Rickettsia parkeri with a Genetically Disrupted Phage Integrase Gene Exhibits Attenuated Virulence and Induces Protective Immunity against Fatal Rickettsioses in Mice
Abstract
1. Introduction
2. Results
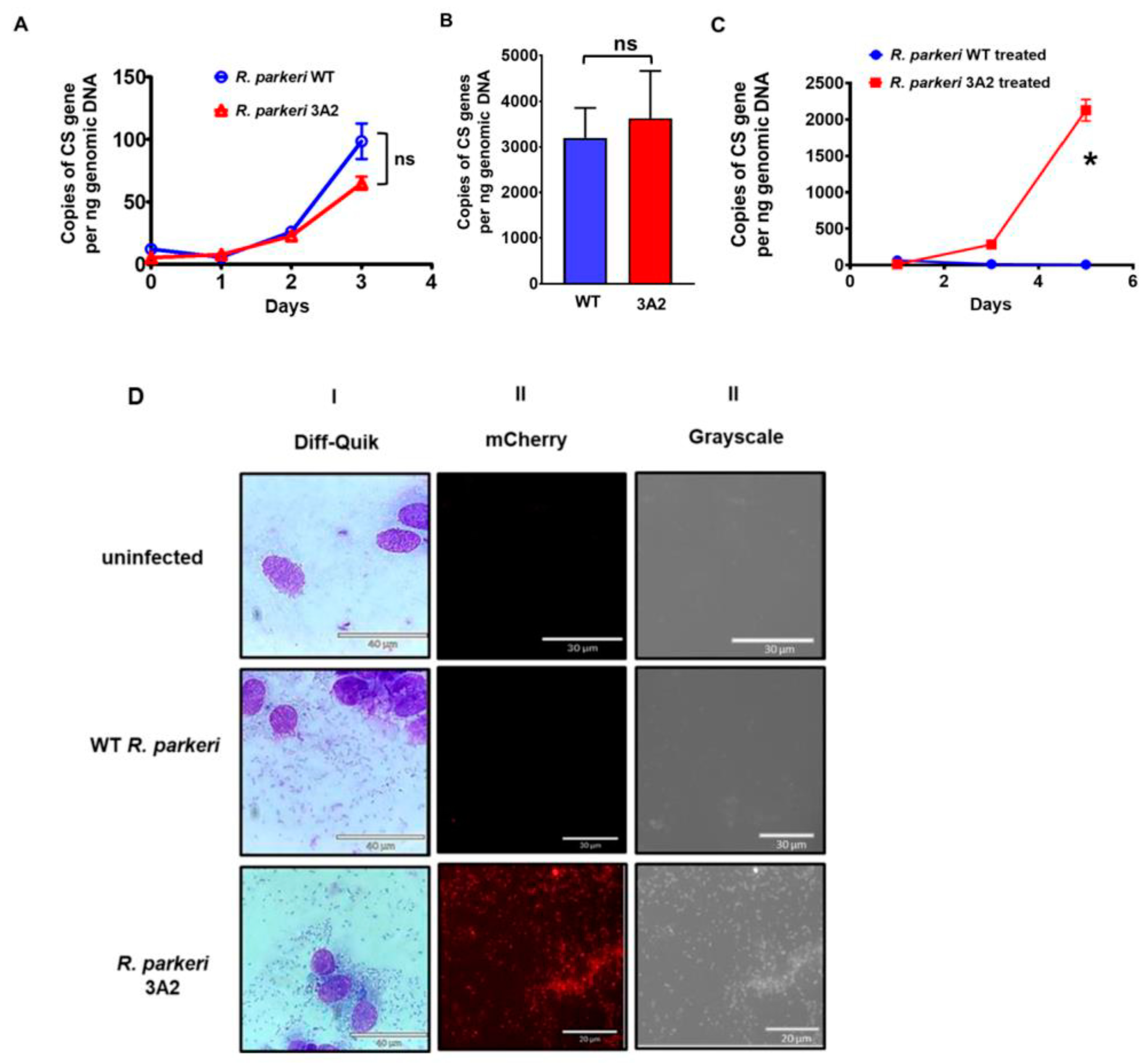
2.1. Mapping of Himar1 Transposon Insertion Site and Phenotypic Characterization of R. parkeri 3A2
2.2. Genetic Disruption of Phage Integrase Family Protein RPATATE_0245 of R. parkeri Results in Virulence Attenuation In Vitro
2.3. Evaluation of Safety and Immunogenicity of R. parkeri 3A2 in a Mouse Model of Rickettsioses
2.4. A Single Dose Immunization of R. parkeri 3A2 Induces Potent and Non-Transient Antibody Response Associated with Rickettsial Clearance In Vivo
2.5. A Single Dose Immunization of R. parkeri 3A2 Confers Protection against Fatal Murine Spotted Fever Rickettsioses
3. Discussion
4. Materials and Methods
4.1. Construction of Random R. parkeri Transposon Mutants
4.2. Genetic Sequencing of R. parkeri 3A2 and Verification of Insertion Region
4.3. In Vitro Growth Kinetics of R. parkeri 3A2
4.4. Imaging Fluorescence-Expressing R. parkeri
4.5. Culture and Purification of R. parkeri and Its Mutant 3A2 As Well As R. conorii
4.6. Plaque Assay
4.7. Growth of R. parkeri 3A2 in Human Macrophage-Like Cells
4.8. Quantification of R. parkeri WT and 3A2 by Real-Time PCR
4.9. Evaluation of the Virulence, Immunogenicity and Protective Efficacy of R. parkeri 3A2 In Vivo
4.10. Cytokine ELISA
4.11. Assessment of Rickettsiae-Specific Antibody Response by IFA
4.12. Evaluation of the Isotype of R. parkeri-Specific IgG Antibody by ELISA
4.13. Histopathological Analyses
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nelder, M.P.; Russell, C.B.; Johnson, S.; Li, Y.; Cronin, K.; Warshawsky, B.; Brandon, N.; Patel, S.N. Assessing human exposure to spotted fever and typhus group rickettsiae in Ontario, Canada (2013–2018): A retrospective, cross-sectional study. BMC Infect. Dis. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, J.S.; Macaluso, K.R.; Smith, N.; Zaki, S.R.; Paddock, C.D.; Davis, J.; Peterson, N.; Azad, A.F.; Rosenberg, R. Fatal spotted fever rickettsiosis, Kenya. Emerg. Infect. Dis. 2004, 10, 910–913. [Google Scholar] [CrossRef]
- Demma, L.J.; Traeger, M.S.; Nicholson, W.L.; Paddock, C.D.; Blau, D.M.; Eremeeva, M.E.; Dasch, G.A.; Levin, M.L.; Singleton, J., Jr.; Zaki, S.R.; et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med. 2005, 353, 587–594. [Google Scholar] [CrossRef]
- De Oliveira, S.V.; Angerami, R.N. Timeliness in the notification of spotted fever in Brazil: Evaluating compulsory reporting strategies and digital disease detection. Int. J. Infect. Dis. 2018, 72, 16–18. [Google Scholar] [CrossRef]
- Szabó, M.P.J.; Pinter, A.; Labruna, M.B. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front. Cell. Infect. Microbiol. 2013, 3, 27. [Google Scholar] [CrossRef]
- Angerami, R.N.; Câmara, M.; Pacola, M.R.; Rezende, R.C.; Duarte, R.M.; Nascimento, E.M.; Colombo, S.; Santos, F.C.; Leite, R.M.; Katz, G.; et al. Features of Brazilian spotted fever in two different endemic areas in Brazil. Ticks Tick-borne Dis. 2012, 3, 346–348. [Google Scholar] [CrossRef]
- Walker, D.H. The realities of biodefense vaccines against Rickettsia. Vaccine 2009, 27, D52–D55. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.A.; Anacker, R.L.; Mann, R.E. Extraction of protective components of Rickettsia rickettsii with n-Octyl-β-D-Glucopyranoside. Rev. Infect. Dis. 1988, 10, S382–S385. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H. Rickettsia rickettsii: As virulent as ever. Am. J. Trop. Med. Hyg. 2002, 66, 448–449. [Google Scholar] [CrossRef]
- Richards, A.L. Rickettsial vaccines: The old and the new. Expert Rev. Vaccines 2004, 3, 541–555. [Google Scholar] [CrossRef]
- Alhassan, A.; Liu, H.; McGill, J.; Cerezo, A.; Jakkula, L.U.M.R.; Nair, A.D.S.; Winkley, E.; Olson, S.; Marlow, D.; Sahni, A.; et al. Rickettsia rickettsii whole-cell antigens offer protection against Rocky Mountain spotted fever in the canine host. Infect. Immun. 2019, 87, e00628-28. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.P.; Jordan, M.E.; Gelfand, H.M. Immunization of man against epidemic typhus by infection with avirulent Rickettsia prowazeki strain E. IV. Persistence of immunity and a note as to differing complement-fixation antigen requirements in post-infection and post-vaccination sera. J. Immunol. 1957, 79, 348–354. [Google Scholar]
- Wisseman, C.L., Jr. Concepts of louse-borne typhus control in developing countries: The use of the living attenuated E strain typhus vaccine in epidemic and endemic situations. Adv. Exp. Med. Biol. 1972, 31, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.W.; Sims, K.G.; Jones, D.C.; Anderson, B.E. Protection of guinea-pigs from experimental Rocky Mountain spotted fever by immunization with baculovirus-expressed Rickettsia rickettsii rOmpA protein. Vaccine 1995, 13, 29–35. [Google Scholar] [CrossRef]
- Vishwanath, S.; McDonald, G.A.; Watkins, N.G. A recombinant Rickettsia conorii vaccine protects guinea pigs from experimental boutonneuse fever and Rocky Mountain spotted fever. Infect. Immun. 1990, 58, 646–653. [Google Scholar] [CrossRef]
- Gazi, M.; Caro-Gomez, E.; Góez, Y.; Cespedes, M.A.; Hidalgo, M.; Correa, P.; Valbuena, G. Discovery of a protective Rickettsia prowazekii antigen recognized by CD8+ T cells, RP884, using an in vivo screening platform. PLoS ONE 2013, 8, e76253. [Google Scholar] [CrossRef]
- Noriea, N.F.; Clark, T.R.; Hackstadt, T. Targeted knockout of the Rickettsia rickettsii OmpA surface antigen does not diminish virulence in a mammalian model system. mBio 2015, 6, e00323-15. [Google Scholar] [CrossRef]
- Lehman, S.S.; Noriea, N.F.; Aistleitner, K.; Clark, T.R.; Dooley, C.A.; Nair, V.; Kaur, S.J.; Rahman, M.S.; Gillespie, J.J.; Azad, A.F.; et al. The rickettsial Ankyrin repeat protein 2 is a Type IV secreted effector that associates with the endoplasmic reticulum. mBio 2018, 9, e00975-18. [Google Scholar] [CrossRef] [PubMed]
- Policastro, P.F.; Hackstadt, T. Differential activity of Rickettsia rickettsii ompA and ompB promoter regions in a heterologous reporter gene system. Microbiology 1994, 140, 2941–2949. [Google Scholar] [CrossRef]
- Radulovic, S.; Troyer, J.M.; Beier, M.S.; Lau, A.O.T.; Azad, A.F. Identification and molecular analysis of the gene encoding Rickettsia typhi hemolysin. Infect. Immun. 1999, 67, 6104–6108. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, T.; Popov, V.L.; Yu, X.-J.; Walker, D.H.; Bouyer, D.H. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica Serovar Typhimurium mediates phagosomal escape. Infect. Immun. 2005, 73, 6668–6673. [Google Scholar] [CrossRef]
- Sunyakumthorn, P.; Petchampai, N.; Kearney, M.T.; Sonenshine, D.E.; Macaluso, K.R. Molecular characterization and tissue-specific gene expression of Dermacentor variabilis α-catenin in response to rickettsial infection. Insect Mol. Biol. 2012, 21, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Rennoll-Bankert, K.E.; Rahman, M.S.; Gillespie, J.J.; Guillotte, M.L.; Kaur, S.J.; Lehman, S.S.; Beier-Sexton, M.; Azad, A.F. Which way in? The RalF Arf-GEF orchestrates Rickettsia host cell invasion. PLoS Pathog. 2015, 11, e1005115. [Google Scholar] [CrossRef]
- Guillotte, M.L.; Gillespie, J.J.; Chandler, C.E.; Rahman, M.S.; Ernst, R.K.; Azad, A.F. Rickettsia lipid A biosynthesis utilizes the late acyltransferase LpxJ for secondary fatty acid addition. J. Bacteriol. 2018, 200, e00334-18. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.J.; Kaur, S.J.; Rahman, M.S.; Rennoll-Bankert, K.; Sears, K.T.; Beier-Sexton, M.; Azad, A.F. Secretome of obligate intracellular Rickettsia. FEMS Microbiol. Rev. 2015, 39, 47–80. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Burkhardt, N.Y.; Felsheim, R.F.; Kurtti, T.J.; Munderloh, U.G. Motility characteristics are altered for Rickettsia bellii transformed to overexpress a heterologous rickA gene. Appl. Environ. Microbiol. 2014, 80, 1170–1176. [Google Scholar] [CrossRef]
- McClure, E.E.; Chávez, A.S.O.; Shaw, D.K.; Carlyon, J.A.; Ganta, R.R.; Noh, S.M.; Wood, D.O.; Bavoil, P.M.; Brayton, K.A.; Martinez, J.J.; et al. Engineering of obligate intracellular bacteria: Progress, challenges and paradigms. Nat. Rev. Microbiol. 2017, 15, 544–558. [Google Scholar] [CrossRef]
- Paddock, C.D.; Sumner, J.W.; Comer, J.A.; Zaki, S.R.; Goldsmith, C.S.; Goddard, J.; McLellan, S.L.F.; Tamminga, C.L.; Ohl, C.A. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004, 38, 805–811. [Google Scholar] [CrossRef]
- Allerdice, M.E.; Hecht, J.A.; Lash, R.R.; Karpathy, S.E.; Paddock, C.D. Rickettsia parkeri and “Candidatus Rickettsia andeanae” in Amblyomma maculatum (Acari: Ixodidae) collected from the Atlanta metropolitan area, Georgia, United States. Ticks Tick-borne Dis. 2019, 10, 1066–1069. [Google Scholar] [CrossRef]
- Paddock, C.D.; Fournier, P.-E.; Sumner, J.W.; Goddard, J.; Elshenawy, Y.; Metcalfe, M.G.; Loftis, A.D.; Varela-Stokes, A. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl. Environ. Microbiol. 2010, 76, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Felsheim, R.F.; Herron, M.J.; Nelson, C.M.; Burkhardt, N.Y.; Barbet, A.F.; Kurtti, T.J.; Munderloh, U.G. Transformation of Anaplasma phagocytophilum. BMC Biotechnol. 2006, 6, 42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, C.; Nair, A.D.S.; Indukuri, V.V.; Gong, S.; Felsheim, R.F.; Jaworski, D.; Munderloh, U.G.; Ganta, R.R. Targeted and random mutagenesis of Ehrlichia chaffeensis for the identification of genes required for in vivo infection. PLoS Pathog. 2013, 9, e1003171. [Google Scholar] [CrossRef]
- Lampe, D.J.; Akerley, B.; Rubin, E.J.; Mekalanos, J.J.; Robertson, H.M. Hyperactive transposase mutants of the Himar1 mariner transposon. Proc. Natl. Acad. Sci. USA 1999, 96, 11428–11433. [Google Scholar] [CrossRef]
- Curto, P.; Simões, I.; Riley, S.P.; Martinez, J.J. Differences in intracellular fate of two spotted fever group Rickettsia in macrophage-like cells. Front. Cell. Infect. Microbiol. 2016, 6, 80. [Google Scholar] [CrossRef]
- Londoño, A.F.; Mendell, N.L.; Valbuena, G.A.; Routh, A.L.; Wood, T.G.; Widen, S.G.; Rodas, J.D.; Walker, D.H.; Bouyer, D.H. Whole-genome sequence of Rickettsia parkeri strain Atlantic Rainforest, isolated from a Colombian tick. Microbiol. Resour. Announc. 2019, 8, e00684-19. [Google Scholar] [CrossRef]
- Firacative, C.; Gressler, A.E.; Schubert, K.; Schulze, B.; Müller, U.; Brombacher, F.; Von Bergen, M.; Alber, G. Identification of T helper (Th)1- and Th2-associated antigens of Cryptococcus neoformans in a murine model of pulmonary infection. Sci. Rep. 2018, 8, 2681. [Google Scholar] [CrossRef]
- Walker, D.H.; Popov, V.L.; Wen, J.; Feng, H.M. Rickettsia conorii infection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab. Investig. 1994, 70, 358–368. [Google Scholar] [PubMed]
- Davidsson, M. The financial implications of a well-hidden and ignored chronic Lyme disease pandemic. Healthcare 2018, 6, 16. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Weinstock, G.; Walker, D.H.; Yu, X.-J. Inactivation of SAM-Methyltransferase is the mechanism of attenuation of a historic louse borne typhus vaccine strain. PLoS ONE 2014, 9, e113285. [Google Scholar] [CrossRef]
- Balayeva, N.M.; Eremeeva, M.E.; Ignatovich, V.F.; Dmitriev, B.A.; Lapina, E.B.; Belousova, L.S. Protein antigens of genetically related Rickettsia prowazekii strains with different virulence. Acta Virol. 1992, 36, 52–56. [Google Scholar]
- Zhang, J.-Z.; Hao, J.-F.; Walker, D.H.; Yu, X.-J. A mutation inactivating the methyltransferase gene in avirulent Madrid E strain of Rickettsia prowazekii reverted to wild type in the virulent revertant strain Evir. Vaccine 2006, 24, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Clavero, G.; Perez-Gallardo, F. Estudio experimental da una cepa apatogenicay immunizante de Rickettsia prowazekii. Rev. Sanidad. Hlg. Pub. 1943, 17, 1–27. [Google Scholar]
- Groth, A.C.; Calos, M.P. Phage Integrases: Biology and Applications. J. Mol. Biol. 2004, 335, 667–678. [Google Scholar] [CrossRef]
- Keravala, A.; Groth, A.C.; Jarrahian, S.; Thyagarajan, B.; Hoyt, J.J.; Kirby, P.J.; Calos, M.P. A diversity of serine phage integrases mediate site-specific recombination in mammalian cells. Mol. Genet. Genom. 2006, 276, 135–146. [Google Scholar] [CrossRef]
- Fang, R.; Ismail, N.; Shelite, T.; Walker, D.H. CD4+ CD25+ Foxp3− T regulatory cells produce both gamma interferon and interleukin-10 during acute severe murine spotted fever rickettsiosis. Infect. Immun. 2009, 77, 3838–3849. [Google Scholar] [CrossRef]
- Smalley, C.; Bechelli, J.; Rockx-Brouwer, D.; Saito, T.; Azar, S.R.; Ismail, N.; Walker, D.H.; Fang, R. Rickettsia australis activates inflammasome in human and murine macrophages. PLoS ONE 2016, 11, e0157231. [Google Scholar] [CrossRef]
- Bechelli, J.; Vergara, L.; Smalley, C.; Buzhdygan, T.P.; Bender, S.; Zhang, W.; Liu, Y.; Popov, V.L.; Wang, J.; Garg, N.; et al. Atg5 supports Rickettsia australis infection in macrophages in vitro and in vivo. Infect. Immun. 2019, 87, e00651-18. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Ismail, N.; Soong, L.; Popov, V.L.; Whitworth, T.; Bouyer, D.H.; Walker, D.H. Differential interaction of dendritic cells with Rickettsia conorii: Impact on host susceptibility to murine spotted fever rickettsiosis. Infect. Immun. 2007, 75, 3112–3123. [Google Scholar] [CrossRef] [PubMed]
- Melehani, J.H.; James, D.B.A.; Dumont, A.L.; Torres, V.J.; Duncan, J.A. Staphylococcus aureus leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLoS Pathog. 2015, 11, e1004970. [Google Scholar] [CrossRef]
- Fang, R.; Houhamdi, L.; Raoult, D. Detection of Rickettsia prowazekii in body lice and their feces by using monoclonal antibodies. J. Clin. Microbiol. 2002, 40, 3358–3363. [Google Scholar] [CrossRef]
- Khakhum, N.; Bharaj, P.; Myers, J.N.; Tapia, D.; Walker, D.H.; Endsley, J.J.; Torres, A.G. Evaluation of Burkholderia mallei ΔtonB Δhcp1 (CLH001) as a live attenuated vaccine in murine models of glanders and melioidosis. PLoS Negl. Trop. Dis. 2019, 13, e0007578. [Google Scholar] [CrossRef] [PubMed]

| Score Illness | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Ruffled Fur | A | A | P | P | P | P |
| Hunched Back | A | A | A | P | P | P |
| Lethargy | A | A | A | P | P | P |
| Squinty Eyes | A | A | A | A | P | P |
| Inability to Reach Food/Drink | A | A | A | A | P | P |
| Weight Loss | A | <5% | 6–10% | 11–15% | 16–20% | >20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyave, E.; Hyseni, I.; Burkhardt, N.; Kuo, Y.-F.; Wang, T.; Munderloh, U.; Fang, R. Rickettsia parkeri with a Genetically Disrupted Phage Integrase Gene Exhibits Attenuated Virulence and Induces Protective Immunity against Fatal Rickettsioses in Mice. Pathogens 2021, 10, 819. https://doi.org/10.3390/pathogens10070819
Arroyave E, Hyseni I, Burkhardt N, Kuo Y-F, Wang T, Munderloh U, Fang R. Rickettsia parkeri with a Genetically Disrupted Phage Integrase Gene Exhibits Attenuated Virulence and Induces Protective Immunity against Fatal Rickettsioses in Mice. Pathogens. 2021; 10(7):819. https://doi.org/10.3390/pathogens10070819
Chicago/Turabian StyleArroyave, Esteban, Ilirjana Hyseni, Nicole Burkhardt, Yong-Fang Kuo, Tian Wang, Ulrike Munderloh, and Rong Fang. 2021. "Rickettsia parkeri with a Genetically Disrupted Phage Integrase Gene Exhibits Attenuated Virulence and Induces Protective Immunity against Fatal Rickettsioses in Mice" Pathogens 10, no. 7: 819. https://doi.org/10.3390/pathogens10070819
APA StyleArroyave, E., Hyseni, I., Burkhardt, N., Kuo, Y.-F., Wang, T., Munderloh, U., & Fang, R. (2021). Rickettsia parkeri with a Genetically Disrupted Phage Integrase Gene Exhibits Attenuated Virulence and Induces Protective Immunity against Fatal Rickettsioses in Mice. Pathogens, 10(7), 819. https://doi.org/10.3390/pathogens10070819







