Mosquito Control Priorities in Florida—Survey Results from Florida Mosquito Control Districts
Abstract
:1. Introduction
2. Results
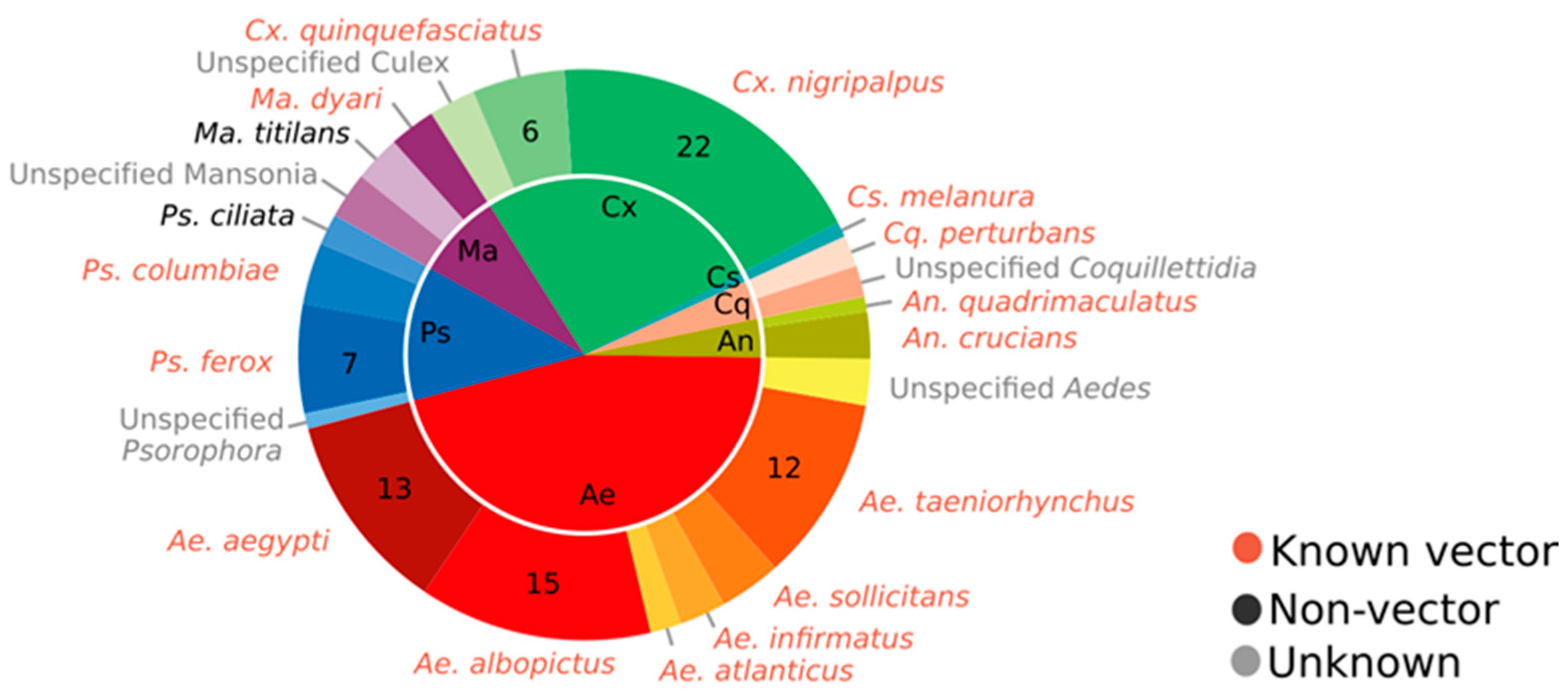
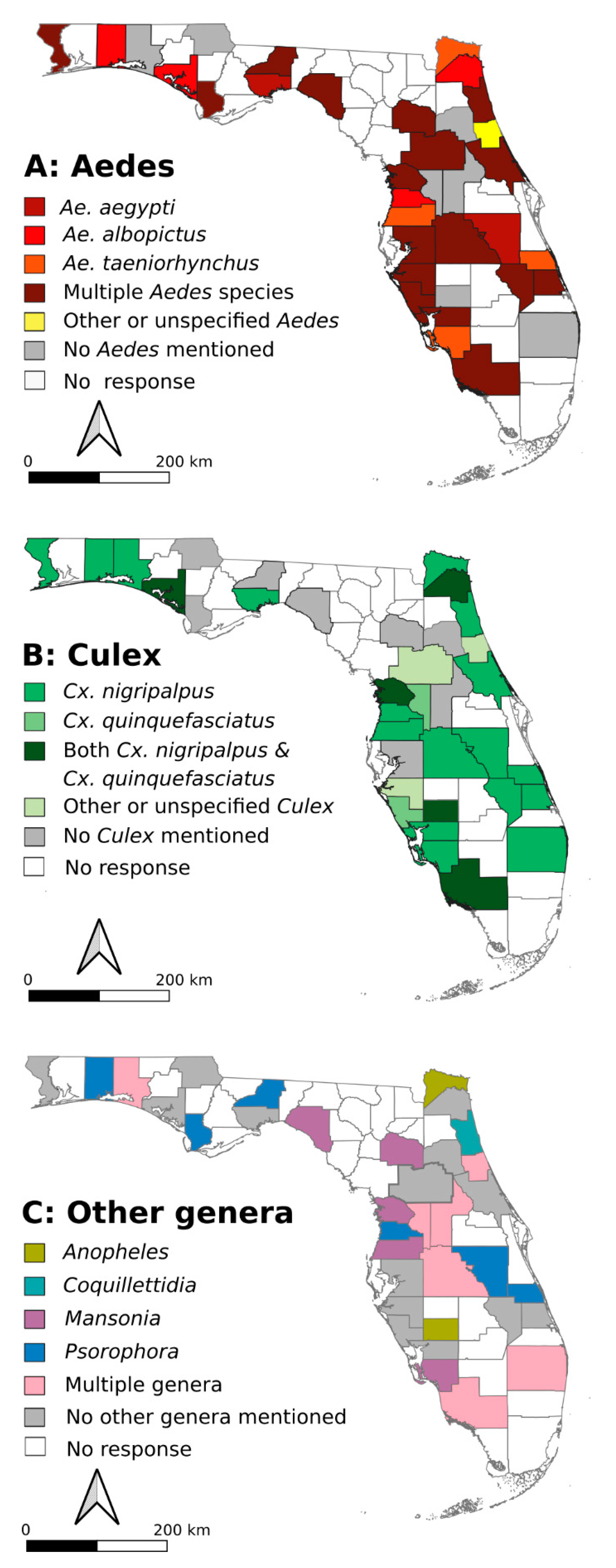
2.1. FMCD Priority Species for Mosquito Control
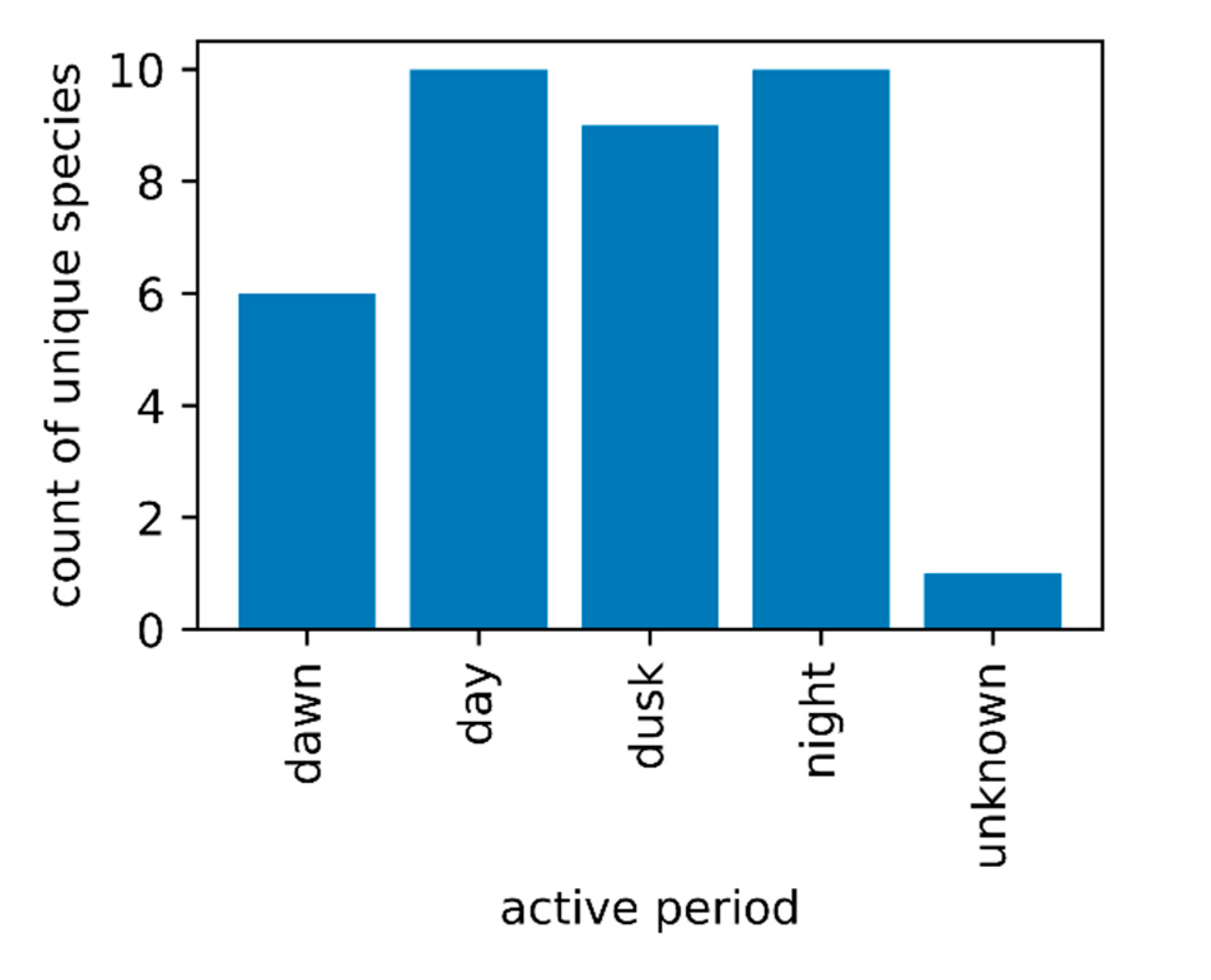
2.2. Activity Times of FMCD Priority Control Species
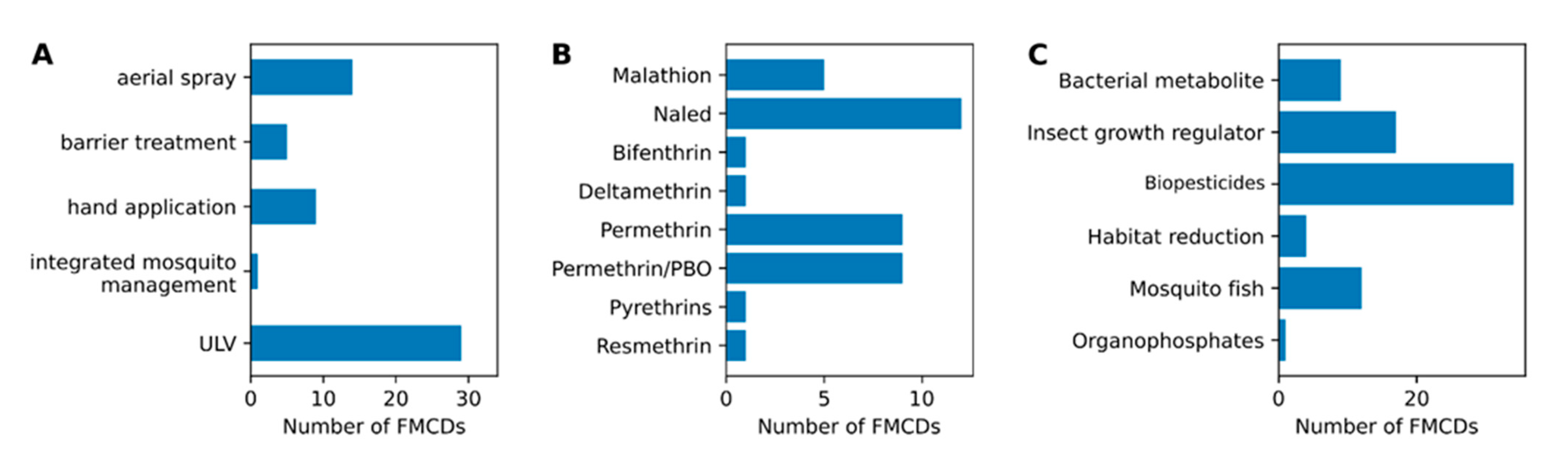
2.3. Common Mosquito Control Methods Used by FMCDs
2.4. Research Questions That Florida Mosquito Control Districts Identified to Be Important
3. Discussion
3.1. Aedes albopictus and Aedes aegypti
3.2. Culex nigripalpus
3.3. Aedes taeniorhynchus
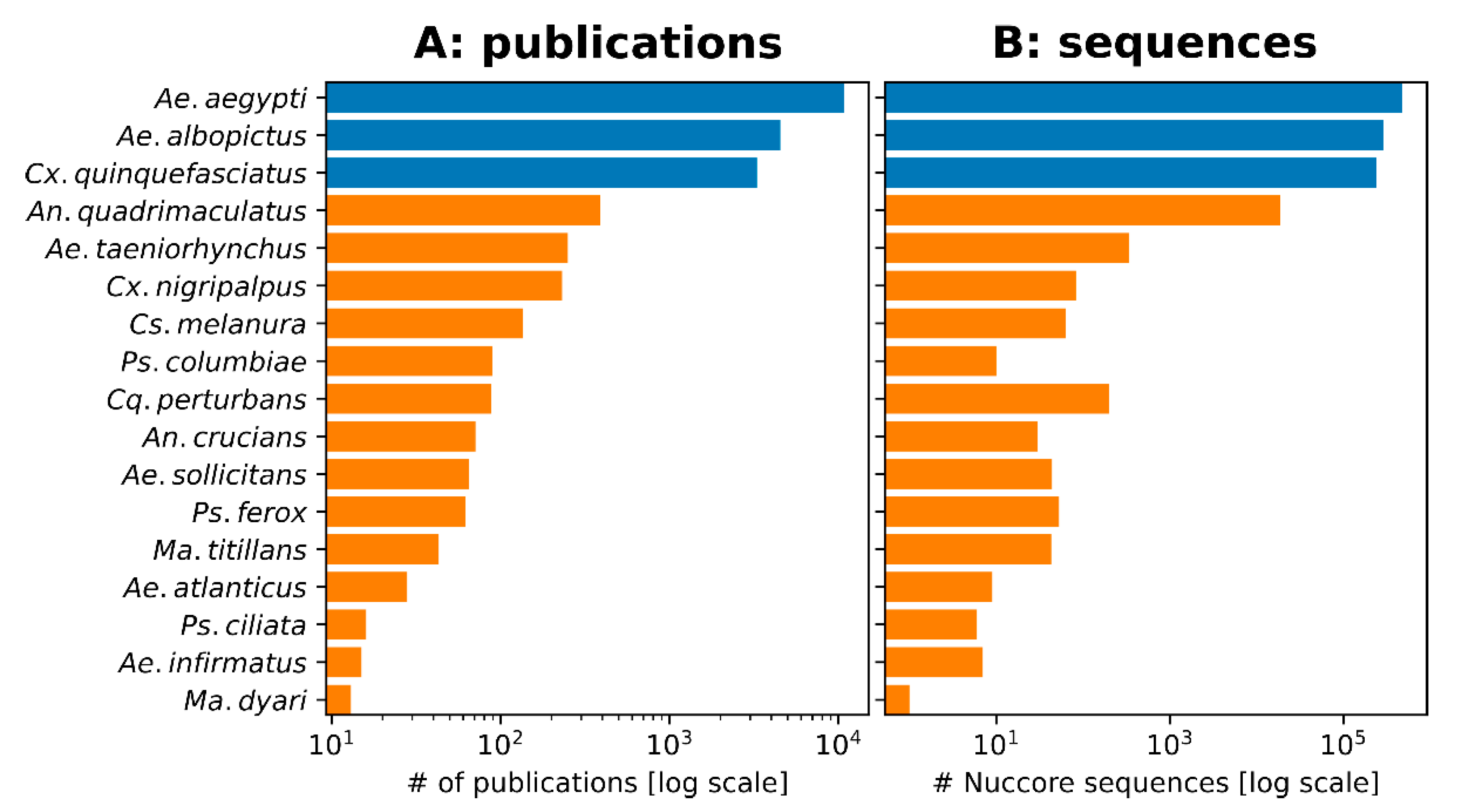
3.4. Mosquito Control Priority Versus Research Interest
3.5. Active Time of Adult Mosquitoes
3.6. Adulticide Choices
3.7. Larval Control Choices
3.8. The Need for Coordination between Mosquito Surveillance, Control, and Research
3.9. Research Questions for Future Investigation
3.10. Study Caveats and Future Directions
4. Materials and Methods
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lloyd, A.M.; Connelly, C.R.; Carlson, D.B. Florida Coordinating Council on Mosquito Control. Florida Mosquito Control: The State of the Mission as Defined by Mosquito Controllers, Regulators, and Environmental Managers; University of Florida, Institute of Food and Agricultural Sciences, Florida Medical Entomology Laboratory: Vero Beach, FL, USA, 2018; Available online: https://fmel.ifas.ufl.edu/florida-mosquito-control/ (accessed on 25 January 2021).
- Riles, M.T.; Connelly, C.R. An update of the mosquito records of Florida counties, USA. J. Am. Mosq. Control Assoc. 2020, 36, 107–111. [Google Scholar] [CrossRef]
- O’Meara, G.F.; Evans, F.D. Seasonal patterns of abundance among three species of Culex mosquitoes in a South Florida wastewater lagoon. Ann. Entomol. Soc. Am. 1983, 76, 130–133. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [Green Version]
- Heberlein-Larson, L.A.; Tan, Y.; Stark, L.M.; Cannons, A.C.; Shilts, M.H.; Unnasch, T.R.; Das, S.R. Complex epidemiological dynamics of Eastern equine encephalitis virus in Florida. Am. J. Trop Med. Hyg. 2019, 100, 1266–1274. [Google Scholar] [CrossRef]
- Miley, K.M.; Downs, J.; Burkett-Cadena, N.D.; West, R.G.; Hunt, B.; Deskins, G.; Kellner, B.; Fisher-Grainger, S.; Unnasch, R.S.; Unnasch, T.R. Field analysis of biological factors associated with sites at high and low to moderate risk for Eastern equine encephalitis virus winter activity in Florida. J. Med. Entomol. 2021, tjab066, 1–13. [Google Scholar] [CrossRef]
- Day, J.F.; Shaman, J. Severe winter freezes enhance St. Louis encephalitis virus amplification and epidemic transmission in peninsular Florida. J. Med. Entomol. 2009, 46, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Ottendorfer, C.L.; Ambrose, J.H.; White, G.S.; Unnasch, T.R.; Stark, L.M. Isolation of genotype V St. Louis encephalitis virus in Florida. Emerg. Infect. Dis. 2009, 15, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Burkett-Cadena, N.D.; McClure, C.J.; Estep, L.K.; Eubanks, M.D. Hosts or habitats: What drives the spatial distribution of mosquitoes? Ecosphere 2013, 4, 30. [Google Scholar] [CrossRef]
- Reno, T. Assessment of Invasive Species in FL. Available online: https://assessment.ifas.ufl.edu/ (accessed on 25 March 2021).
- National Plant Board (NPB). Safeguarding American Plant Resources: A Stakeholder Review of the APHIS-PPQ Safeguarding System; National Plant Board, United States Department of Agriculture Animal and Plant Health Inspection Service Plant Protection and Quarantine: Washington, DC, USA, 1999; Available online: https://nationalplantboard.org/wp-content/uploads/docs/safe_summ.pdf (accessed on 25 January 2021).
- Darsie, R.F., Jr.; Vlach, J.J.; Fussell, E.M. New addition to the mosquito fauna of United States, Anopheles grabhamii (Diptera: Culicidae). J. Med. Entomol. 2002, 39, 430–431. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.P.; Walsh, J.D.; Cope, E.H.; Tennant, R.A., Jr.; Kozak, J.A., 3rd; Darsie, R.F., Jr. Culex coronator Dyar and Knab: A new Florida species record. J. Am. Mosq. Control Assoc. 2006, 22, 330–332. [Google Scholar] [CrossRef]
- Blosser, E.M.; Burkett-Cadena, N.D. Culex (Melanoconion) panocossa from peninsular Florida, USA. Acta Trop. 2017, 167, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Burkett-Cadena, N.D.; Blosser, E.M. Aedeomyia squamipennis (Diptera: Culicidae) in Florida, USA, a New State and Country Record. J. Med. Entomol. 2017, 54, 788–792. [Google Scholar] [CrossRef]
- Riles, M.T.; Smith, J.P.; Burkett-Cadena, N.; Connelly, C.R.; Morse, G.W., Jr.; Byrd, B.D. First record of Aedes japonicus In Florida. J. Am. Mosq. Control Assoc. 2017, 33, 340–344. [Google Scholar] [CrossRef]
- Reeves, L.E.; Medina, J.; Miqueli, E.; Sloyer, K.E.; Petrie, W.; Vasquez, C.; Burkett-Cadena, N.D. Establishment of Aedes (Ochlerotatus) scapularis (Diptera: Culicidae) in Mainland Florida, With Notes on the Ochlerotatus Group in the United States. J. Med. Entomol. 2021, 58, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Shroyer, D.A.; Harrison, B.A.; Bintz, B.J.; Wilson, M.R.; Sither, C.B.; Byrd, B.D. Aedes pertinax, a newly recognized mosquito species in the United States. J. Am. Mosq. Control Assoc. 2015, 31, 97–100. [Google Scholar] [CrossRef]
- Darsie, R.F., Jr.; Shroyer, D.A. Culex (Culex) declarator, a mosquito species new to Florida. J. Am. Mosq. Control Assoc. 2004, 20, 224–227. [Google Scholar] [PubMed]
- Shin, D.; O’Meara, G.F.; Civana, A.; Shroyer, D.A.; Miqueli, E. Culex interrogator (Diptera: Culicidae), a mosquito species new to Florida. J. Vector Ecol. 2016, 41, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Philip, C.; Novick, C.G.; Novick, L.F. Local transmission of Zika virus in Miami-Dade county: The Florida Department of Health rises to the challenge. J. Public Health Manag. Pract. 2019, 25, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Beier, J.C.; Cantrell, R.S.; Cosner, C.; Fuller, D.O.; Guan, Y.; Zhang, G.; Ruan, S. Modeling the importation and local transmission of vector-borne diseases in Florida: The case of Zika outbreak in 2016. J. Biol. 2018, 455, 342–356. [Google Scholar] [CrossRef]
- Norman, S.L.; Patterson, G. The mosquito wars: A history of mosquito control in Florida. J. South. Hist. 2005, 71, 928. [Google Scholar] [CrossRef]
- Richardson, E.A.; Abruzzo, N.O.; Taylor, C.E.; Stevens, B.R.; Cuda, J.P.; Weeks, E.N. Methionine as an effective mosquito larvicide in natural water sources. Fla. Entomol. 2021, 103, 479–483. [Google Scholar] [CrossRef]
- Bonds, J.A.S.; Mulla, C.; Hunter, E. Characterization of the effects of droplet size, air blast strength, and angle on spray distribution and efficacy of a Buffalo turbine barrier treatment sprayer. J. ASTM Int. 2011, 8, 1–6. [Google Scholar]
- Connelly, C.R. Hurricanes and Mosquitoes. EDIS 2004, 2004, 1. [Google Scholar] [CrossRef]
- Parker, C.; Ramirez, D.; Connelly, C.R. State-wide survey of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Florida. J. Vector Ecol. 2019, 44, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Sprenger, D.; Wuithiranyagool, T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J. Am. Mosq. Control Assoc. 1986, 2, 217–219. [Google Scholar] [PubMed]
- Christophers, S.R. Aëdes aegypti (L.), the Yellow Fever Mosquito; Its Life History, Bionomics, and Structure; University Press: Cambridge, UK, 1960; Volume xii, 738p. [Google Scholar]
- Tabachnick, W.J. Evolutionary genetics and arthropod-borne disease: The yellow fever mosquito. Am. Entomol. 1991, 37, 14–24. [Google Scholar] [CrossRef]
- Cornel, A.J.; Holeman, J.; Nieman, C.C.; Lee, Y.; Smith, C.; Amorino, M.; Brisco, K.K.; Barrera, R.; Lanzaro, G.C.; Mulligan Iii, F.S. Surveillance, insecticide resistance and control of an invasive Aedes aegypti (Diptera: Culicidae) population in California. F1000Research 2016, 5, 194. [Google Scholar] [CrossRef] [PubMed]
- Center for Diseaase Control and Prevension (CDC). Potential Ranges of Aedes Mosquitoes. Available online: https://www.cdc.gov/mosquitoes/mosquito-control/professionals/range.html (accessed on 15 July 2021).
- Giordano, B.V.; Gasparotto, A.; Liang, P.; Nelder, M.P.; Russell, C.; Hunter, F.F. Discovery of an Aedes (Stegomyia) albopictus population and first records of Aedes (Stegomyia) aegypti in Canada. Med. Vet. Entomol. 2020, 34, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Schmidt, H.; Collier, T.C.; Conner, W.R.; Hanemaaijer, M.J.; Slatkin, M.; Marshall, J.M.; Chiu, J.C.; Smartt, C.T.; Lanzaro, G.C.; et al. Genome-wide divergence among invasive populations of Aedes aegypti in California. BMC Genom. 2019, 20, 204. [Google Scholar] [CrossRef] [Green Version]
- Guagliardo, S.A.J.; Lee, Y.; Pierce, A.A.; Wong, J.; Chu, Y.Y.; Morrison, A.C.; Astete, H.; Brosi, B.; Vazquez-Prokopec, G.; Scott, T.W.; et al. The genetic structure of Aedes aegypti populations is driven by boat traffic in the Peruvian Amazon. PLoS Negl. Trop. Dis. 2019, 13, e0007552. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.B.; Kasai, S.; Scott, J.G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 2016, 133, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Ramirez, D.; Thomas, C.; Connelly, C.R. Baseline susceptibility status of Florida populations of Aedes aegypti (Diptera: Culicidae) and Aedes albopictus. J. Med. Entomol. 2020, 57, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Estep, A.S.; Sanscrainte, N.D.; Waits, C.M.; Bernard, S.J.; Lloyd, A.M.; Lucas, K.J.; Buckner, E.A.; Vaidyanathan, R.; Morreale, R.; Conti, L.A.; et al. Quantification of permethrin resistance and kdr alleles in Florida strains of Aedes aegypti (L.) and Aedes albopictus (Skuse). PLoS Negl. Trop. Dis. 2018, 12, e0006544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, J.F. Florida SLE Mosquito—Culex nigripalpus Theobald. EDIS 2017. Available online: https://edis.ifas.ufl.edu/publication/IN136 (accessed on 15 July 2021).
- Rutledge, C.R.; Day, J.F.; Lord, C.C.; Stark, L.M.; Tabachnick, W.J. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J. Med. Entomol. 2003, 40, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.W. History of St. Louis encephalitis. In St. Louis Encephalitis; Monath, T.P., Ed.; American Public Health Association: Washington, DC, USA, 1980; p. 680. [Google Scholar]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito microbiota and implications for disease control. Trends Parasitol. 2020, 36, 98–111. [Google Scholar] [CrossRef]
- Wu, P.; Sun, P.; Nie, K.; Zhu, Y.; Shi, M.; Xiao, C.; Liu, H.; Liu, Q.; Zhao, T.; Chen, X.; et al. A Gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe 2019, 25, 101–112 e105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, J.L.; Short, S.M.; Bahia, A.C.; Saraiva, R.G.; Dong, Y.; Kang, S.; Tripathi, A.; Mlambo, G.; Dimopoulos, G. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog. 2014, 10, e1004398. [Google Scholar] [CrossRef]
- Boissiere, A.; Tchioffo, M.T.; Bachar, D.; Abate, L.; Marie, A.; Nsango, S.E.; Shahbazkia, H.R.; Awono-Ambene, P.H.; Levashina, E.A.; Christen, R.; et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012, 8, e1002742. [Google Scholar] [CrossRef] [Green Version]
- Duguma, D.; Hall, M.W.; Smartt, C.T.; Neufeld, J.D. Effects of organic amendments on microbiota associated with the Culex nigripalpus mosquito vector of the Saint Louis encephalitis and West Nile viruses. mSphere 2017, 2, e00387-16. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.L.; Anderson, S.L.; Lord, C.C.; Tabachnick, W.J. Impact of West Nile virus dose and incubation period on vector competence of Culex nigripalpus (Diptera: Culicidae). Vector Borne Zoonotic Dis. 2011, 11, 1487–1491. [Google Scholar] [CrossRef] [Green Version]
- Agramonte, N.M.; Connelly, C.R. Black salt marsh mosquito Aedes taeniorhynchus (Wiedemann) (Insecta: Diptera: Culicidae). EDIS 2018, EENY591, 1–6. Available online: https://edis.ifas.ufl.edu/publication/in1035 (accessed on 15 July 2021).
- Harrison, B.A.; Byrd, B.D.; Sither, C.B.; Whitt, P.B. The Mosquitoes of the Mid-Atlantic Region: An Identification Guide, 1st ed.; Western Carolina University: Cullowhee, NC, USA, 2016. [Google Scholar]
- Ritchie, S.A.; Addison, D.S. Oviposition preferences of Aedes taeniorhynchus (Diptera: Culicidae) in Florida mangrove forests. Env. Entomol. 1992, 21, 737–744. [Google Scholar] [CrossRef]
- Provost, M.W. The dispersal of Aedes taeniorhynchus I. Preliminary Studies. Mosq. News 1952, 12, 174–190. [Google Scholar]
- Smith, D.R.; Arrigo, N.C.; Leal, G.; Muehlberger, L.E.; Weaver, S.C. Infection and dissemination of Venezuelan equine encephalitis virus in the epidemic mosquito vector, Aedes taeniorhynchus. Am. J. Trop. Med. Hyg. 2007, 77, 176–187. [Google Scholar] [CrossRef]
- Corrin, T.; Ackford, R.; Mascarenhas, M.; Greig, J.; Waddell, L.A. Eastern equine encephalitis virus: A scoping review of the global evidence. Vector Borne Zoonotic Dis. 2021, 21, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Hribar, L.J.; Vlach, J.J.; Demay, D.J.; Stark, L.M.; Stoner, R.L.; Godsey, M.S.; Burkhalter, K.L.; Spoto, M.C.; James, S.S.; Smith, J.M.; et al. Mosquitoes infected with West Nile virus in the Florida Keys, Monroe County, Florida, USA. J. Med. Entomol. 2003, 40, 361–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastwood, G.; Goodman, S.J.; Cunningham, A.A.; Kramer, L.D. Aedes taeniorhynchus vectorial capacity informs a pre-emptive assessment of West Nile virus establishment in Galapagos. Sci. Rep. 2013, 3, 1519. [Google Scholar] [CrossRef] [PubMed]
- Center for Diseaase Control and Prevension (CDC). Eastern Equine Encephalitis (EEE). Available online: https://www.cdc.gov/easternequineencephalitis/tech/epi.html#moremapschartstables (accessed on 15 July 2021).
- Kent, R.J.; Thuma, P.E.; Mharakurwa, S.; Norris, D.E. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am. J. Trop. Med. Hyg. 2007, 76, 267–274. [Google Scholar] [CrossRef]
- Main, B.J.; Lee, Y.; Ferguson, H.M.; Kreppel, K.S.; Kihonda, A.; Govella, N.J.; Collier, T.C.; Cornel, A.J.; Eskin, E.; Kang, E.Y.; et al. The genetic basis of host preference and resting behavior in the major African malaria vector, Anopheles arabiensis. PLoS Genet. 2016, 12, e1006303. [Google Scholar] [CrossRef]
- Pokhrel, V.; DeLisi, N.A.; Danka, R.G.; Walker, T.W.; Ottea, J.A.; Healy, K.B. Effects of truck-mounted, ultra low volume mosquito adulticides on honey bees (Apis mellifera) in a suburban field setting. PLoS ONE 2018, 13, e0193535. [Google Scholar] [CrossRef] [Green Version]
- Bonds, J.A. Ultra-low-volume space sprays in mosquito control: A critical review. Med. Vet. Entomol. 2012, 26, 121–130. [Google Scholar] [CrossRef]
- Kaufman, M.G.; Fonseca, D.M. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu. Rev. Entomol. 2014, 59, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Kawada, H.; Takemura, S.Y.; Arikawa, K.; Takagi, M. Comparative study on nocturnal behavior of Aedes aegypti and Aedes albopictus. J. Med. Entomol. 2005, 42, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingham, G.; Strode, C.; Tran, L.; Khoa, P.T.; Jamet, H.P. Can piperonyl butoxide enhance the efficacy of pyrethroids against pyrethroid-resistant Aedes aegypti? Trop. Med. Int. Health 2011, 16, 492–500. [Google Scholar] [CrossRef]
- Loke, S.R.; Andy-Tan, W.A.; Benjamin, S.; Lee, H.L.; Sofian-Azirun, M. Susceptibility of field-collected Aedes aegypti (L.) (Diptera: Culicidae) to Bacillus thuringiensis israelensis and temephos. Trop. Biomed. 2010, 27, 493–503. [Google Scholar]
- Kahindi, S.C.; Muriu, S.; Derua, Y.A.; Wang, X.; Zhou, G.; Lee, M.C.; Mwangangi, J.; Atieli, H.; Githeko, A.K.; Yan, G. Efficacy and persistence of long-lasting microbial larvicides against malaria vectors in western Kenya highlands. Parasit. Vectors 2018, 11, 438. [Google Scholar] [CrossRef] [Green Version]
- Floore, T.G. Mosquito larval control practices: Past and present. J. Am. Mosq. Control Assoc. 2006, 22, 527–533. [Google Scholar] [CrossRef]
- Walker, A.N.; Bush, P.; Puritz, J.; Wilson, T.; Chang, E.S.; Miller, T.; Holloway, K.; Horst, M.N. Bioaccumulation and metabolic effects of the endocrine disruptor methoprene in the lobster, Homarus americanus. Integr. Comp. Biol. 2005, 45, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.P. Environmental safety review of methoprene and bacterially-derived pesticides commonly used for sustained mosquito control. Ecotoxicol. Environ. Saf. 2017, 139, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kern, W.H. Some small native freshwater fish recommended for mosquito and midge control in ornamental ponds. Ask IFAS 2020, ENY2057, 1–12. Available online: https://edis.ifas.ufl.edu/publication/IN456 (accessed on 15 July 2021).
- Cassiano, E.J.; Hill, J.; Tuckett, Q.; Watson, C. Eastern mosquitofish, Gambusia holbrooki, for control of mosquito larvae. Ask IFAS 2021, FA202, 1–5. Available online: https://edis.ifas.ufl.edu/publication/FA202 (accessed on 15 July 2021). [CrossRef]
- Walton, W.E. Larvivorous fish including Gambusia. J. Am. Mosq. Control Assoc. 2007, 23, 184–220. [Google Scholar] [CrossRef]
- Juliano, S.A. Population dynamics. J. Am. Mosq. Control Assoc. 2007, 23, 265–275. [Google Scholar] [CrossRef]
- Smith, J.P.; Taylor, T.J.; Adams, C.; Tennant, R.; Cope, E.; Walsh, J.; Nomann, B.; Horn, C.; Johnson, F.; Hilson, G.; et al. Updated county mosquito species records for northwest Florida. J. Fla. Mosq. Control Assoc. 2020, 67, 1–9. [Google Scholar]
- Adams, B.; Kapan, D.D. Man bites mosquito: Understanding the contribution of human movement to vector-borne disease dynamics. PLoS ONE 2009, 4, e6763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, L.S.; Nave, C.; Au, D.D.; Guda, T.; Chevez, J.A.; Ray, A.; Holmes, T.C. Circadian regulation of light-evoked attraction and avoidance behaviors in daytime- versus nighttime-biting mosquitoes. Curr. Biol. 2020, 30, 3252–3259 e3253. [Google Scholar] [CrossRef]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef]
- Moise, I.K.; Xue, R.D.; Zulu, L.C.; Beier, J.C. A survey of program capacity and skills of Florida mosquito control districts to conduct arbovirus surveillance and control. J. Am. Mosq. Control Assoc. 2020, 36, 99–106. [Google Scholar] [CrossRef]
- Kluyver, T.; Ragan-Kelley, B.; Pérez, F.; Granger, B.; Bussonnier, M.; Frederic, J.; Kelley, K.; Hamrick, J.; Grout, J.; Corlay, S.; et al. Jupyter Notebooks—A publishing format for reproducible computational workflows. In Positioning and Power in Academic Publishing: Players, Agents and Agendas; Loizides, F., Schmidt, B., Eds.; IOS Press BV: Amsterdam, The Netherlands, 2016. [Google Scholar]
- van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- McKinney, W. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; Volume 445, pp. 51–56. [Google Scholar]
- Staff. QGIS: A Free and Open Source Geographic Information System. Available online: https://www.qgis.org/ (accessed on 15 May 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondapaneni, R.; Malcolm, A.N.; Vazquez, B.M.; Zeng, E.; Chen, T.-Y.; Kosinski, K.J.; Romero-Weaver, A.L.; Giordano, B.V.; Allen, B.; Riles, M.T.; et al. Mosquito Control Priorities in Florida—Survey Results from Florida Mosquito Control Districts. Pathogens 2021, 10, 947. https://doi.org/10.3390/pathogens10080947
Kondapaneni R, Malcolm AN, Vazquez BM, Zeng E, Chen T-Y, Kosinski KJ, Romero-Weaver AL, Giordano BV, Allen B, Riles MT, et al. Mosquito Control Priorities in Florida—Survey Results from Florida Mosquito Control Districts. Pathogens. 2021; 10(8):947. https://doi.org/10.3390/pathogens10080947
Chicago/Turabian StyleKondapaneni, Rishi, Ashley N. Malcolm, Brian M. Vazquez, Eric Zeng, Tse-Yu Chen, Kyle J. Kosinski, Ana L. Romero-Weaver, Bryan V. Giordano, Benjamin Allen, Michael T. Riles, and et al. 2021. "Mosquito Control Priorities in Florida—Survey Results from Florida Mosquito Control Districts" Pathogens 10, no. 8: 947. https://doi.org/10.3390/pathogens10080947
APA StyleKondapaneni, R., Malcolm, A. N., Vazquez, B. M., Zeng, E., Chen, T.-Y., Kosinski, K. J., Romero-Weaver, A. L., Giordano, B. V., Allen, B., Riles, M. T., Killingsworth, D., Campbell, L. P., Caragata, E. P., & Lee, Y. (2021). Mosquito Control Priorities in Florida—Survey Results from Florida Mosquito Control Districts. Pathogens, 10(8), 947. https://doi.org/10.3390/pathogens10080947







