Alphavirus-Induced Membrane Rearrangements during Replication, Assembly, and Budding
Abstract
1. Introduction
2. Overview of the Alphavirus Lifecycle
3. Replication-Induced Membrane Rearrangements
3.1. Alphavirus Replication Proteins
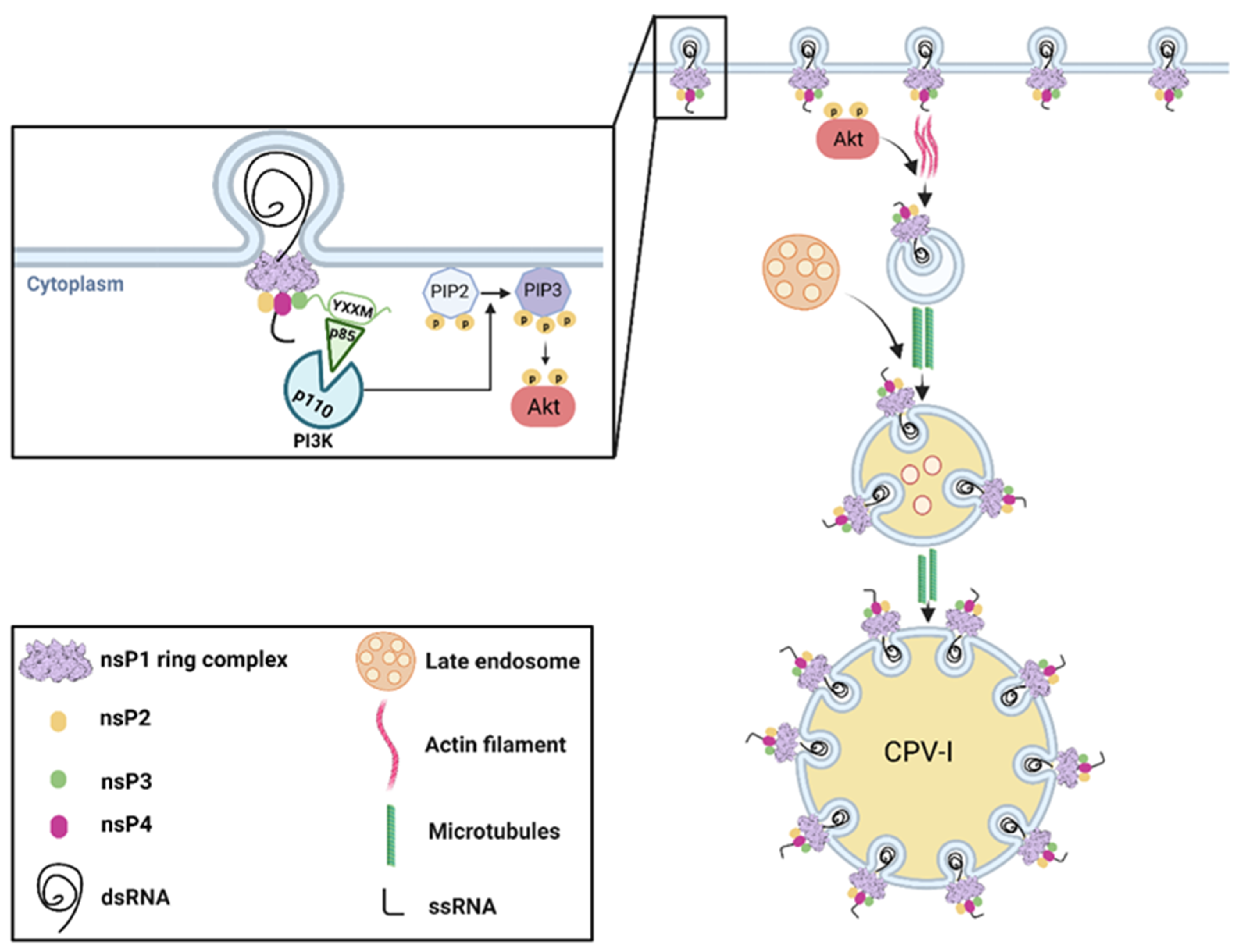
3.2. nsP1-Mediated Membrane Anchorage of the Replication Complex
3.3. Viral and Host Factors Involved in the Formation of Replication Spherules
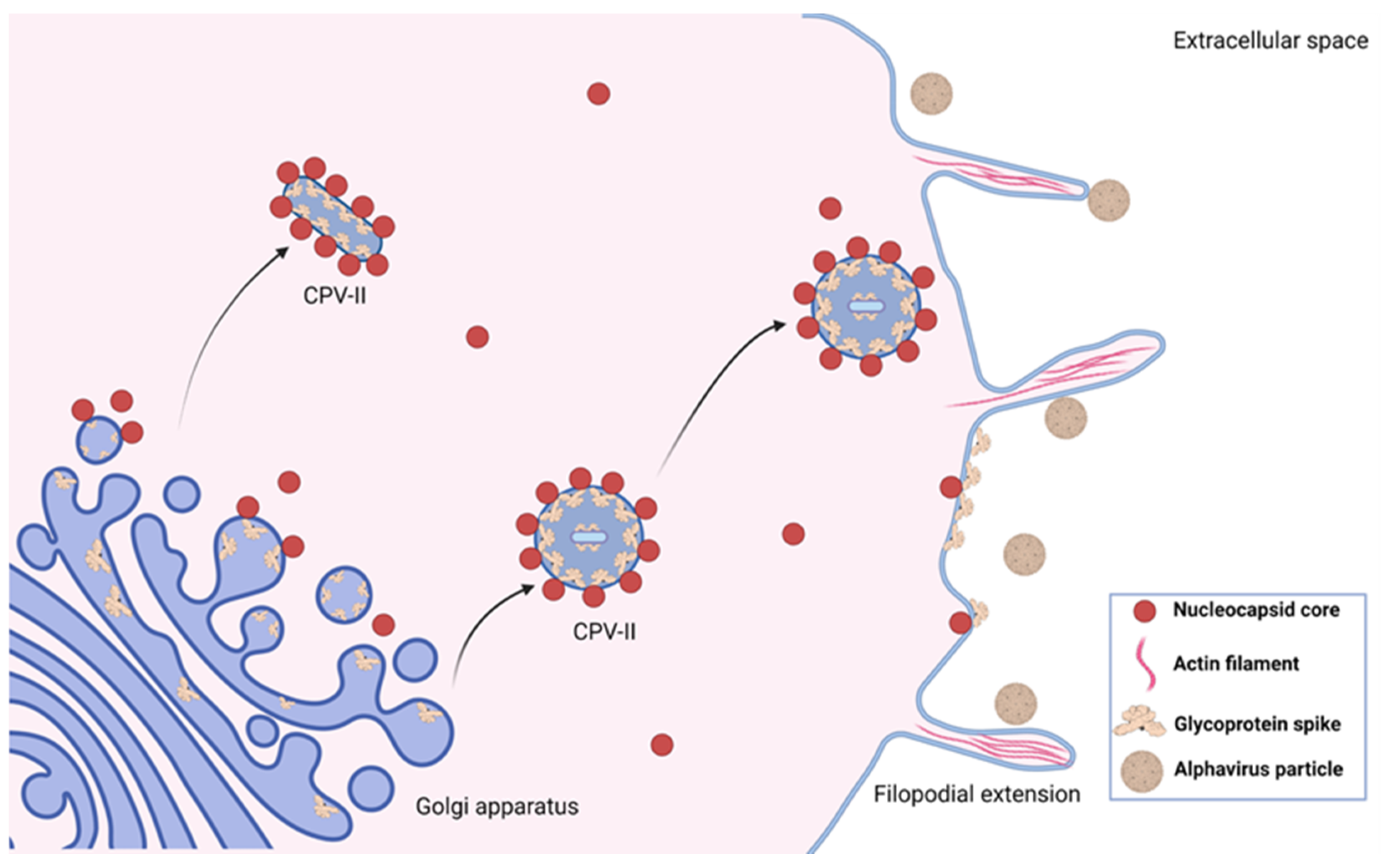
4. Assembly Induced Membrane Rearrangements
5. Filopodial Extensions
6. Alphavirus-Induced Structural Modifications in Mosquito Cells
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, R.; Mukhopadhyay, S.; Merits, A.; Bolling, B.; Nasar, F.; Coffey, L.L.; Powers, A.; Weaver, S.C.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Togaviridae. J. Gen. Virol. 2018, 99, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to Virus Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2019). Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Snyder, J.E.; Kuhn, R.J. A Structural and Functional Perspective of Alphavirus Replication and Assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Watts, D.M.; Russell, K.L.; Damodaran, C.; Calampa, C.; Cabezas, C.; Ramirez, G.; Vasquez, B.; Hayes, C.G.; Rossi, C.A.; et al. Mayaro Virus Disease: An Emerging Mosquito-Borne Zoonosis in Tropical South America. Clin. Infect. Dis. 1999, 28, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.-C.; Carrera, J.-P.; Liria, J.; Auguste, A.J.; Weaver, S.C. Alphaviruses in Latin America and the Introduction of Chikungunya Virus. In Human Virology in Latin America: From Biology to Control; Ludert, J.E., Pujol, F.H., Arbiza, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 169–192. ISBN 978-3-319-54567-7. [Google Scholar]
- Brown, R.S.; Wan, J.J.; Kielian, M. The Alphavirus Exit Pathway: What We Know and What We Wish We Knew. Viruses 2018, 10, 89. [Google Scholar] [CrossRef]
- Snyder, J.E.; Kulcsar, K.A.; Schultz, K.L.W.; Riley, C.P.; Neary, J.T.; Marr, S.; Jose, J.; Griffin, D.E.; Kuhn, R.J. Functional Characterization of the Alphavirus TF Protein. J. Virol. 2013, 87, 8511–8523. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. The Alphaviruses: Gene Expression, Replication, and Evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Holmes, A.C.; Basore, K.; Fremont, D.H.; Diamond, M.S. A Molecular Understanding of Alphavirus Entry. PLoS Pathog. 2020, 16, e1008876. [Google Scholar] [CrossRef]
- Glycoprotein Organization of Chikungunya Virus Particles Revealed by X-Ray Crystallography | Nature. Available online: https://www.nature.com/articles/nature09555 (accessed on 15 June 2021).
- Basore, K.; Kim, A.S.; Nelson, C.A.; Zhang, R.; Smith, B.K.; Uranga, C.; Vang, L.; Cheng, M.; Gross, M.L.; Smith, J.; et al. Cryo-EM Structure of Chikungunya Virus in Complex with the Mxra8 Receptor. Cell 2019, 177, 1725–1737.e16. [Google Scholar] [CrossRef]
- Song, H.; Zhao, Z.; Chai, Y.; Jin, X.; Li, C.; Yuan, F.; Liu, S.; Gao, Z.; Wang, H.; Song, J.; et al. Molecular Basis of Arthritogenic Alphavirus Receptor MXRA8 Binding to Chikungunya Virus Envelope Protein. Cell 2019, 177, 1714–1724.e12. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, A.P.; Griffin, D.E. Large-Plaque Mutants of Sindbis Virus Show Reduced Binding to Heparan Sulfate, Heightened Viremia, and Slower Clearance from the Circulation. J. Virol. 2000, 74, 644–651. [Google Scholar] [CrossRef]
- Tanaka, A.; Tumkosit, U.; Nakamura, S.; Motooka, D.; Kishishita, N.; Priengprom, T.; Sa-Ngasang, A.; Kinoshita, T.; Takeda, N.; Maeda, Y. Genome-Wide Screening Uncovers the Significance of N-Sulfation of Heparan Sulfate as a Host Cell Factor for Chikungunya Virus Infection. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Gardner, C.L.; Ebel, G.D.; Ryman, K.D.; Klimstra, W.B. Heparan Sulfate Binding by Natural Eastern Equine Encephalitis Viruses Promotes Neurovirulence. Proc. Natl. Acad. Sci. USA 2011, 108, 16026–16031. [Google Scholar] [CrossRef]
- Ryman, K.D.; Gardner, C.L.; Burke, C.W.; Meier, K.C.; Thompson, J.M.; Klimstra, W.B. Heparan Sulfate Binding Can Contribute to the Neurovirulence of Neuroadapted and Nonneuroadapted Sindbis Viruses. J. Virol. 2007, 81, 3563–3573. [Google Scholar] [CrossRef]
- Ferguson, M.C.; Saul, S.; Fragkoudis, R.; Weisheit, S.; Cox, J.; Patabendige, A.; Sherwood, K.; Watson, M.; Merits, A.; Fazakerley, J.K. Ability of the Encephalitic Arbovirus Semliki Forest Virus To Cross the Blood-Brain Barrier Is Determined by the Charge of the E2 Glycoprotein. J. Virol. 2015, 89, 7536–7549. [Google Scholar] [CrossRef] [PubMed]
- Froelich, S.; Tai, A.; Kennedy, K.; Zubair, A.; Wang, P. Pseudotyping Lentiviral Vectors with Aura Virus Envelope Glycoproteins for DC-SIGN–Mediated Transduction of Dendritic Cells. Hum. Gene Ther. 2011, 22, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, W.B.; Nangle, E.M.; Smith, M.S.; Yurochko, A.D.; Ryman, K.D. DC-SIGN and L-SIGN Can Act as Attachment Receptors for Alphaviruses and Distinguish between Mosquito Cell- and Mammalian Cell-Derived Viruses. J. Virol. 2003, 77, 12022–12032. [Google Scholar] [CrossRef] [PubMed]
- Jemielity, S.; Wang, J.J.; Chan, Y.K.; Ahmed, A.A.; Li, W.; Monahan, S.; Bu, X.; Farzan, M.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-Family Proteins Promote Infection of Multiple Enveloped Viruses through Virion-Associated Phosphatidylserine. PLoS Pathog. 2013, 9, e1003232. [Google Scholar] [CrossRef]
- Moller-Tank, S.; Kondratowicz, A.S.; Davey, R.A.; Rennert, P.D.; Maury, W. Role of the Phosphatidylserine Receptor TIM-1 in Enveloped-Virus Entry. J. Virol. 2013, 87, 8327–8341. [Google Scholar] [CrossRef]
- Vancini, R.; Hernandez, R.; Brown, D. Alphavirus Entry into Host Cells. Prog. Mol. Biol. Transl. Sci. 2015, 129, 33–62. [Google Scholar] [CrossRef]
- Rose, P.P.; Hanna, S.L.; Spiridigliozzi, A.; Wannissorn, N.; Beiting, D.P.; Ross, S.R.; Hardy, R.W.; Bambina, S.A.; Heise, M.T.; Cherry, S. Natural Resistance-Associated Macrophage Protein Is a Cellular Receptor for Sindbis Virus in Both Insect and Mammalian Hosts. Cell Host Microbe 2011, 10, 97–104. [Google Scholar] [CrossRef]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 Is a Receptor for Multiple Arthritogenic Alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef]
- Zhang, R.; Hryc, C.F.; Cong, Y.; Liu, X.; Jakana, J.; Gorchakov, R.; Baker, M.L.; Weaver, S.C.; Chiu, W. 4.4 Å Cryo-EM Structure of an Enveloped Alphavirus Venezuelan Equine Encephalitis Virus. EMBO J. 2011, 30, 3854–3863. [Google Scholar] [CrossRef]
- Wu, S.-R.; Haag, L.; Hammar, L.; Wu, B.; Garoff, H.; Xing, L.; Murata, K.; Cheng, R.H. The Dynamic Envelope of a Fusion Class II Virus: PREFUSION STAGES OF SEMLIKI FOREST VIRUS REVEALED BY ELECTRON CRYOMICROSCOPY. J. Biol. Chem. 2007, 282, 6752–6762. [Google Scholar] [CrossRef] [PubMed]
- Roussel, A.; Lescar, J.; Vaney, M.-C.; Wengler, G.; Wengler, G.; Rey, F.A. Structure and Interactions at the Viral Surface of the Envelope Protein E1 of Semliki Forest Virus. Structure 2006, 14, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Kielian, M.; Chanel-Vos, C.; Liao, M. Alphavirus Entry and Membrane Fusion. Viruses 2010, 2, 796–825. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Fuchs, R.; Helenius, A. Acidification of the Endocytic and Exocytic Pathways. Annu. Rev. Biochem. 1986, 55, 663–700. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Fuchs, R.; Kielian, M.; Helenius, A.; Mellman, I. Acidification of Endosome Subpopulations in Wild-Type Chinese Hamster Ovary Cells and Temperature-Sensitive Acidification-Defective Mutants. J. Cell Biol. 1989, 108, 1291–1300. [Google Scholar] [CrossRef]
- Helenius, A.; Kartenbeck, J.; Simons, K.; Fries, E. On the Entry of Semliki Forest Virus into BHK-21 Cells. J. Cell Biol. 1980, 84, 404–420. [Google Scholar] [CrossRef] [PubMed]
- PH-Induced Alterations in the Fusogenic Spike Protein of Semliki Forest Virus. J. Cell Biol. 1985, 101, 2284–2291. [CrossRef] [PubMed]
- Justman, J.; Klimjack, M.R.; Kielian, M. Role of Spike Protein Conformational Changes in Fusion of Semliki Forest Virus. J. Virol. 1993, 67, 7597–7607. [Google Scholar] [CrossRef]
- Bron, R.; Wahlberg, J.M.; Garoff, H.; Wilschut, J. Membrane Fusion of Semliki Forest Virus in a Model System: Correlation between Fusion Kinetics and Structural Changes in the Envelope Glycoprotein. EMBO J. 1993, 12, 693–701. [Google Scholar] [CrossRef]
- Klimjack, M.R.; Jeffrey, S.; Kielian, M. Membrane and Protein Interactions of a Soluble Form of the Semliki Forest Virus Fusion Protein. J. Virol. 1994, 68, 6940–6946. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; Bittman, R.; Wilschut, J. Low-PH-Dependent Fusion of Sindbis Virus with Receptor-Free Cholesterol- and Sphingolipid-Containing Liposomes. J. Virol. 1999, 73, 8476–8484. [Google Scholar] [CrossRef]
- White, J.; Helenius, A. PH-Dependent Fusion between the Semliki Forest Virus Membrane and Liposomes. Proc. Natl. Acad. Sci. USA 1980, 77, 3273–3277. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, J.M.; Bron, R.; Wilschut, J.; Garoff, H. Membrane Fusion of Semliki Forest Virus Involves Homotrimers of the Fusion Protein. J. Virol. 1992, 66, 7309–7318. [Google Scholar] [CrossRef]
- Wahlberg, J.M.; Garoff, H. Membrane Fusion Process of Semliki Forest Virus. I: Low PH-Induced Rearrangement in Spike Protein Quaternary Structure Precedes Virus Penetration into Cells. J. Cell Biol. 1992, 116, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Lanzrein, M.; Weingart, R.; Kempf, C. PH-Dependent Pore Formation in Semliki Forest Virus-Infected Aedes Albopictus Cells. Virology 1993, 193, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Omar, A.; Jentsch, P.; Morell, A.; Kempf, C. Semliki Forest Virus Envelope Proteins Function as Proton Channels. Biosci. Rep. 1991, 11, 243–255. [Google Scholar] [CrossRef]
- Waarts, B.-L.; Smit, J.M.; Aneke, O.J.C.; McInerney, G.M.; Liljeström, P.; Bittman, R.; Wilschut, J. Reversible Acid-Induced Inactivation of the Membrane Fusion Protein of Semliki Forest Virus. J. Virol. 2005, 79, 7942–7948. [Google Scholar] [CrossRef][Green Version]
- Sousa, I.P.; Carvalho, C.A.M.; Mendes, Y.S.; Weissmuller, G.; Oliveira, A.C.; Gomes, A.M.O. Fusion of a New World Alphavirus with Membrane Microdomains Involving Partially Reversible Conformational Changes in the Viral Spike Proteins. Biochemistry 2017, 56, 5823–5830. [Google Scholar] [CrossRef]
- Glanville, N.; Ranki, M.; Morser, J.; Kääriäinen, L.; Smith, A.E. Initiation of Translation Directed by 42S and 26S RNAs from Semliki Forest Virus in Vitro. Proc. Natl. Acad. Sci. USA 1976, 73, 3059–3063. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Helenius, A. Role of Ribosomes in Semliki Forest Virus Nucleocapsid Uncoating. J. Virol. 1992, 66, 7049–7058. [Google Scholar] [CrossRef] [PubMed]
- Wengler, G.; Würkner, D.; Wengler, G. Identification of a Sequence Element in the Alphavirus Core Protein Which Mediates Interaction of Cores with Ribosomes and the Disassembly of Cores. Virology 1992, 191, 880–888. [Google Scholar] [CrossRef]
- Pietilä, M.K.; Hellström, K.; Ahola, T. Alphavirus Polymerase and RNA Replication. Virus Res. 2017, 234, 44–57. [Google Scholar] [CrossRef]
- Kallio, K.; Hellström, K.; Jokitalo, E.; Ahola, T. RNA Replication and Membrane Modification Require the Same Functions of Alphavirus Nonstructural Proteins. J. Virol. 2016, 90, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Rupp, J.C.; Sokoloski, K.J.; Gebhart, N.N.; Hardy, R.W. Alphavirus RNA Synthesis and Non-Structural Protein Functions. J. Gen. Virol. 2015, 96, 2483–2500. [Google Scholar] [CrossRef] [PubMed]
- Grimley, P.M.; Berezesky, I.K.; Friedman, R.M. Cytoplasmic Structures Associated with an Arbovirus Infection: Loci of Viral Ribonucleic Acid Synthesis. J. Virol. 1968, 2, 1326–1338. [Google Scholar] [CrossRef]
- Friedman, R.M.; Levin, J.G.; Grimley, P.M.; Berezesky, I.K. Membrane-Associated Replication Complex in Arbovirus Infection. J. Virol. 1972, 10, 504–515. [Google Scholar] [CrossRef]
- Pietilä, M.K.; van Hemert, M.J.; Ahola, T. Purification of Highly Active Alphavirus Replication Complexes Demonstrates Altered Fractionation of Multiple Cellular Membranes. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Nicola, A.V.; Chen, W.; Helenius, A. Co-Translational Folding of an Alphavirus Capsid Protein in the Cytosol of Living Cells. Nat. Cell Biol. 1999, 1, 341–345. [Google Scholar] [CrossRef]
- Choi, H.K.; Tong, L.; Minor, W.; Dumas, P.; Boege, U.; Rossmann, M.G.; Wengler, G. Structure of Sindbis Virus Core Protein Reveals a Chymotrypsin-like Serine Proteinase and the Organization of the Virion. Nature 1991, 354, 37–43. [Google Scholar] [CrossRef]
- Choi, H.K.; Lu, G.; Lee, S.; Wengler, G.; Rossmann, M.G. Structure of Semliki Forest Virus Core Protein. Proteins 1997, 27, 345–359. [Google Scholar] [CrossRef]
- Frolova, E.; Frolov, I.; Schlesinger, S. Packaging Signals in Alphaviruses. J. Virol. 1997, 71, 248–258. [Google Scholar] [CrossRef] [PubMed]
- White, C.L.; Thomson, M.; Dimmock, N.J. Deletion Analysis of a Defective Interfering Semliki Forest Virus RNA Genome Defines a Region in the NsP2 Sequence That Is Required for Efficient Packaging of the Genome into Virus Particles. J. Virol. 1998, 72, 4320–4326. [Google Scholar] [CrossRef] [PubMed]
- Frolov, I.; Frolova, E.; Schlesinger, S. Sindbis Virus Replicons and Sindbis Virus: Assembly of Chimeras and of Particles Deficient in Virus RNA. J. Virol. 1997, 71, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Garoff, H.; Huylebroeck, D.; Robinson, A.; Tillman, U.; Liljeström, P. The Signal Sequence of the P62 Protein of Semliki Forest Virus Is Involved in Initiation but Not in Completing Chain Translocation. J. Cell Biol. 1990, 111, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Erdei, S.; Keränen, S.; Saraste, J.; Kääriäinen, L. Evidence for a Separate Signal Sequence for the Carboxy-Terminal Envelope Glycoprotein E1 of Semliki Forest Virus. J. Virol. 1981, 38, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Anthony, R.P.; Paredes, A.M.; Brown, D.T. Disulfide Bonds Are Essential for the Stability of the Sindbis Virus Envelope. Virology 1992, 190, 330–336. [Google Scholar] [CrossRef]
- Mulvey, M.; Brown, D.T. Formation and Rearrangement of Disulfide Bonds during Maturation of the Sindbis Virus E1 Glycoprotein. J. Virol. 1994, 68, 805–812. [Google Scholar] [CrossRef]
- Pletnev, S.V.; Zhang, W.; Mukhopadhyay, S.; Fisher, B.R.; Hernandez, R.; Brown, D.T.; Baker, T.S.; Rossmann, M.G.; Kuhn, R.J. Locations of Carbohydrate Sites on Alphavirus Glycoproteins Show That E1 Forms an Icosahedral Scaffold. Cell 2001, 105, 127–136. [Google Scholar] [CrossRef]
- Ivanova, L.; Schlesinger, M.J. Site-Directed Mutations in the Sindbis Virus E2 Glycoprotein Identify Palmitoylation Sites and Affect Virus Budding. J. Virol. 1993, 67, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Sefton, B.M. Immediate Glycosylation of Sindbis Virus Membrane Proteins. Cell 1977, 10, 659–668. [Google Scholar] [CrossRef]
- Sánchez-San Martín, C.; Liu, C.Y.; Kielian, M. Dealing with Low PH: Entry and Exit of Alphaviruses and Flaviviruses. Trends Microbiol. 2009, 17, 514–521. [Google Scholar] [CrossRef]
- Zhang, X.; Kielian, M. Mutations That Promote Furin-Independent Growth of Semliki Forest Virus Affect P62-E1 Interactions and Membrane Fusion. Virology 2004, 327, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fugère, M.; Day, R.; Kielian, M. Furin Processing and Proteolytic Activation of Semliki Forest Virus. J. Virol. 2003, 77, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lindqvist, B.; Garoff, H.; von Bonsdorff, C.H.; Liljeström, P. A Tyrosine-Based Motif in the Cytoplasmic Domain of the Alphavirus Envelope Protein Is Essential for Budding. EMBO J. 1994, 13, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Soonsawad, P.; Xing, L.; Milla, E.; Espinoza, J.M.; Kawano, M.; Marko, M.; Hsieh, C.; Furukawa, H.; Kawasaki, M.; Weerachatyanukul, W.; et al. Structural Evidence of Glycoprotein Assembly in Cellular Membrane Compartments Prior to Alphavirus Budding. J. Virol. 2010, 84, 11145–11151. [Google Scholar] [CrossRef]
- Lu, Y.E.; Eng, C.H.; Shome, S.G.; Kielian, M. In Vivo Generation and Characterization of a Soluble Form of the Semliki Forest Virus Fusion Protein. J. Virol. 2001, 75, 8329–8339. [Google Scholar] [CrossRef]
- Lu, Y.E.; Kielian, M. Semliki Forest Virus Budding: Assay, Mechanisms, and Cholesterol Requirement. J. Virol. 2000, 74, 7708–7719. [Google Scholar] [CrossRef]
- Suomalainen, M.; Liljeström, P.; Garoff, H. Spike Protein-Nucleocapsid Interactions Drive the Budding of Alphaviruses. J. Virol. 1992, 66, 4737–4747. [Google Scholar] [CrossRef]
- Duffus, W.A.; Levy-Mintz, P.; Klimjack, M.R.; Kielian, M. Mutations in the Putative Fusion Peptide of Semliki Forest Virus Affect Spike Protein Oligomerization and Virus Assembly. J. Virol. 1995, 69, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, M.; Garoff, H. Interactions between the Transmembrane Segments of the Alphavirus E1 and E2 Proteins Play a Role in Virus Budding and Fusion. J. Virol. 2003, 77, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Byrd, E.A.; Kielian, M. An Alphavirus E2 Membrane-Proximal Domain Promotes Envelope Protein Lateral Interactions and Virus Budding. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Von Bonsdorff, C.H.; Harrison, S.C. Sindbis Virus Glycoproteins Form a Regular Icosahedral Surface Lattice. J. Virol. 1975, 16, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, H.V.; Coimbra, L.D.; Cassago, A.; Rocha, R.P.F.; da Silva Guerra, J.V.; de Felicio, R.; Carnieli, C.M.; Leme, L.; Padilha, A.C.; Paes Leme, A.F.; et al. Cryo-EM Structure of the Mature and Infective Mayaro Virus at 4.4 Å Resolution Reveals Features of Arthritogenic Alphaviruses. Nat. Commun. 2021, 12, 3038. [Google Scholar] [CrossRef]
- Jose, J.; Przybyla, L.; Edwards, T.J.; Perera, R.; Burgner, J.W.; Kuhn, R.J. Interactions of the Cytoplasmic Domain of Sindbis Virus E2 with Nucleocapsid Cores Promote Alphavirus Budding. J. Virol. 2012, 86, 2585–2599. [Google Scholar] [CrossRef]
- Garoff, H.; Sjöberg, M.; Cheng, R.H. Budding of Alphaviruses. Virus Res. 2004, 106, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Galaz-Montoya, J.G.; Sherman, M.B.; Sun, S.Y.; Goldsmith, C.S.; O’Toole, E.T.; Ackerman, L.; Carlson, L.-A.; Weaver, S.C.; Chiu, W.; et al. Neutralizing Antibodies Inhibit Chikungunya Virus Budding at the Plasma Membrane. Cell Host Microbe 2018, 24, 417–428.e5. [Google Scholar] [CrossRef]
- Firth, A.E.; Chung, B.Y.; Fleeton, M.N.; Atkins, J.F. Discovery of Frameshifting in Alphavirus 6K Resolves a 20-Year Enigma. Virol. J. 2008, 5, 108. [Google Scholar] [CrossRef]
- Liljeström, P.; Lusa, S.; Huylebroeck, D.; Garoff, H. In Vitro Mutagenesis of a Full-Length CDNA Clone of Semliki Forest Virus: The Small 6000-Molecular-Weight Membrane Protein Modulates Virus Release. J. Virol. 1991, 65, 4107–4113. [Google Scholar] [CrossRef]
- Lusa, S.; Garoff, H.; Liljeström, P. Fate of the 6K Membrane Protein of Semliki Forest Virus during Virus Assembly. Virology 1991, 185, 843–846. [Google Scholar] [CrossRef]
- Loewy, A.; Smyth, J.; von Bonsdorff, C.H.; Liljeström, P.; Schlesinger, M.J. The 6-Kilodalton Membrane Protein of Semliki Forest Virus Is Involved in the Budding Process. J. Virol. 1995, 69, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.J.; Jones-Burrage, S.; Maury, W.; Mukhopadhyay, S. TF Protein of Sindbis Virus Antagonizes Host Type I Interferon Responses in a Palmitoylation-Dependent Manner. Virology 2020, 542, 63–70. [Google Scholar] [CrossRef]
- Dey, D.; Siddiqui, S.I.; Mamidi, P.; Ghosh, S.; Kumar, C.S.; Chattopadhyay, S.; Ghosh, S.; Banerjee, M. The Effect of Amantadine on an Ion Channel Protein from Chikungunya Virus. PLoS Negl. Trop. Dis. 2019, 13, e0007548. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xie, S.; Sun, B. Viral Proteins Function as Ion Channels. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 510–515. [Google Scholar] [CrossRef]
- Button, J.M.; Qazi, S.A.; Wang, J.C.-Y.; Mukhopadhyay, S. Revisiting an Old Friend: New Findings in Alphavirus Structure and Assembly. Curr. Opin. Virol. 2020, 45, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.R. Seeking Membranes: Positive-Strand RNA Virus Replication Complexes. PLoS Biol. 2008, 6. [Google Scholar] [CrossRef]
- Miller, S.; Krijnse-Locker, J. Modification of Intracellular Membrane Structures for Virus Replication. Nat. Rev. Microbiol. 2008, 6, 363–374. [Google Scholar] [CrossRef]
- Scutigliani, E.M.; Kikkert, M. Interaction of the Innate Immune System with Positive-Strand RNA Virus Replication Organelles. Cytokine Growth Factor Rev. 2017, 37, 17–27. [Google Scholar] [CrossRef]
- Harak, C.; Lohmann, V. Ultrastructure of the Replication Sites of Positive-Strand RNA Viruses. Virology 2015, 479–480, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Melia, C.E.; Snijder, E.J.; Bárcena, M. Double-Membrane Vesicles as Platforms for Viral Replication. Trends Microbiol. 2020, 28, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Limpens, R.W.A.L.; Zevenhoven-Dobbe, J.C.; Laugks, U.; Zheng, S.; de Jong, A.W.M.; Koning, R.I.; Agard, D.A.; Grünewald, K.; Koster, A.J.; et al. A Molecular Pore Spans the Double Membrane of the Coronavirus Replication Organelle. Science 2020, 369, 1395–1398. [Google Scholar] [CrossRef]
- Du Toit, A. Coronavirus Replication Factories. Nat. Rev. Microbiol. 2020, 18, 411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, Y.; Sanyal, S. Membrane Heist: Coronavirus Host Membrane Remodeling during Replication. Biochimie 2020, 179, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.; Kikkert, M.; van den Worm, S.H.E.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J. SARS-Coronavirus Replication Is Supported by a Reticulovesicular Network of Modified Endoplasmic Reticulum. PLoS Biol. 2008, 6, e226. [Google Scholar] [CrossRef]
- De Wilde, A.H.; Raj, V.S.; Oudshoorn, D.; Bestebroer, T.M.; van Nieuwkoop, S.; Limpens, R.W.A.L.; Posthuma, C.C.; van der Meer, Y.; Bárcena, M.; Haagmans, B.L.; et al. MERS-Coronavirus Replication Induces Severe in Vitro Cytopathology and Is Strongly Inhibited by Cyclosporin A or Interferon-α Treatment. J. Gen. Virol. 2013, 94, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Romero-Brey, I.; Merz, A.; Chiramel, A.; Lee, J.-Y.; Chlanda, P.; Haselman, U.; Santarella-Mellwig, R.; Habermann, A.; Hoppe, S.; Kallis, S.; et al. Three-Dimensional Architecture and Biogenesis of Membrane Structures Associated with Hepatitis C Virus Replication. PLoS Pathog. 2012, 8, e1003056. [Google Scholar] [CrossRef]
- Chukkapalli, V.; Randall, G. Hepatitis C Virus Replication Compartment Formation: Mechanism and Drug Target. Gastroenterology 2014, 146, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Quiner, C.A.; Jackson, W.T. Fragmentation of the Golgi Apparatus Provides Replication Membranes for Human Rhinovirus 1A. Virology 2010, 407, 185–195. [Google Scholar] [CrossRef]
- Belov, G.A.; Nair, V.; Hansen, B.T.; Hoyt, F.H.; Fischer, E.R.; Ehrenfeld, E. Complex Dynamic Development of Poliovirus Membranous Replication Complexes. J. Virol. 2012, 86, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Suhy, D.A.; Giddings, T.H.; Kirkegaard, K. Remodeling the Endoplasmic Reticulum by Poliovirus Infection and by Individual Viral Proteins: An Autophagy-like Origin for Virus-Induced Vesicles. J. Virol. 2000, 74, 8953–8965. [Google Scholar] [CrossRef] [PubMed]
- Melia, C.E.; van der Schaar, H.M.; de Jong, A.W.M.; Lyoo, H.R.; Snijder, E.J.; Koster, A.J.; van Kuppeveld, F.J.M.; Bárcena, M. The Origin, Dynamic Morphology, and PI4P-Independent Formation of Encephalomyocarditis Virus Replication Organelles. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Doerflinger, S.Y.; Cortese, M.; Romero-Brey, I.; Menne, Z.; Tubiana, T.; Schenk, C.; White, P.A.; Bartenschlager, R.; Bressanelli, S.; Hansman, G.S.; et al. Membrane Alterations Induced by Nonstructural Proteins of Human Norovirus. PLoS Pathog. 2017, 13, e1006705. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoeven, B.; Oudshoorn, D.; Koster, A.J.; Snijder, E.J.; Kikkert, M.; Bárcena, M. Biogenesis and Architecture of Arterivirus Replication Organelles. Virus Res. 2016, 220, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.; Bárcena, M.; Limpens, R.W.A.L.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J. Ultrastructural Characterization of Arterivirus Replication Structures: Reshaping the Endoplasmic Reticulum To Accommodate Viral RNA Synthesis. J. Virol. 2012, 86, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Goellner, S.; Acosta, E.G.; Neufeldt, C.J.; Oleksiuk, O.; Lampe, M.; Haselmann, U.; Funaya, C.; Schieber, N.; Ronchi, P.; et al. Ultrastructural Characterization of Zika Virus Replication Factories. Cell Rep. 2017, 18, 2113–2123. [Google Scholar] [CrossRef]
- Welsch, S.; Miller, S.; Romero-Brey, I.; Merz, A.; Bleck, C.K.E.; Walther, P.; Fuller, S.D.; Antony, C.; Krijnse-Locker, J.; Bartenschlager, R. Composition and Three-Dimensional Architecture of the Dengue Virus Replication and Assembly Sites. Cell Host Microbe 2009, 5, 365–375. [Google Scholar] [CrossRef]
- Neufeldt, C.J.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring Cellular Networks by Members of the Flaviviridae Family. Nat. Rev. Microbiol. 2018, 16, 125–142. [Google Scholar] [CrossRef]
- Frolova, E.I.; Gorchakov, R.; Pereboeva, L.; Atasheva, S.; Frolov, I. Functional Sindbis Virus Replicative Complexes Are Formed at the Plasma Membrane. J. Virol. 2010, 84, 11679–11695. [Google Scholar] [CrossRef]
- Grimley, P.M.; Levin, J.G.; Berezesky, I.K.; Friedman, R.M. Specific Membranous Structures Associated with the Replication of Group A Arboviruses 1. J. Virol. 1972, 10, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Kopek, B.G.; Perkins, G.; Miller, D.J.; Ellisman, M.H.; Ahlquist, P. Three-Dimensional Analysis of a Viral RNA Replication Complex Reveals a Virus-Induced Mini-Organelle. PLoS Biol. 2007, 5, e220. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Chen, J.; Janda, M.; Sullivan, M.; den Boon, J.; Ahlquist, P. A Positive-Strand RNA Virus Replication Complex Parallels Form and Function of Retrovirus Capsids. Mol. Cell 2002, 9, 505–514. [Google Scholar] [CrossRef]
- Noueiry, A.O.; Ahlquist, P. BROME MOSAIC VIRUS RNA REPLICATION: Revealing the Role of the Host in RNA Virus Replication. Annu. Rev. Phytopathol. 2003, 41, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Froshauer, S.; Kartenbeck, J.; Helenius, A. Alphavirus RNA Replicase Is Located on the Cytoplasmic Surface of Endosomes and Lysosomes. J. Cell Biol. 1988, 107, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Ahola, T.; Kääriäinen, L. Reaction in Alphavirus MRNA Capping: Formation of a Covalent Complex of Nonstructural Protein NsP1 with 7-Methyl-GMP. Proc. Natl. Acad. Sci. USA 1995, 92, 507–511. [Google Scholar] [CrossRef]
- Ahola, T.; Lampio, A.; Auvinen, P.; Kääriäinen, L. Semliki Forest Virus MRNA Capping Enzyme Requires Association with Anionic Membrane Phospholipids for Activity. EMBO J. 1999, 18, 3164–3172. [Google Scholar] [CrossRef]
- Li, C.; Guillén, J.; Rabah, N.; Blanjoie, A.; Debart, F.; Vasseur, J.-J.; Canard, B.; Decroly, E.; Coutard, B. MRNA Capping by Venezuelan Equine Encephalitis Virus NsP1: Functional Characterization and Implications for Antiviral Research. J. Virol. 2015, 89, 8292–8303. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Bragagnolo, G.; Arranz, R.; Reguera, J. Capping Pores of Alphavirus NsP1 Gate Membranous Viral Replication Factories. Nature 2021, 589, 615–619. [Google Scholar] [CrossRef]
- Zhang, K.; Law, Y.-S.; Law, M.C.Y.; Tan, Y.B.; Wirawan, M.; Luo, D. Structural Insights into Viral RNA Capping and Plasma Membrane Targeting by Chikungunya Virus Nonstructural Protein 1. Cell Host Microbe 2021, 29, 757–764.e3. [Google Scholar] [CrossRef]
- Russo, A.T.; White, M.A.; Watowich, S.J. The Crystal Structure of the Venezuelan Equine Encephalitis Alphavirus NsP2 Protease. Structure 2006, 14, 1449–1458. [Google Scholar] [CrossRef]
- Hu, X.; Compton, J.R.; Leary, D.H.; Olson, M.A.; Lee, M.S.; Cheung, J.; Ye, W.; Ferrer, M.; Southall, N.; Jadhav, A.; et al. Kinetic, Mutational, and Structural Studies of the Venezuelan Equine Encephalitis Virus Nonstructural Protein 2 Cysteine Protease. Biochemistry 2016, 55, 3007–3019. [Google Scholar] [CrossRef]
- Gomez de Cedrón, M.; Ehsani, N.; Mikkola, M.L.; García, J.A.; Kääriäinen, L. RNA Helicase Activity of Semliki Forest Virus Replicase Protein NSP2. FEBS Lett. 1999, 448, 19–22. [Google Scholar] [CrossRef]
- Vasiljeva, L.; Merits, A.; Auvinen, P.; Kääriäinen, L. Identification of a Novel Function of the Alphavirus Capping Apparatus. RNA 5’-Triphosphatase Activity of Nsp2. J. Biol. Chem. 2000, 275, 17281–17287. [Google Scholar] [CrossRef]
- Hardy, W.R.; Strauss, J.H. Processing the Nonstructural Polyproteins of Sindbis Virus: Nonstructural Proteinase Is in the C-Terminal Half of NsP2 and Functions Both in Cis and in Trans. J. Virol. 1989, 63, 4653–4664. [Google Scholar] [CrossRef] [PubMed]
- Lulla, A.; Lulla, V.; Tints, K.; Ahola, T.; Merits, A. Molecular Determinants of Substrate Specificity for Semliki Forest Virus Nonstructural Protease. J. Virol. 2006, 80, 5413–5422. [Google Scholar] [CrossRef] [PubMed]
- Garmashova, N.; Gorchakov, R.; Frolova, E.; Frolov, I. Sindbis Virus Nonstructural Protein NsP2 Is Cytotoxic and Inhibits Cellular Transcription. J. Virol. 2006, 80, 5686–5696. [Google Scholar] [CrossRef]
- Mayuri; Geders, T.W.; Smith, J.L.; Kuhn, R.J. Role for Conserved Residues of Sindbis Virus Nonstructural Protein 2 Methyltransferase-like Domain in Regulation of Minus-Strand Synthesis and Development of Cytopathic Infection. J. Virol. 2008, 82, 7284–7297. [Google Scholar] [CrossRef] [PubMed]
- Götte, B.; Liu, L.; McInerney, G.M. The Enigmatic Alphavirus Non-Structural Protein 3 (NsP3) Revealing Its Secrets at Last. Viruses 2018, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Lark, T.; Keck, F.; Narayanan, A. Interactions of Alphavirus NsP3 Protein with Host Proteins. Front. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Yost, S.A.; Miller, M.T.; Elrod, E.J.; Grakoui, A.; Marcotrigiano, J. Structural and Functional Insights into Alphavirus Polyprotein Processing and Pathogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 16534–16539. [Google Scholar] [CrossRef] [PubMed]
- Malet, H.; Coutard, B.; Jamal, S.; Dutartre, H.; Papageorgiou, N.; Neuvonen, M.; Ahola, T.; Forrester, N.; Gould, E.A.; Lafitte, D.; et al. The Crystal Structures of Chikungunya and Venezuelan Equine Encephalitis Virus NsP3 Macro Domains Define a Conserved Adenosine Binding Pocket. J. Virol. 2009, 83, 6534–6545. [Google Scholar] [CrossRef]
- Eckei, L.; Krieg, S.; Bütepage, M.; Lehmann, A.; Gross, A.; Lippok, B.; Grimm, A.R.; Kümmerer, B.M.; Rossetti, G.; Lüscher, B.; et al. The Conserved Macrodomains of the Non-Structural Proteins of Chikungunya Virus and Other Pathogenic Positive Strand RNA Viruses Function as Mono-ADP-Ribosylhydrolases. Sci. Rep. 2017, 7, 41746. [Google Scholar] [CrossRef]
- Gao, Y.; Goonawardane, N.; Ward, J.; Tuplin, A.; Harris, M. Multiple Roles of the Non-Structural Protein 3 (NsP3) Alphavirus Unique Domain (AUD) during Chikungunya Virus Genome Replication and Transcription. PLoS Pathog. 2019, 15, e1007239. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.; Gorchakov, R.; Garmashova, N.; Atasheva, S.; Vergara, L.A.; Frolov, I. Formation of NsP3-Specific Protein Complexes during Sindbis Virus Replication. J. Virol. 2006, 80, 4122–4134. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Reynaud, J.M.; Rasalouskaya, A.; Akhrymuk, I.; Mobley, J.A.; Frolov, I.; Frolova, E.I. New World and Old World Alphaviruses Have Evolved to Exploit Different Components of Stress Granules, FXR and G3BP Proteins, for Assembly of Viral Replication Complexes. PLoS Pathog. 2016, 12, e1005810. [Google Scholar] [CrossRef]
- Frolov, I.; Kim, D.Y.; Akhrymuk, M.; Mobley, J.A.; Frolova, E.I. Hypervariable Domain of Eastern Equine Encephalitis Virus NsP3 Redundantly Utilizes Multiple Cellular Proteins for Replication Complex Assembly. J. Virol. 2017, 91, e00371-17. [Google Scholar] [CrossRef]
- Scholte, F.E.M.; Tas, A.; Albulescu, I.C.; Žusinaite, E.; Merits, A.; Snijder, E.J.; van Hemert, M.J. Stress Granule Components G3BP1 and G3BP2 Play a Proviral Role Early in Chikungunya Virus Replication. J. Virol. 2015, 89, 4457–4469. [Google Scholar] [CrossRef]
- Weston, J.; Villoing, S.; Brémont, M.; Castric, J.; Pfeffer, M.; Jewhurst, V.; McLoughlin, M.; Rødseth, O.; Christie, K.E.; Koumans, J.; et al. Comparison of Two Aquatic Alphaviruses, Salmon Pancreas Disease Virus and Sleeping Disease Virus, by Using Genome Sequence Analysis, Monoclonal Reactivity, and Cross-Infection. J. Virol. 2002, 76, 6155–6163. [Google Scholar] [CrossRef]
- Tomar, S.; Hardy, R.W.; Smith, J.L.; Kuhn, R.J. Catalytic Core of Alphavirus Nonstructural Protein NsP4 Possesses Terminal Adenylyltransferase Activity. J. Virol. 2006, 80, 9962–9969. [Google Scholar] [CrossRef] [PubMed]
- Rubach, J.K.; Wasik, B.R.; Rupp, J.C.; Kuhn, R.J.; Hardy, R.W.; Smith, J.L. Characterization of Purified Sindbis Virus NsP4 RNA-Dependent RNA Polymerase Activity in Vitro. Virology 2009, 384, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.B.; Lello, L.S.; Liu, X.; Law, Y.-S.; Kang, C.; Lescar, J.; Zheng, J.; Merits, A.; Luo, D. A Crystal Structure of Alphavirus Nonstructural Protein 4 (NsP4) Reveals an Intrinsically Dynamic RNA-Dependent RNA Polymerase. bioRxiv 2021. [Google Scholar] [CrossRef]
- Peränen, J.; Laakkonen, P.; Hyvönen, M.; Kääriäinen, L. The Alphavirus Replicase Protein NsP1 Is Membrane-Associated and Has Affinity to Endocytic Organelles. Virology 1995, 208, 610–620. [Google Scholar] [CrossRef]
- Salonen, A.; Vasiljeva, L.; Merits, A.; Magden, J.; Jokitalo, E.; Kääriäinen, L. Properly Folded Nonstructural Polyprotein Directs the Semliki Forest Virus Replication Complex to the Endosomal Compartment. J. Virol. 2003, 77, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Peränen, J.; Rikkonen, M.; Liljeström, P.; Kääriäinen, L. Nuclear Localization of Semliki Forest Virus-Specific Nonstructural Protein NsP2. J. Virol. 1990, 64, 1888–1896. [Google Scholar] [CrossRef]
- Spuul, P.; Salonen, A.; Merits, A.; Jokitalo, E.; Kääriäinen, L.; Ahola, T. Role of the Amphipathic Peptide of Semliki Forest Virus Replicase Protein NsP1 in Membrane Association and Virus Replication. J. Virol. 2007, 81, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, P.; Ahola, T.; Kääriäinen, L. The Effects of Palmitoylation on Membrane Association of Semliki Forest Virus RNA Capping Enzyme. J. Biol. Chem. 1996, 271, 28567–28571. [Google Scholar] [CrossRef] [PubMed]
- Ahola, T.; Kujala, P.; Tuittila, M.; Blom, T.; Laakkonen, P.; Hinkkanen, A.; Auvinen, P. Effects of Palmitoylation of Replicase Protein NsP1 on Alphavirus Infection. J. Virol. 2000, 74, 6725–6733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gottipati, K.; Woodson, M.; Choi, K.H. Membrane Binding and Rearrangement by Chikungunya Virus Capping Enzyme NsP1. Virology 2020, 544, 31–41. [Google Scholar] [CrossRef]
- Spuul, P.; Balistreri, G.; Hellström, K.; Golubtsov, A.V.; Jokitalo, E.; Ahola, T. Assembly of Alphavirus Replication Complexes from RNA and Protein Components in a Novel Trans-Replication System in Mammalian Cells. J. Virol. 2011, 85, 4739–4751. [Google Scholar] [CrossRef]
- Kallio, K.; Hellström, K.; Balistreri, G.; Spuul, P.; Jokitalo, E.; Ahola, T. Template RNA Length Determines the Size of Replication Complex Spherules for Semliki Forest Virus. J. Virol. 2013, 87, 9125–9134. [Google Scholar] [CrossRef]
- Kopek, B.G.; Settles, E.W.; Friesen, P.D.; Ahlquist, P. Nodavirus-Induced Membrane Rearrangement in Replication Complex Assembly Requires Replicase Protein a, RNA Templates, and Polymerase Activity. J. Virol. 2010, 84, 12492–12503. [Google Scholar] [CrossRef]
- Hellström, K.; Kallio, K.; Utt, A.; Quirin, T.; Jokitalo, E.; Merits, A.; Ahola, T. Partially Uncleaved Alphavirus Replicase Forms Spherule Structures in the Presence and Absence of RNA Template. J. Virol. 2017, 91, e00787-17. [Google Scholar] [CrossRef]
- Neuvonen, M.; Kazlauskas, A.; Martikainen, M.; Hinkkanen, A.; Ahola, T.; Saksela, K. SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus NsP3 Promotes Viral RNA Replication. PLoS Pathog. 2011, 7, e1002383. [Google Scholar] [CrossRef]
- Tossavainen, H.; Aitio, O.; Hellman, M.; Saksela, K.; Permi, P. Structural Basis of the High Affinity Interaction between the Alphavirus Nonstructural Protein-3 (NsP3) and the SH3 Domain of Amphiphysin-2. J. Biol. Chem. 2016, 291, 16307–16317. [Google Scholar] [CrossRef]
- Zech, B.; Kurtenbach, A.; Krieger, N.; Strand, D.; Blencke, S.; Morbitzer, M.; Salassidis, K.; Cotten, M.; Wissing, J.; Obert, S.; et al. Identification and Characterization of Amphiphysin II as a Novel Cellular Interaction Partner of the Hepatitis C Virus NS5A Protein. J. Gen. Virol. 2003, 84, 555–560. [Google Scholar] [CrossRef]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.G.; Evans, P.R.; McMahon, H.T. BAR Domains as Sensors of Membrane Curvature: The Amphiphysin BAR Structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Spuul, P.; Balistreri, G.; Kääriäinen, L.; Ahola, T. Phosphatidylinositol 3-Kinase-, Actin-, and Microtubule-Dependent Transport of Semliki Forest Virus Replication Complexes from the Plasma Membrane to Modified Lysosomes. J. Virol. 2010, 84, 7543–7557. [Google Scholar] [CrossRef] [PubMed]
- Thaa, B.; Biasiotto, R.; Eng, K.; Neuvonen, M.; Götte, B.; Rheinemann, L.; Mutso, M.; Utt, A.; Varghese, F.; Balistreri, G.; et al. Differential Phosphatidylinositol-3-Kinase-Akt-MTOR Activation by Semliki Forest and Chikungunya Viruses Is Dependent on NsP3 and Connected to Replication Complex Internalization. J. Virol. 2015, 89, 11420–11437. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Castro, C.; Thaa, B.; Liu, L.; Mutso, M.; Liu, X.; Mahalingam, S.; Griffin, J.L.; Marsh, M.; McInerney, G.M. Alphavirus-Induced Hyperactivation of PI3K/AKT Directs pro-Viral Metabolic Changes. PLoS Pathog. 2018, 14, e1006835. [Google Scholar] [CrossRef] [PubMed]
- Garoff, H.; Wilschut, J.; Liljeström, P.; Wahlberg, J.M.; Bron, R.; Suomalainen, M.; Smyth, J.; Salminen, A.; Barth, B.U.; Zhao, H. Assembly and Entry Mechanisms of Semliki Forest Virus. Arch. Virol. Suppl. 1994, 9, 329–338. [Google Scholar] [CrossRef]
- Griffiths, G.; Quinn, P.; Warren, G. Dissection of the Golgi Complex. I. Monensin Inhibits the Transport of Viral Membrane Proteins from Medial to Trans Golgi Cisternae in Baby Hamster Kidney Cells Infected with Semliki Forest Virus. J. Cell Biol. 1983, 96, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.; Simons, K.; Warren, G.; Tokuyasu, K.T. Immunoelectron Microscopy Using Thin, Frozen Sections: Application to Studies of the Intracellular Transport of Semliki Forest Virus Spike Glycoproteins. Methods Enzymol. 1983, 96, 466–485. [Google Scholar] [CrossRef]
- Jose, J.; Taylor, A.B.; Kuhn, R.J. Spatial and Temporal Analysis of Alphavirus Replication and Assembly in Mammalian and Mosquito Cells. mBio 2017, 8. [Google Scholar] [CrossRef]
- Owen, K.E.; Kuhn, R.J. Alphavirus Budding Is Dependent on the Interaction between the Nucleocapsid and Hydrophobic Amino Acids on the Cytoplasmic Domain of the E2 Envelope Glycoprotein. Virology 1997, 230, 187–196. [Google Scholar] [CrossRef]
- Liu, L.N.; Lee, H.; Hernandez, R.; Brown, D.T. Mutations in the Endo Domain of Sindbis Virus Glycoprotein E2 Block Phosphorylation, Reorientation of the Endo Domain, and Nucleocapsid Binding. Virology 1996, 222, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Dodonova, S.O.; Aderhold, P.; Kopp, J.; Ganeva, I.; Röhling, S.; Hagen, W.J.H.; Sinning, I.; Wieland, F.; Briggs, J.A.G. 9Å Structure of the COPI Coat Reveals That the Arf1 GTPase Occupies Two Contrasting Molecular Environments. eLife 2017, 6, e26691. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lacomis, L.; Erdjument-Bromage, H.; Tempst, P.; Stamnes, M. Cytosol-Derived Proteins Are Sufficient for Arp2/3 Recruitment and ARF/Coatomer-Dependent Actin Polymerization on Golgi Membranes. FEBS Lett. 2004, 566, 281–286. [Google Scholar] [CrossRef]
- Fucini, R.V.; Navarrete, A.; Vadakkan, C.; Lacomis, L.; Erdjument-Bromage, H.; Tempst, P.; Stamnes, M. Activated ADP-Ribosylation Factor Assembles Distinct Pools of Actin on Golgi Membranes. J. Biol. Chem. 2000, 275, 18824–18829. [Google Scholar] [CrossRef] [PubMed]
- Kroschewski, R.; Hall, A.; Mellman, I. Cdc42 Controls Secretory and Endocytic Transport to the Basolateral Plasma Membrane of MDCK Cells. Nat. Cell Biol. 1999, 1, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.D.; Johnsen, I.B.; Stiberg, K.A.; Sherstova, T.; Wakita, T.; Richard, G.M.; Kandasamy, R.K.; Meurs, E.F.; Anthonsen, M.W. Hepatitis C Virus Triggers Golgi Fragmentation and Autophagy through the Immunity-Related GTPase M. Proc. Natl. Acad. Sci. USA 2017, 114, E3462–E3471. [Google Scholar] [CrossRef]
- Freundt, E.C.; Yu, L.; Goldsmith, C.S.; Welsh, S.; Cheng, A.; Yount, B.; Liu, W.; Frieman, M.B.; Buchholz, U.J.; Screaton, G.R.; et al. The Open Reading Frame 3a Protein of Severe Acute Respiratory Syndrome-Associated Coronavirus Promotes Membrane Rearrangement and Cell Death. J. Virol. 2010, 84, 1097–1109. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Machamer, C.E. The SARS CoV-1 3a Protein Disrupts Golgi Complex Morphology and Cargo Trafficking. bioRxiv 2021. [Google Scholar] [CrossRef]
- Donaldson, J.G.; Honda, A.; Weigert, R. Multiple Activities for Arf1 at the Golgi Complex. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2005, 1744, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Murphy, B.J.; Law, M. Vaccinia Virus Motility. Annu. Rev. Microbiol. 2003, 57, 323–342. [Google Scholar] [CrossRef]
- Alvarez, D.E.; Agaisse, H. A Role for the Small GTPase Rac1 in Vaccinia Actin-Based Motility. Small GTPases 2014, 5, e29038. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schudt, G.; Dolnik, O.; Kolesnikova, L.; Biedenkopf, N.; Herwig, A.; Becker, S. Transport of Ebolavirus Nucleocapsids Is Dependent on Actin Polymerization: Live-Cell Imaging Analysis of Ebolavirus-Infected Cells. J. Infect. Dis. 2015, 212, S160–S166. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, T.; Welch, M.D. Baculovirus Actin-Based Motility Drives Nuclear Envelope Disruption and Nuclear Egress. Curr. Biol. 2018, 28, 2153–2159.e4. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, P.; Auvinen, P.; Kujala, P.; Kääriäinen, L. Alphavirus Replicase Protein NSP1 Induces Filopodia and Rearrangement of Actin Filaments. J. Virol. 1998, 72, 10265–10269. [Google Scholar] [CrossRef]
- Martinez, M.G.; Kielian, M. Intercellular Extensions Are Induced by the Alphavirus Structural Proteins and Mediate Virus Transmission. PLoS Pathog. 2016, 12, e1006061. [Google Scholar] [CrossRef]
- Martinez, M.G.; Snapp, E.-L.; Perumal, G.S.; Macaluso, F.P.; Kielian, M. Imaging the Alphavirus Exit Pathway. J. Virol. 2014, 88, 6922–6933. [Google Scholar] [CrossRef]
- Jose, J.; Tang, J.; Taylor, A.B.; Baker, T.S.; Kuhn, R.J. Fluorescent Protein-Tagged Sindbis Virus E2 Glycoprotein Allows Single Particle Analysis of Virus Budding from Live Cells. Viruses 2015, 7, 6182–6199. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kam, Y.-W.; Fric, J.; Malleret, B.; Koh, E.G.L.; Prakash, C.; Huang, W.; Lee, W.W.L.; Lin, C.; Lin, R.T.P.; et al. Chikungunya Virus Neutralization Antigens and Direct Cell-to-Cell Transmission Are Revealed by Human Antibody-Escape Mutants. PLoS Pathog. 2011, 7, e1002390. [Google Scholar] [CrossRef]
- Raghow, R.S.; Grace, T.D.; Filshie, B.K.; Bartley, W.; Dalgarno, L. Ross River Virus Replication in Cultured Mosquito and Mammalian Cells: Virus Growth and Correlated Ultrastructural Changes. J. Gen. Virol. 1973, 21, 109–122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmasri, Z.; Nasal, B.L.; Jose, J. Alphavirus-Induced Membrane Rearrangements during Replication, Assembly, and Budding. Pathogens 2021, 10, 984. https://doi.org/10.3390/pathogens10080984
Elmasri Z, Nasal BL, Jose J. Alphavirus-Induced Membrane Rearrangements during Replication, Assembly, and Budding. Pathogens. 2021; 10(8):984. https://doi.org/10.3390/pathogens10080984
Chicago/Turabian StyleElmasri, Zeinab, Benjamin L. Nasal, and Joyce Jose. 2021. "Alphavirus-Induced Membrane Rearrangements during Replication, Assembly, and Budding" Pathogens 10, no. 8: 984. https://doi.org/10.3390/pathogens10080984
APA StyleElmasri, Z., Nasal, B. L., & Jose, J. (2021). Alphavirus-Induced Membrane Rearrangements during Replication, Assembly, and Budding. Pathogens, 10(8), 984. https://doi.org/10.3390/pathogens10080984





