LAMP Assay for the Detection of Echinococcus multilocularis Eggs Isolated from Canine Faeces by a Cost-Effective NaOH-Based DNA Extraction Method
Abstract
1. Introduction
2. Results
2.1. Optimisation, Specificity, Analytical Sensitivity, Limit of Detection, and Stability of LAMP
2.2. Examination of Field Samples with Protocols 1A and 1B
2.2.1. Foxes
2.2.2. Dogs
2.3. Examination of Field Samples with Protocols 1A and 1B According to Worm Burden
2.4. DNA Extraction from Whole Faeces (Protocol 2)
3. Discussion
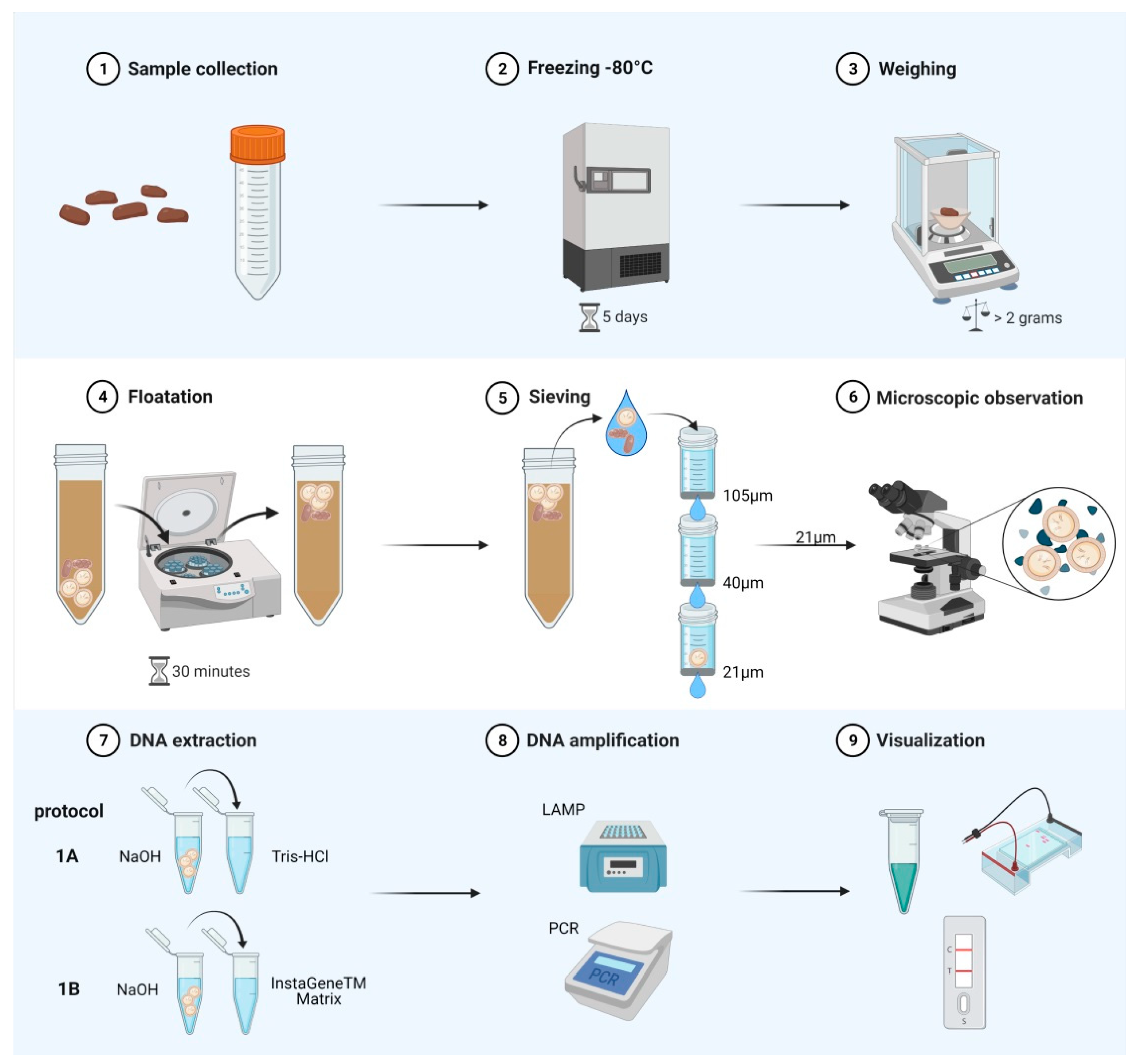
4. Materials and Methods
4.1. Parasites
4.2. LAMP
4.2.1. Primer Design
4.2.2. Specificity and Analytical Sensitivity
4.2.3. LAMP Assay
4.2.4. Visualisation of the LAMP Reaction
4.2.5. Stability of the LAMP Master Mix
4.2.6. Limit of Detection of LAMP
4.3. DNA Extraction (Protocols 1A and 1B)
4.4. Application of LAMP and Multiplex-PCR in Field Samples
4.4.1. Fox Faecal Samples
4.4.2. Dog Faecal Samples
4.5. Application of LAMP and Multiplex-PCR in Whole Faeces (Protocols 2A and 2B)
4.6. Sensitivity and Specificity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef]
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; De La Rue, M. Ecology and life cycle patterns of Echinococcus species. Adv. Parasitol. 2017, 95, 213–314. [Google Scholar] [CrossRef]
- Alvarez Rojas, C.A.; Mathis, A.; Deplazes, P. Assessing the contamination of food and the environment with Taenia and Echinococcus eggs and their zoonotic transmission. Curr. Clin. Microbiol. Rep. 2018, 5, 154–163. [Google Scholar] [CrossRef]
- Eckert, J.; Deplazes, P.; Craig, P.S.; Gemmell, M.A.; Gottstein, B.; Heath, D.; Jenkins, D.J.; Kamiya, M.; Lightowlers, M. Chapter 3 Echinococcosis in animals: Clinical aspects, diagnosis and treatment. In WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; Eckert, J., Gemmell, M.A., Meslin, F.X., Pawlowski, Z.S., Eds.; World Organisation for Animal Health: Paris, France, 2001. [Google Scholar]
- Deplazes, P.; Gottstein, B.; Eckert, J.; Jenkins, D.J.; Ewald, D.; Jimenez-Palacios, S. Detection of Echinococcus coproantigens by enzyme-linked immunosorbent assay in dogs, dingoes and foxes. Parasitol. Res. 1992, 78, 303–308. [Google Scholar] [CrossRef]
- Deplazes, P.; Alther, P.; Tanner, I.; Thompson, R.C.A.; Eckert, J. Echinococcus multilocularis coproantigen detection by enzyme-linked immunosorbent assay in fox, dog, and cat populations. J. Parasitol. 1999, 85, 115–121. [Google Scholar] [CrossRef]
- Sakai, H.; Nonaka, N.; Yagi, K.; Oku, Y.; Kamiya, M. Coproantigen detection in a survey of Echinococcus multilocularis infection among red foxes, Vulpes vulpes schrencki, in Hokkaido, Japan. J. Vet. Med. Sci. 1998, 60, 639–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Machnicka, B.; Dziemian, E.; Rocki, B.; Kołodziej-Sobocińska, M. Detection of Echinococcus multilocularis antigens in faeces by ELISA. Parasitol. Res. 2003, 91, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Dinkel, A.; Von Nickisch-Rosenegk, M.; Bilger, B.; Merli, M.; Lucius, R.; Romig, T. Detection of Echinococcus multilocularis in the definitive host: Coprodiagnosis by PCR as an alternative to necropsy. J. Clin. Microbiol. 1998, 36, 1871–1876. [Google Scholar] [CrossRef]
- Mathis, A.; Deplazes, P.; Eckert, J. An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J. Helminthol. 1996, 70, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Bretagne, S.; Guillou, J.P.; Morand, M.; Houin, R. Detection of Echinococcus multilocularis DNA in fox faeces using DNA amplification. Parasitology 1993, 106, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Dinkel, A.; Mathis, A. Molecular tools for studies on the transmission biology of Echinococcus multilocularis. Parasitology 2003, 127, S53–S61. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Casulli, A.; Conraths, F.J.; Müller, N. Chapter Three—Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Adv. Parasitol. 2017, 96, 159–257. [Google Scholar] [PubMed]
- Yimam, A.E.; Nonaka, N.; Oku, Y.; Kamiya, M. Prevalence and intensity of Echinococcus multilocularis in red foxes (Vulpes vulpes schrencki) and raccoon dogs (Nyctereutes procyonoides albus) in Otaru City, Hokkaido, Japan. Jpn. J. Vet. Res. 2002, 49, 287–296. [Google Scholar]
- Trachsel, D.; Deplazes, P.; Mathis, A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 2007, 134, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, P.; Schares, G.; Press, S.; Fröhlich, A.; Basso, W.; Herzig, M.; Conraths, F.J. Comparison of different commercial DNA extraction kits and PCR protocols for the detection of Echinococcus multilocularis eggs in faecal samples from foxes. Vet. Parasitol. 2017, 237, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Videnska, P.; Smerkova, K.; Zwinsova, B.; Popovici, V.; Micenkova, L.; Sedlar, K.; Budinska, E. Stool sampling and DNA isolation kits affect DNA quality and bacterial composition following 16S rRNA gene sequencing using MiSeq Illumina platform. Sci. Rep. 2019, 9, 3837. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Grab, D.J.; Lonsdale-Eccles, J.; Inoue, N. Lamp for tadpoles. Nat. Methods 2005, 2, 635. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.-J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef]
- Ni, X.; Mcmanus, D.P.; Yan, H.; Yang, J.; Lou, Z.; Li, H.; Li, L.; Lei, M.; Cai, J.; Fan, Y.; et al. Loop-mediated isothermal amplification (LAMP) assay for the identification of Echinococcus multilocularis infections in canine definitive hosts. Parasites Vectors 2014, 7, 254. [Google Scholar] [CrossRef]
- Feng, K.; Li, W.; Guo, Z.; Duo, H.; Fu, Y.; Shen, X.; Tie, C.; Xiao, C.; Luo, Y.; Qi, G.; et al. Development of LAMP assays for the molecular detection of taeniid infection in canine in Tibetan rural area. J. Vet. Med. Sci. 2017, 79, 1986–1993. [Google Scholar] [CrossRef]
- Avila, H.G.; Mozzoni, C.; Trangoni, M.D.; Cravero, S.L.P.; Pérez, V.M.; Valenzuela, F.; Gertiser, M.L.; Butti, M.J.; Kamenetzky, L.; Jensen, O.; et al. Development of a copro-LAMP assay for detection of several species of Echinococcus granulosus sensu lato complex. Vet. Parasitol. 2020, 277, 109017. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-W.; Mcmanus, D.P.; Lou, Z.-Z.; Yang, J.-F.; Yan, H.-B.; Li, L.; Li, H.-M.; Liu, Q.-Y.; Li, C.-H.; Shi, W.-G.; et al. A comparison of loop-mediated isothermal amplification (LAMP) with other surveillance tools for Echinococcus granulosus diagnosis in canine definitive hosts. PLoS ONE 2014, 9, e100877. [Google Scholar] [CrossRef] [PubMed]
- Salant, H.; Abbasi, I.; Hamburger, J. The development of a loop-mediated isothermal amplification method (LAMP) for Echinococcus granulosus coprodetection. Am. J. Trop. Med. Hyg. 2012, 87, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, M.; Mackenstedt, U.; Romig, T. A loop-mediated isothermal amplification (LAMP) method for the identification of species within the Echinococcus granulosus complex. Vet. Parasitol. 2014, 200, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.M.; Song, H.B.; Jin, Y.; Oh, J.-K.; Lim, M.K.; Hong, S.-T.; Choi, M.-H. Application of a loop-mediated isothermal amplification (LAMP) assay targeting cox1 gene for the detection of Clonorchis sinensis in human fecal samples. PLoS Negl. Trop. Dis. 2017, 11, e0005995. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Fernández-Medina, C.; Cruz-Fernández, S.; Crego-Vicente, B.; Febrer-Sendra, B.; García-Bernalt Diego, J.; Gorgojo-Galindo, Ó.; López-Abán, J.; Vicente Santiago, B.; Muro Álvarez, A. Whip-LAMP: A novel LAMP assay for the detection of Trichuris muris-derived DNA in stool and urine samples in a murine experimental infection model. Parasites Vectors 2020, 13, 552. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Sánchez-Hernández, A.; Gandasegui, J.; Bajo Santos, C.; López-Abán, J.; Saugar, J.M.; Rodríguez, E.; Vicente, B.; Muro, A. Strong-LAMP: A LAMP Assay for Strongyloides spp. Detection in Stool and Urine Samples. Towards the Diagnosis of Human Strongyloidiasis Starting from a Rodent Model. PLoS Negl. Trop. Dis. 2016, 10, e0004836. [Google Scholar] [CrossRef]
- Mugambi, R.M.; Agola, E.L.; Mwangi, I.N.; Kinyua, J.; Shiraho, E.A.; Mkoji, G.M. Development and evaluation of a Loop Mediated Isothermal Amplification (LAMP) technique for the detection of hookworm (Necator americanus) infection in fecal samples. Parasites Vectors 2015, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Nkouawa, A.; Sako, Y.; Nakao, M.; Nakaya, K.; Ito, A. Loop-mediated isothermal amplification method for differentiation and rapid detection of Taenia species. J. Clin. Microbiol. 2009, 47, 168–174. [Google Scholar] [CrossRef]
- Steiner, J.J.; Polemba, C.J.; Fjellstrom, R.G.; Elliott, L.F. A rapid one-tube genomic DNA extraction process for PCR and RAPD analyses. Nucleic Acids Res. 1995, 23, 2569–2570. [Google Scholar] [CrossRef]
- De Lamballerie, X.; Zandotti, C.; Vignoli, C.; Bollet, C.; De Micco, P. A one-step microbial DNA extraction method using “Chelex 100” suitable for gene amplification. Res. Microbiol. 1992, 143, 785–790. [Google Scholar] [CrossRef]
- Kawasaki, E.S. Sample preparation from blood, cells and other fluids. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 146–152. [Google Scholar]
- Wang, H.; Qi, M.; Cutler, A.J. A simple method of preparing plant samples for PCR. Nucleic Acids Res. 1993, 21, 4153–4154. [Google Scholar] [CrossRef] [PubMed]
- Osmundson, T.W.; Eyre, C.A.; Hayden, K.M.; Dhillon, J.; Garbelotto, M.M. Back to basics: An evaluation of NaOH and alternative rapid DNA extraction protocols for DNA barcoding, genotyping, and disease diagnostics from fungal and oomycete samples. Mol. Ecol. Resour. 2013, 13, 66–74. [Google Scholar] [CrossRef]
- Satya, P.; Mitra, S.; Ray, D.P.; Mahapatra, B.S.; Karan, M.; Jana, S.; Sharma, A.K. Rapid and inexpensive NaOH based direct PCR for amplification of nuclear and organelle DNA from ramie (Boehmeria nivea), a bast fibre crop containing complex polysaccharides. Ind. Crop. Prod. 2013, 50, 532–536. [Google Scholar] [CrossRef]
- Werner, O.; Ros, R.M.; Guerra, J. Direct amplification and NaOH extraction: Two rapid and simple methods for preparing bryophyte DNA for polymerase chain reaction (PCR). J. Bryol. 2002, 24, 127–131. [Google Scholar] [CrossRef]
- Hüttner, M.; Nakao, M.; Wassermann, T.; Siefert, L.; Boomker, J.D.F.; Dinkel, A.; Sako, Y.; Mackenstedt, U.; Romig, T.; Ito, A. Genetic characterization and phylogenetic position of Echinococcus felidis Ortlepp, 1937 (Cestoda: Taeniidae) from the African lion. Int. J. Parasitol. 2008, 38, 861–868. [Google Scholar] [CrossRef]
- Mulinge, E.; Magambo, J.; Odongo, D.; Njenga, S.; Zeyhle, E.; Mbae, C.; Kagendo, D.; Addy, F.; Ebi, D.; Wassermann, M.; et al. Molecular characterization of Echinococcus species in dogs from four regions of Kenya. Vet. Parasitol. 2018, 255, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Sako, Y.; Ito, A. Isolation of polymorphic microsatellite loci from the tapeworm Echinococcus multilocularis. Infect. Genet. Evol. 2003, 3, 159–163. [Google Scholar] [CrossRef]
- Wassermann, M.; Aschenborn, O.; Aschenborn, J.; Mackenstedt, U.; Romig, T. A sylvatic lifecycle of Echinococcus equinus in the Etosha National Park, Namibia. Int. J. Parasitol. Parasites Wildl. 2015, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Rong, R.; Zhang, H.Q.; Shi, C.J.; Zhu, X.Q.; Xia, C.M. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int. J. Parasitol. 2010, 40, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Q.; Xu, M.J.; Wang, Y.H.; Qiu, D.Y.; Liu, G.X.; Lin, A.; Tang, J.D.; Zhang, R.L.; Zhu, X.Q. Sensitive and rapid detection of Clonorchis sinensis infection in fish by loop-mediated isothermal amplification (LAMP). Parasitol. Res. 2010, 106, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Karamon, J.; Samorek-Pieróg, M.; Dąbrowska, J.; Kochanowski, M.; Sroka, J.; Bilska-Zając, E.; Cencek, T. Comparison of two DNA extraction methods and two PCRs for detection of Echinococcus multilocularis in the stool samples of naturally infected red foxes. Animals 2020, 10, 2381. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Ma, L.; Kontogeorgos, I.; Xueyong, Z.; Li, X.; Karanis, P. Contamination of wastewater with Echinococcus multilocularis—Possible implications for drinking water resources in the QTP China. Water Res. 2020, 170, 115334. [Google Scholar] [CrossRef]
- Chamai, M.; Omadang, L.; Erume, J.; Ocaido, M.; Oba, P.; Othieno, E.; Bonaventure, S.; Kitibwa, A. Identification of Echinococcus granulosus strains using polymerase chain reaction–restriction fragment length polymorphism amongst livestock in Moroto district, Uganda. Onderstepoort J. Vet. Res. 2016, 83, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapel, C.M.O.; Torgerson, P.R.; Thompson, R.C.A.; Deplazes, P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int. J. Parasitol. 2006, 36, 79–86. [Google Scholar] [CrossRef]
- Guggisberg, A.R.; Alvarez Rojas, C.A.; Kronenberg, P.A.; Miranda, N.; Deplazes, P. A sensitive, one-way sequential sieving method to isolate helminths’ eggs and protozoal oocysts from lettuce for genetic identification. Pathogens 2020, 9, 624. [Google Scholar] [CrossRef]
- Bodar, C.W.M.; Pronk, M.E.J.; Sijm, D.T.H.M. The European Union risk assessment on zinc and zinc compounds: The process and the facts. Integr. Environ. Assess. Manag. 2005, 1, 301–319. [Google Scholar] [CrossRef]
- Matsuo, K.; Kamiya, H. Modified sugar centrifugal flotation technique for recovering Echinococcus multilocularis eggs from soil. J. Parasitol. 2005, 91, 208–209. [Google Scholar] [CrossRef]
- Mathis, A.; Deplazes, P. Copro-DNA tests for diagnosis of animal taeniid cestodes. Parasitol. Int. 2006, 55, S87–S90. [Google Scholar] [CrossRef]
- Meeker, N.D.; Hutchinson, S.A.; Ho, L.; Trede, N.S. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques 2007, 43, 610–614. [Google Scholar] [CrossRef]
- Boubaker, G.; Macchiaroli, N.; Prada, L.; Cucher, M.A.; Rosenzvit, M.C.; Ziadinov, I.; Deplazes, P.; Saarma, U.; Babba, H.; Gottstein, B.; et al. A Multiplex PCR for the simultaneous detection and genotyping of the Echinococcus granulosus complex. PLoS Negl. Trop. Dis. 2013, 7, e2017. [Google Scholar] [CrossRef] [PubMed]
- Sean Walsh, P.; Metzger, D.A.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 2013, 54, 134–139. [Google Scholar] [CrossRef]
- Singh, U.A.; Kumari, M.; Iyengar, S. Method for improving the quality of genomic DNA obtained from minute quantities of tissue and blood samples using Chelex 100 resin. Biol. Proced. Online 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, E.; Mcintosh, C.; Ralls, K.; Fleischer, R. A noninvasive method for distinguishing among canid species: Amplification and enzyme restriction of DNA from dung. Mol. Ecol. 1997, 6, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Stefanic, S.; Shaikenov, B.S.; Deplazes, P.; Dinkel, A.; Torgerson, P.R.; Mathis, A. Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol. Res. 2004, 92, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Laurimäe, T.; Kronenberg, P.A.; Alvarez Rojas, C.A.; Ramp, T.W.; Eckert, J.; Deplazes, P. Long-term (35 years) cryopreservation of Echinococcus multilocularis metacestodes. Parasitology 2020, 147, 1048–1054. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; Mcmanus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef]
- ECC Ltd. PrimerExplorer V5. Available online: http://primerexplorer.jp/lampv5e/index.html (accessed on 24 August 2020).
- PREMIER Biosoft. LAMP Designer. Available online: http://www.premierbiosoft.com/isothermal/index.html (accessed on 24 August 2020).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Kamber, T.; Koekemoer, L.L.; Mathis, A. Loop-mediated isothermal amplification (LAMP) assays for Anopheles funestus group and Anopheles gambiae complex species. Med. Vet. Entomol. 2020, 34, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Rojas, C.A.; Kronenberg, P.A.; Aitbaev, S.; Omorov, R.A.; Abdykerimov, K.K.; Paternoster, G.; Mullhaupt, B.; Torgerson, P.; Deplazes, P. Genetic diversity of Echinococcus multilocularis and Echinococcus granulosus sensu lato in Kyrgyzstan: The A2 haplotype of E. multilocularis is the predominant variant infecting humans. PLoS Negl. Trop. Dis. 2020, 14, e0008242. [Google Scholar] [CrossRef] [PubMed]
| Host | Number of Animals Confirmed Positive (+) or Without (–) Detected E. multilocularis Infection (Method for Diagnosis) | Protocol 1A: NaOH/Dilution 1:50 with Tris-HCl | Protocol 1B: NaOH + InstaGeneTM Matrix | ||||
|---|---|---|---|---|---|---|---|
| Microscopic Detection of Taeniid Eggs 2 | LAMP Positive | Multiplex-PCR 1 Positive | Microscopic Detection of Taeniid Eggs 2 | LAMP Positive | Multiplex-PCR 1 Positive | ||
| Foxes | 44 + (necropsy/SCT) | 30 positive | 25 | 29 | 31 positive | 28 | 27 |
| 14 negative | 1 | 3 | 13 negative | 4 | 3 | ||
| 18 − (necropsy/SCT) | 5 positive | 1 | 1 | 5 positive | 1 | 1 | |
| 13 negative | 0 | 0 | 13 negative | 0 | 0 | ||
| Dogs | 22 + (multiplex-PCR 1) | 20 positive | 20 | 20 | 21 positive | 21 | 21 |
| 2 negative | 0 | 0 | 1 negative | 0 | 0 | ||
| 10 − (multiplex-PCR 1) | 10 negative | 0 | 0 | 10 negative | 0 | 0 | |
| Foxes Protocol 1A | Foxes Protocol 1B | Dogs Protocol 1A | Dogs Protocol 1B | |||||
|---|---|---|---|---|---|---|---|---|
| LAMP | Multiplex-PCR | LAMP | Multiplex-PCR | LAMP | Multiplex-PCR | LAMP | Multiplex-PCR | |
| Sensitivity (IC 95%) | 59 (43.2–73.6) | 72.7 (57.2–85) | 72.7 (57.2–85) | 68.1 (52.4–81.3) | 90.9 (70.8–98.8) | 90.9 (70.8–98.8) | 95.4 (77.1–99.8) | 95.4 (77.1–99.8) |
| Specificity (IC 95%) | 94.4 (72.7–99.8) | 94.4 (72.7–99.8) | 94.4 (72.7–99.8) | 94.4 (72.7–99.8) | 100 (69.1–100) | 100 (69.1–100) | 100 (69.1–100) | 100 (69.1–100) |
| Protocol 1A: NaOH/Dilution 1:50 Tris-HCl | Protocol 1B: NaOH + InstaGeneTM Matrix | ||||
|---|---|---|---|---|---|
| # E. multilocularis at SCT | # Examined | LAMP Positive (Sensitivity) | Multiplex-PCR Positive (Sensitivity) | LAMP Positive (Sensitivity) | Multiplex-PCR Positive (Sensitivity) |
| 0 | 17 | 1 | 1 | 1 | 1 |
| 2–20 | 9 | 1 (11.1%) | 1 (11.1%) | 2 (18.1%) | 2 (18.1%) |
| 21–100 | 7 | 4 (57.1%) | 6 (85.7%) | 6 (85.7%) | 7 (100%) |
| >100 | 12 | 10 (83.3%) | 12 (100%) | 12 (100%) | 12 (100%) |
| Host | Number of Animals Confirmed Positive (+) or Without (–) Detected E. multilocularis infection (Method for Diagnosis) | Protocol 2A: NaOH + InstaGeneTM Matrix | Protocol 2B: QIAamp DNA Stool Mini Kit | ||
|---|---|---|---|---|---|
| LAMP Positive | Multiplex-PCR 1 Positive | LAMP Positive | Multiplex-PCR 1 Positive | ||
| Foxes | 30 + (necropsy/SCT) | 10 2 | 17 | 16 2 | 20 |
| 5 − (necropsy/SCT) | 0 | 0 | 0 | 0 | |
| Dogs | 18 + (multiplex-PCR) | 2 2 | 7 | 7 | 6 |
| 10 − (multiplex-PCR) | 0 | 0 | 0 | 0 | |
| Primer | Sequence (5′–3′) | 5′-Modification (LFD) |
|---|---|---|
| Em-F3 | GCTTGTTGTTGTTTCCATTGA | - |
| Em-B3 | ACAAAACCACCACCAACC | - |
| Em-FIP | TCCCTTTCAGACTCCCCATAATCA-TTTTTGGTGTGTGTGCTATG | FAM |
| Em-BIP | AGCGGTATATACTTTACGTGTTTGT-TCATTACAACAATCAACCATGA | DIG |
| Em-LB | TTGCTTGTGAGTATATAGTTGTATATGTGT | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucher, B.J.; Muchaamba, G.; Kamber, T.; Kronenberg, P.A.; Abdykerimov, K.K.; Isaev, M.; Deplazes, P.; Alvarez Rojas, C.A. LAMP Assay for the Detection of Echinococcus multilocularis Eggs Isolated from Canine Faeces by a Cost-Effective NaOH-Based DNA Extraction Method. Pathogens 2021, 10, 847. https://doi.org/10.3390/pathogens10070847
Bucher BJ, Muchaamba G, Kamber T, Kronenberg PA, Abdykerimov KK, Isaev M, Deplazes P, Alvarez Rojas CA. LAMP Assay for the Detection of Echinococcus multilocularis Eggs Isolated from Canine Faeces by a Cost-Effective NaOH-Based DNA Extraction Method. Pathogens. 2021; 10(7):847. https://doi.org/10.3390/pathogens10070847
Chicago/Turabian StyleBucher, Barbara J., Gillian Muchaamba, Tim Kamber, Philipp A. Kronenberg, Kubanychbek K. Abdykerimov, Myktybek Isaev, Peter Deplazes, and Cristian A. Alvarez Rojas. 2021. "LAMP Assay for the Detection of Echinococcus multilocularis Eggs Isolated from Canine Faeces by a Cost-Effective NaOH-Based DNA Extraction Method" Pathogens 10, no. 7: 847. https://doi.org/10.3390/pathogens10070847
APA StyleBucher, B. J., Muchaamba, G., Kamber, T., Kronenberg, P. A., Abdykerimov, K. K., Isaev, M., Deplazes, P., & Alvarez Rojas, C. A. (2021). LAMP Assay for the Detection of Echinococcus multilocularis Eggs Isolated from Canine Faeces by a Cost-Effective NaOH-Based DNA Extraction Method. Pathogens, 10(7), 847. https://doi.org/10.3390/pathogens10070847







