Honeybee exposure to veterinary drugs: How is the gut microbiota affected? Microbiol. Spectr. 2021, 2021, e0017621. [Google Scholar] [CrossRef]
- Huang, W.F.; Solter, L.F. Comparative development and tissue tropism of Nosema apis and Nosema ceranae. J. Invertebr. Pathol. 2013, 113, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Alberoni, D.; Favaro, R.; Baffoni, L.; Angeli, S.; Di Gioia, D. Neonicotinoids in the agroecosystem: In-field long-term assessment on honey bee colony strength and microbiome. Sci. Total Environ. 2021, 762, 144116. [Google Scholar] [CrossRef]
- Cilia, G.; Sagona, S.; Giusti, M.; Dos Santos, P.E.J.; Nanetti, A.; Felicioli, A. Nosema ceranae infection in honey bee samples from Tuscanian Archipelago (Central Italy) investigated by two qPCR methods. Saudi J. Biol. Sci. 2019, 26, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Cabbri, R.; Maiorana, G.; Cardaio, I.; Dall’Olio, R.; Nanetti, A. A Novel TaqMan® assay for Nosema ceranae quantification in honey bee, based on the protein coding gene Hsp70. Eur. J. Protistol. 2018, 63, 44–50. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 23 March 2021).
- Cook, R.D. Influential observations in linear regression. J. Am. Stat. Assoc. 1979, 74, 169–174. [Google Scholar] [CrossRef]
- Pătruică, S.; Bogdan, A.T.; Bura, M.; Popovici, D. Research on the effect of acidifying substances on bee colonies development and health in spring. Agrobuletin AGIR 2011, 2, 124–130. [Google Scholar]
- Pătruică, S.; Dumitrescu, G.; Popescu, R.; Filimon, N.M. The effect of prebiotic and probiotic products used in feed to stimulate the bee colony (Apis mellifera) on intestines of working bees. J. Food. Agricul. Environ. 2013, 11, 2461–2464. [Google Scholar] [CrossRef]
- Frizzera, D.; Fabbro, S.D.; Ortis, G.; Zanni, V.; Bortolomeazzi, R.; Nazzi, F.; Annoscia, D. Possible side effects of sugar supplementary nutrition on honey bee health. Apidologie 2020, 51, 594–608. [Google Scholar] [CrossRef]
- Claing, G.; Dubreuil, P.; Ferland, J.; Bernier, M.; Arsenault, J. Beekeeping management practices in southwestern Quebec. Can. J. Vet. Res. 2019, 1, 103. [Google Scholar]
- Mederle, N.; Kaya, A.; Morariu, S.; CrăCiun, C.; Balint, A.; Radulov, I.; Dărăbuș, G. Innovative therapeutic approaches for the control and prevention of honey bee nosemosis: Implications in the bee’s life and the quality of their products. Geogr. Timisiensis 2017, 26, 31–40. [Google Scholar]
- Brighenti, D.M.; Brighenti, C.R.; Carvalho, C.F. Life spans of Africanized honey bees fed sucrose diets enhanced with citric acid or lemon juice. J. Apicul. Res. 2017, 56, 91–99. [Google Scholar] [CrossRef]
- Forsgren, E.; Fries, I. Acidic Food and Nosema Disease. In Proceedings of the 38th International Apimondia Congress in Ljubljana, Ljubljana, Slovenia, 24–29 August 2003; Volume 488. [Google Scholar]
- Gätschenberger, H.; Azzami, K.; Tautz, J.; Beier, H. Antibacterial immune competence of honey bees (Apis mellifera) is adapted to different life stages and environmental risks. PLoS ONE 2013, 8, e66415. [Google Scholar] [CrossRef]
- Crailsheim, K. Dependence of protein metabolism on age and season in the honey bee (Apis mellifica carnica Pollm). J. Insect Physiol. 1986, 32, 629–634. [Google Scholar] [CrossRef]
- Kešnerová, L.; Emery, O.; Troilo, M.; Liberti, J.; Erkosar, B.; Engel, P. Gut microbiota structure differs between honey bees in winter and summer. ISME J. 2020, 14, 801–814. [Google Scholar] [CrossRef]
- USEPA. Acetic Acid and Sodium Diacetate Interim Registration Review Decision, Docket Number EPA-HQ-OPP-2008-0016. Available online: https://www.regulations.gov/document?D=EPA-HQ-OPP-2008-0016-0017 (accessed on 7 July 2021).
- Theobald, A.; Müller, A.; Anklam, E. Determination of 5- hydroxymethylfurfural in vinegar samples by HLPC. J. Agric. Food Chem. 1998, 46, 1850–1854. [Google Scholar] [CrossRef]
- LeBlanc, B.W.; Eggleston, G.; Sammataro, D.; Cornett, C.; Dufault, R.; Deeby, T.; St. Cyr, E. Formation of hydroxymethylfurfural in domestic high-fructose corn syrup and its toxicity to the honey bee (Apis mellifera). J. Agric. Food Chem. 2009, 57, 7369–7376. [Google Scholar] [CrossRef] [PubMed]
- Zirbes, L.; Nguyen, B.K.; de Graaf, D.C.; de Meulenaer, B.; Reybroeck, W.; Haubruge, E.; Saegerman, C. Hydroxymethylfurfural: A possible emergent cause of honey bee mortality? J. Agric. Food Chem. 2013, 61, 11865–11870. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Borsuk, G.; Mułenko, W.; Olszewski, K. Impact of ethanol on Nosema spp. infected bees. Med. Weter 2013, 69, 736–740. [Google Scholar]
- Hranitz, J.M.; Abramson, C.I.; Carter, R.P. Ethanol increases HSP70 concentrations in honey bee (Apis mellifera L.) brain tissue. Alcohol 2010, 44, 275–282. [Google Scholar] [CrossRef]
- Szawarski, N.; Giménez-Martínez, P.; Mitton, G.; Negri, P.; Meroi-Arcerito, F.; Moliné, M.P.; Fuselli, S.; Eguaras, M.; Lamattina, L.; Maggi, M. Short communication: Antimicrobial activity of indoleacetic, gibberellic and coumaric acids against Paenibacillus larvae and its toxicity against Apis mellifera. Span. J. Agric. Res. 2020, 18, e05SC01. [Google Scholar] [CrossRef]
- Amdam, G.V.; Hartfelder, K.; Norberg, K.; Hagen, A.; Omholt, S.W. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested by the mite Varroa destructor (Acari: Varroidae): A factor in colony loss during over-wintering? J. Econ. Entomol. 2004, 97, 741–747. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Omholt, S.W.; Kryger, P.; Lourenco, A.P.; Bitondi, M.M.G.; Simões, Z.L.P. Higher vitellogenin concentrations in honey bee workers may be an adaptation to life in temperate climates. Insectes Soc. 2005, 52, 316–319. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef] [PubMed]
- Münch, D.; Amdam, G.V.; Wolschin, F. Ageing in a eusocial insect: Molecular and physiological characteristics of life span plasticity in the honey bee. Funct. Ecol. 2008, 22, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R. Within-nest temporal polyethism in the honey bee. Behav. Ecol. Sociobiol. 2008, 62, 777–784. [Google Scholar] [CrossRef]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; HS, A. Dietary phytochemicals, honey bee longevity and pathogen tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef]
- Negri, P.; Maggi, M.; Ramirez, L.; De Feudis, L.; Szwarski, N.; Quintana, S.; Eguaras, M.; Lamattina, L. Abscisic acid enhances the immune response in Apis mellifera and contributes to the colony fitness. Apidologie 2015, 46, 542–557. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honey bee nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
- Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E. Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 342–344. [Google Scholar] [CrossRef]
- Alpatov, V.V.; Kirikova, V.A. Apicultural Abstracts. Bee World 1954, 35, 181–192. [Google Scholar]
- Prenner, E.J.; Lewis, R.N.; McElhaney, R.N. The interaction of the antimicrobial peptide gramicidin S with lipid bilayer model and biological membranes. Biochim. Biophys. Acta-Biomembr. 1999, 1462, 201–221. [Google Scholar] [CrossRef]
- Kessler, N.; Schuhmann, H.; Morneweg, S.; Linne, U.; Marahiel, M.A. The linear pentadecapeptide gramicidin is assembled by four multimodular nonribosomal peptide synthetases that comprise 16 modules with 56 catalytic domains. J. Biol. Chem. 2004, 279, 7413–7419. [Google Scholar] [CrossRef]
- Wang, F.; Qin, L.; Pace, C.J.; Wong, P.; Malonis, R.; Gao, J. Solubilized gramicidin A as potential systemic antibiotics. Chem. BioChem. 2012, 3, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Macdonald, C.A.; Cook, J.; Anderson, I.C.; Singh, B.K. An ecological loop: Host microbiomes across multitrophic interactions. Trends Ecol. Evol. 2019, 34, 1118–1130. [Google Scholar] [CrossRef]
- Abe, T.; Toyokawa, Y.; Sugimoto, Y.; Azuma, H.; Tsukahara, K.; Nasuno, R.; Watanabe, D.; Tsukahara, M.; Takagi, H. Characterization of a new Saccharomyces cerevisiae isolated from hibiscus flower and its mutant with l-leucine accumulation for awamori brewing. J. Front. Genet. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Tauber, J.P.; Nguyen, V.; Lopez, D.; Evans, J.D. Effects of a resident yeast from the honey bee gut on immunity, microbiota, and Nosema disease. Insects 2019, 10, 296. [Google Scholar] [CrossRef]
- Damiani, N.; Fernández, N.J.; Porrini, M.P.; Gende, L.B.; Álvarez, E.; Buffa, F.; Brasesco, C.; Maggi, D.M.; Marcangeli, A.J.; Eguaras, M.J. Laurel leaf extracts for honey bee pest and disease management: Antimicrobial, microsporicidal, and acaricidal activity. Parasit. Res. 2014, 113, 701–709. [Google Scholar] [CrossRef]
- Castillo, S.L.; Heredia, N.; Contreras, J.F.; García, S. Extracts of edible and medicinal plants in inhibition of growth, adherence, and cytotoxin production of Campylobacter jejuni and Campylobacter coli. J. Food Sci. 2011, 76, M421–M426. [Google Scholar] [CrossRef]
- Ismail, A.; Ktari, L.; Ahmed, M.; Bolhuis, H.; Boudabbous, A.; Stal, L.J.; Cretoiu, M.S.; El Bour, M. Antimicrobial activities of bacteria associated with the brown alga Padina pavonica. Front. Microbiol. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed]
| Experimental Theses | Concentration of Ingredient Per Treatment | Reference (When Available) | ||
|---|---|---|---|---|
| Ingredient | Treatment Code | Source or Producers | ||
| Test on Organic Acids | ||||
| Acetic acid lower concentration | AA_L | Acetic Acid; | 84 mM | [40,41] |
| Acetic acid higher concentration | AA_H | Merck | 0.35 M | [40,41] |
| Abscisic acid | ABA | S-(+)-Abscisic Acid Fanda Chem | 50 µM | [42] |
| p-Coumaric acid | pCA | trans-4-Hydroxycinnamic acid; Merck | 31.4 µM | [43] |
| Test onSaccharomycesand Antibiotics | ||||
| Saccharomycessp. strain KIA1 | SC | Isolated by authors from soil | 10 CFU/mL | - |
| Gramicidin D lower concentration | GRA_L | Gramicidin from Bacillus aneurinolyticus; Merck | 7.7 mM | - |
| Gramicidin D higher concentration | GRA_H | Gramicidin from Bacillus aneurinolyticus; Merck | 15.4 mM | - |
| Nisin lower concentration | NisA_L | Nisin from Lactococcus lactis Merck | 7.45 mM | - |
| Nisin higher concentration | NisA_H | Nisin from Lactococcus lactis Merck | 74.5 mM | - |
| Test on Wine Derivatives | ||||
| Ethanol | EtOH | Ethanol; Carlo Erba Reagents | 0.69 M | - |
| Wine Sulphites (precipitates of potassium pyrosulfite) | SPH | Produced from red wine by a local winemaker and gifted | 4 mM | - |
| Wine vinegar | WA | Produced from red wine by a local winemaker and gifted | 0.3 M | - |
| Test on Plant Extracts | ||||
| Extract ofOpuntia ficus-indica | OPT | Produced by authors | 0.005 µL/mL | - |
| Extract ofPadina pavonica | PP | Produced by authors | 0.005 µL/mL | - |
| Steam distilled Manuka and Tea tree essential oil | MT | Optima Naturalis and ESI s.r.l., respectively | 0.75 µL/mL + 0.1 µL/mL | - |
| Positive and Negative Controls (included in all tests) | ||||
| Fumagillin | DCH | Fumagilin-B; Medivet Ltd. | 2.59 mM a | Medivet Ltd. guidelines |
| Untreated control | CTR | - | - | - |
| Reference Figure | Log NcU ± St.Dev in [CTR] | Experimental Conditions | Log NcU ± St.Dev | p-Value |
|---|---|---|---|---|
| ORGANIC ACIDS | ||||
| First Test | ||||
| 2A | 7.69 ± 0.24 | Acetic Acid_Low [AA_L] | 7.57 ± 0.19 | |
| Abscissic acid [ABA] | 7.55 ± 0.21 | |||
| Para-coumaric acid [pCA] | 7.69 ± 0.29 | |||
| Fumagillin [DCH] | 4.86 ± 0.29 | *** | ||
| Derived Test | ||||
| 2B | 6.64 ± 0.30 | Acetic Acid_Low [AA_L] | 5.90 ± 1.20 | |
| Abscissic acid [ABA] | 6.86 ± 0.28 | |||
| Para-coumaric acid [pCA] | 4.23 ± 0.26 | *** | ||
| Fumagillin [DCH] | 4.11 ± 0.32 | *** | ||
| 2C | 7.08 ± 1.40 | Acetic Acid_High [AA_H] | 4.39 ± 1.20 | *** |
| Fumagillin [DCH] | 4.27 ± 0.91 | *** | ||
| 2D | 7.36 ± 0.18 | Acetic Acid_High [AA_H] | 7.15 ± 0.07 | |
| Para-coumaric acid [pCA] | 7.36 ± 0.36 | |||
| Fumagillin [DCH] | 4.72 ± 0.47 | *** | ||
| SaccharomycesAND ANTIBIOTICS | ||||
| First Test | ||||
| 2E | 7.42 ± 0.24 | Saccharomyces sp. strain KIA1 [SC] | 7.13 ± 0.65 | *** |
| mix, Low concentration [GRA_L] | 7.78 ± 0.25 | |||
| Nisin A, Low concentration [NisA_L] | 7.53 ± 0.27 | |||
| Fumagillin [DCH] | 4.86 ± 0.29 | |||
| Derived Test | ||||
| 2F | 7.42 ± 0.24 | Gramicidin mix, High concentration [GRA_H] | 7.65 ± 0.85 | |
| Nisin A, High concentration [NisA_H] | 6.45 ± 1.48 | *** | ||
| Nisin A, Low concentration [NisA_L] | 7.18 ± 0.99 | |||
| Fumagillin [DCH] | 4.51 ± 0.58 | *** | ||
| 2G | 6.64 ± 0.28 | mix, High concentration [GRA_H] | 6.67 ± 0.14 | |
| mix, Low concentration [GRA_L] | 6.57 ± 0.30 | |||
| Fumagillin [DCH_A] | 4.11 ± 0.32 | *** | ||
| 2H | 7.36 ± 0.18 | Saccharomyces sp. strain KIA1 [SC] | 7.35 ± 0.09 | |
| Nisin A, High concentration [NisA_H] | 6.99 ± 0.59 | |||
| Fumagillin [DCH] | 4.72 ± 0.47 | *** | ||
| WINE DERIVATES | ||||
| First Test | ||||
| 3A | 7.69 ± 0.24 | Ethanol [EtOH] | 7.78 ± 0.99 | |
| Sulphites [SPH] | 7.68 ± 0.40 | |||
| Wine vinegar [WA] | 7.99 ± 0.24 | *** | ||
| Fumagillin [DCH] | 4.86 ± 0.29 | *** | ||
| Derived Test | ||||
| 3B | 6.64 ± 0.31 | Ethanol [EtOH] | 6.83 ± 0.18 | |
| Sulphites [SPH] | 6.69 ± 0.16 | |||
| Wine vinegar [WA] | 6.67 ± 0.24 | |||
| Fumagillin [DCH] | 4.11 ± 0.32 | *** | ||
| PLANT EXTRACTS | ||||
| First Test | ||||
| 3C | 7.97 ± 0.24 | Opuntia ficus-indica extract [OPT] | 7.92 ± 0.43 | |
| Padina pavonica extract [PP] | 8.20 ± 0.32 | |||
| Manuka and tea oil [MT] | 7.12 ± 1.06 | |||
| Fumagillin [DCH] | 5.82 ± 0.20 | *** | ||
| Derived Test | ||||
| 3D | 7.36 ± 0.18 | Opuntia ficus-indica extract [OPT] | - | |
| Padina pavonica extract [PP] | - | |||
| Manuka and tea oil [MT] | 7.28 ± 0.11 | |||
| Fumagillin [DCH] | 4.72 ± 0.47 | *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braglia, C.; Alberoni, D.; Porrini, M.P.; Garrido, P.M.; Baffoni, L.; Di Gioia, D. Screening of Dietary Ingredients against the Honey Bee Parasite Nosema ceranae. Pathogens 2021, 10, 1117. https://doi.org/10.3390/pathogens10091117
Braglia C, Alberoni D, Porrini MP, Garrido PM, Baffoni L, Di Gioia D. Screening of Dietary Ingredients against the Honey Bee Parasite Nosema ceranae. Pathogens. 2021; 10(9):1117. https://doi.org/10.3390/pathogens10091117
Chicago/Turabian StyleBraglia, Chiara, Daniele Alberoni, Martin Pablo Porrini, Paula Melisa Garrido, Loredana Baffoni, and Diana Di Gioia. 2021. "Screening of Dietary Ingredients against the Honey Bee Parasite Nosema ceranae" Pathogens 10, no. 9: 1117. https://doi.org/10.3390/pathogens10091117
APA StyleBraglia, C., Alberoni, D., Porrini, M. P., Garrido, P. M., Baffoni, L., & Di Gioia, D. (2021). Screening of Dietary Ingredients against the Honey Bee Parasite Nosema ceranae. Pathogens, 10(9), 1117. https://doi.org/10.3390/pathogens10091117










