Benznidazole Treatment: Time- and Dose-Dependence Varies with the Trypanosoma cruzi Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
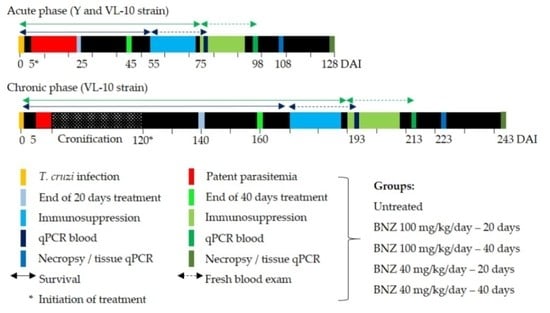
2.2. Therapeutic Schemes
2.3. Cure Criteria
2.3.1. Survival
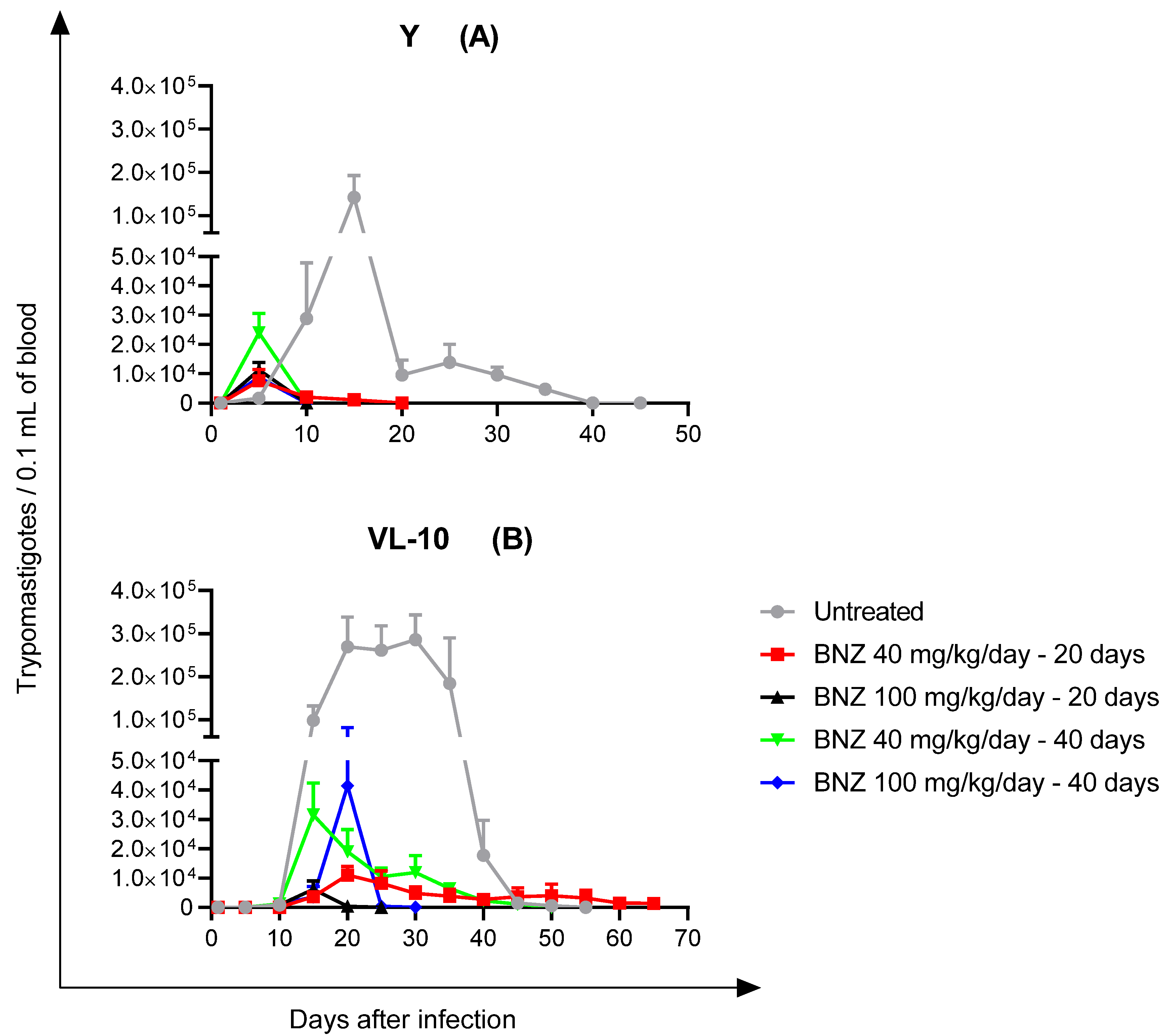
2.3.2. Parasitemia
2.3.3. Immunosuppression and Fresh Blood Examination
2.3.4. Real-Time Polymerase Chain Reaction (qPCR)
qPCR in Total Blood
qPCR in Tissue
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Integrating Neglected Tropical Diseases into Global Health and Development: 4th WHO Report on Neglected Tropical Diseases; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Chagas, C. Nova tripanozomiaze humana. Estudo sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Karnkowska, A.; Votýpka, J.; Tashyreva, D.; Maciszewski, K.; Yurchenko, V.; Lukeš, J. Euglenozoa: Taxonomy, diversity and ecology, symbioses and viruses. Open Biol. 2021, 11, 200407. [Google Scholar] [CrossRef] [PubMed]
- Brener, Z. Pathogenesis and immunopathology of chronic chagas’ disease. Mem. Inst. Oswaldo Cruz 1987, 82, 205–213. [Google Scholar] [CrossRef]
- Prata, A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 2001, 1, 92–100. [Google Scholar] [CrossRef]
- Molina, I.; Salvador, F.; Sánchez-Montalvá, A.; Treviño, B.; Serre, N.; Avils, A.S.; Almirante, B. Toxic profile of benznidazole in patients with chronic chagas disease: Risk factors and comparison of the product from two different manufacturers. Antimicrob. Agents Chemother. 2015, 59, 6125–6131. [Google Scholar] [CrossRef]
- Andrade, Z.A. The pathology of Chagas disease in man. Ann. Soc. Belg. Med. Trop. 1985, 65, 15–30. [Google Scholar]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef]
- Molina, I.; Gómez i Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Andrade, A.L.D.; Zicker, F.; Oliveira, R.M.D.; e Silva, S.A.; Luquetti, A.; Travassos, L.R.; Almeida, I.C.; Andrade, S.S.; Andrade, J.G.; Martelli, C.M.T. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 1996, 348, 1407–1413. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Vigliano, C.; Lococo, B.; Petti, M.; Bertocchi, G.; Viotti, R. Transactions of the royal society of tropical medicine and hygiene seronegative conversion after incomplete benznidazole treatment in chronic Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 636–638. [Google Scholar] [CrossRef]
- Altcheh, J.; Moscatelli, G.; Mastrantonio, G.; Moroni, S.; Giglio, N.; Marson, M.E.; Ballering, G.; Bisio, M.; Koren, G.; García-Bournissen, F. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PloS Negl. Trop. Dis. 2014, 8, e2907. [Google Scholar] [CrossRef] [PubMed]
- Soy, D.; Aldasoro, E.; Guerrero, L.; Posada, E.; Serret, N.; Mejia, T.; Urbina, J.A.; Gascónd, J. Population pharmacokinetics of benznidazole in adult patients with Chagas disease. Antimicrob. Agents Chemother. 2015, 59, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.M.; Tarleton, R.L. Potential new clinical therapies for Chagas disease. Expert Rev. Clin. Pharmacol. 2014, 7, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.M.; Sanchez-Valdez, F.; Padilla, A.M.; White, B.; Wang, W.; Tarleton, R.L. A modified drug regimen clears active and dormant Trypanosomes in mouse models of Chagas disease. Sci. Transl. Med. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Mazzeti, A.L.; Diniz, L.D.F.; Gonçalves, K.R.; Nascimento, A.F.S.; Spósito, P.A.F.; Mosqueira, V.C.; Machado-Coelho, G.L.L.; Ribeiro, I.; Bahia, M.T. Time and dose-dependence evaluation of nitroheterocyclic drugs for improving efficacy following Trypanosoma cruzi infection: A pre-clinical study. Biochem. Pharmacol. 2018, 148, 213–221. [Google Scholar] [CrossRef]
- Maximiano, F.P.; Costa, G.H.Y.; Souza, J.D.; Cunha-Filho, M.S.S.D. Caracterização Físico-química do fármaco antichagásico benznidazol. Quim Nov. 2010, 33, 1714–1719. [Google Scholar] [CrossRef]
- Martins, T.A.F.; Figueiredo, L.D.D.; Mazzeti, A.L.; Nascimento, A.F.S.; Caldas, I.S.; de Andrade, I.M.; Ribeiro, I.; Bahia, M.T. Benznidazole/Itraconazole combination treatment enhances anti-Trypanosoma cruzi activity in experimental Chagas disease. PLoS ONE 2015, 10, e0128707. [Google Scholar]
- Diniz, L.F.; Urbina, J.A.; Andrade, I.M.D.; Mazzeti, A.L.; Martins, T.A.F.; Caldas, I.S.; Talvani, A.; Ribeiro, I.; Bahia, M.T. Benznidazole and posaconazole in experimental Chagas disease: Positive interaction in concomitant and sequential treatments. PloS Negl. Trop. Dis. 2013, 7, e2367. [Google Scholar] [CrossRef]
- Romanha, A.J.; Castro, S.L.; Soeiro, M.N.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; OlivieriI, B.; ZaniI, C.; et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 1962, 4, 389–396. [Google Scholar]
- Caldas, S.; Santos, F.M.; Lana, M.D.; Diniz, L.F.; Machado-Coelho, G.L.L.; Veloso, V.M.; Bahia, M.T. Trypanosoma cruzi: Acute and long-term infection in the vertebrate host can modify the response to benznidazole. Exp. Parasitol. 2008, 118, 315–323. [Google Scholar] [CrossRef]
- Filardi, L.S.; Brener, Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Hernández, Y.; Bertocchi, G.; Fernández, M.; Lococo, B.; Ramírez, C.; Cura, C.; Lopez Albizu, C.; Schijman, A.; Abril, M.; et al. New scheme of intermittent benznidazole administration in patients chronically infected with Trypanosoma cruzi: A pilot short-term follow-up study with adult patients. Antimicrob. Agents Chemother. 2016, 60, 833–837. [Google Scholar] [CrossRef]
- Yun, O.; Lima, M.A.; Ellman, T.; Chambi, W.; Castillo, S.; Flevaud, L.; Roddy, P.; Parreño, F.; Viñas, P.A.; Palma, P.P. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10-Year experience of médecins sans frontières. PloS Negl. Trop. Dis. 2009, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; Garcia, W.; et al. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): A phase 2, double-blind, randomised trial. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Perin, L.; Silva, R.M.R.; Fonseca, K.S.; Cardoso, J.M.O.; Mathias, F.A.S.; Reis, L.E.S.; Molina, I.; Correa-Oliveira, R.; Vieira, P.M.A.; Carneiro, C.M. Pharmacokinetics and tissue distribution of benznidazole after oral administration in mice. Antimicrob. Agents Chemother. 2017, 61, e02410–e02416. [Google Scholar] [CrossRef]
- Perin, L.; Pinto, L.; Nardotto, G.H.; Fonseca, K.S.; Paiva, B.O.; Bastos, T.; Molina, I.; Correa-Oliveira, R.; Vieira, P.M.A.; Carneiro, C.M. Population pharmacokinetics and biodistribution of benznidazole in mice. J. Antimicrob. Chemother. 2020, 75, 1–9. [Google Scholar] [CrossRef]
- Molina, I.; Salvador, F.; Sánchez-Montalvá, A.; Artaza, M.A.; Moreno, R.; Perin, L.; Esquisabel, A.; Pinto, L.L.; Pedraz, J.L. Pharmacokinetics of benznidazole in healthy volunteers and implications in future clinical trials. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Lewis, M.D.; Francisco, A.F.; Taylor, M.C.; Burrell-Saward, H.; McLatchie, A.P.; Miles, M.A.; Kelly, J.M. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol. 2014, 16, 1285–1300. [Google Scholar] [CrossRef]
- Perin, L.; Fonseca, K.S.; Carvalho, T.V.; Carvalho, L.M.; Madeira, J.V.; Medeiros, L.F.; Molina, I.; Correa-Oliveira, R.; Carneiro, C.M.; Vieira, P.M.A. Low-dose of benznidazole promotes therapeutic cure in experimental chronic Chagas’ disease with absence of parasitism in blood, heart and colon. Exp. Parasitol. 2020, 210, 107834. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Carvalho, T.V.; Ferraz, A.T.; Marques, F.S.; Roatt, B.M.; Fonseca, K.S.; Reis, L.E.S.; Carneiro, C.M.; Vieira, P.M.A. Histopathological changes in the gastrointestinal tract and systemic alterations triggered by experimental oral infection with Trypanosoma cruzi. Exp. Parasitol. 2020, 218, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bice, D.E.; Zeledon, R. Comparison of infectivity of strains of Trypanosoma cruzi (Chagas, 1909). J. Parasitol. 1970, 56, 663. [Google Scholar] [CrossRef] [PubMed]
- Caldas, I.S.; Diniz, L.F.; Guedes, P.M.M.; Nascimento, A.F.S.; Galvão, L.M.C.; Lima, W.G.; Caldas, S.; Bahia, M.T. Myocarditis in different experimental models infected by Trypanosoma cruzi is correlated with the production of IgG1 isotype. Acta Trop. 2017, 167, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.; Brener, Z. Tissue tropism of different Trypanosoma cruzi strains. J. Parasitol. 1978, 64, 475–482. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Lewis, M.D.; White, K.L.; Shacklefor, D.M.; Chen, G.; Saunders, J.; Osuna-Cabello, M.; Read, K.D.; Charman, S.A.; et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci. Rep. 2016, 6, 35351. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.G.C.D.; Branquinho, R.T.; Oliveira, M.T.; Milagre, M.M.; Saúde-Guimarães, D.A.; Mosqueira, V.C.F.; Lana, M. Efficacy of lychnopholide polymeric nanocapsules after oral and intravenous administration in murine experimental Chagas disease. Antimicrob. Agents Chemother. 2016, 60, 5215–5222. [Google Scholar] [CrossRef]
- Martins, H.R.; Figueiredo, L.M.; Valamiel-Silva, J.C.O.; Carneiro, C.M.; Machado-Coelho, G.L.L.; Vitelli-Avelar, D.M.; Bahia, M.T.; Martins-Filho, O.A.; Macedo, A.M.; Lana, M. Persistence of PCR-positive tissue in benznidazole-treated mice with negative blood parasitological and serological tests in dual infections with Trypanosoma cruzi stocks from different genotypes. J. Antimicrob. Chemother. 2008, 61, 1319–1327. [Google Scholar] [CrossRef]
- Chatelain, E.; Konar, N. Translational challenges of animal models in chagas disease drug development: A review. Drug Des. Devel. Ther. 2015, 9, 4807–4823. [Google Scholar] [CrossRef]
- Keenan, M.; Chaplin, J.H.; Alexander, P.W.; Abbott, M.J.; Best, W.M.; Khong, A.; Botero, A.; Perez, C.; Cornwall, S.; Thompson, A.R.; et al. Two analogues of fenarimol show curative activity in an experimental model of Chagas disease. J. Med. Chem. 2013, 56, 10158–10170. [Google Scholar] [CrossRef]
- Ndao, M.; Beaulieu, C.; Black, W.C.; Isabel, E.; Vasquez-Camargo, F.; Nath-Chowdhury, M.; Massé, F.; Mellon, C.; Methot, N.; Nicoll-Griffit, D.A. Reversible cysteine protease inhibitors show promise for a chagas disease cure. Antimicrob. Agents Chemother. 2014, 58, 1167–1178. [Google Scholar] [CrossRef]
- Jones, E.M.; Colley, D.G.; Tostes, S.; Lopes, E.R.; Vnencak-Jones, C.L.; McCurley, T.L. Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am. J. Trop. Med. Hyg. 1993, 48, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, R.L. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 2001, 31, 550–554. [Google Scholar] [CrossRef]
 ” represents a significant difference among the different phases of infection, but for the same treatment and strain (p < 0.05).
” represents a significant difference among the different phases of infection, but for the same treatment and strain (p < 0.05).
 ” represents a significant difference among the different phases of infection, but for the same treatment and strain (p < 0.05).
” represents a significant difference among the different phases of infection, but for the same treatment and strain (p < 0.05).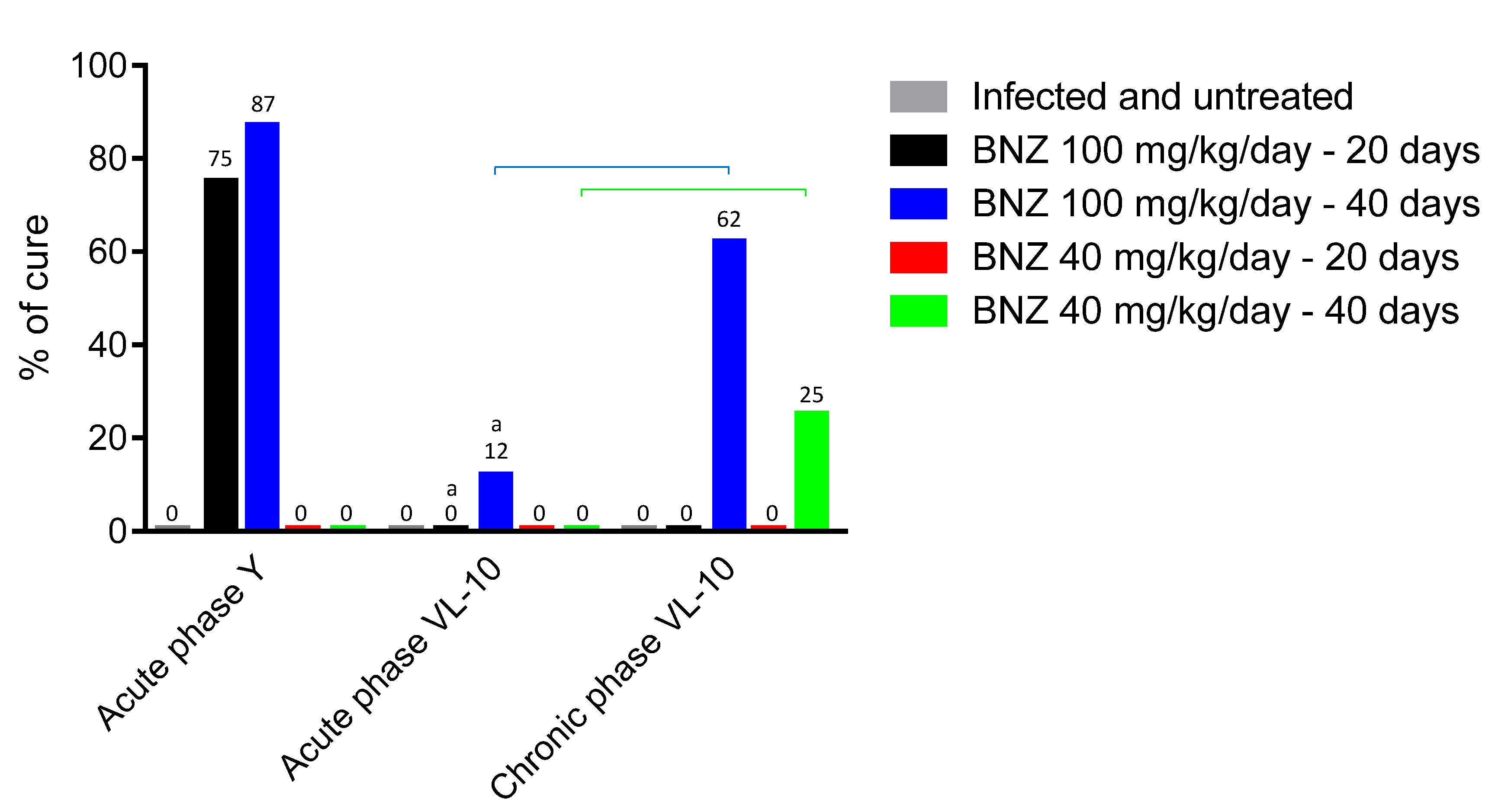
| Strain £ | Group | Survival § | Positive | Positive qPCR ɸ | Total Positive | Cure # | ||
|---|---|---|---|---|---|---|---|---|
| FBE ¶ | Blood | Heart | Colon | Mice | ||||
| Y | Untreated | 5/8 (63%) | 5/5 (100%) | * | - | - | 5/5 (100%) | 0 |
| BNZ 100 mg/kg/day—20 days | 8/8 (100%) | 0/8 (0%) | 1/8 (13%) | 1/8 (13%) | 2/8 (25%) | 2/8 (25%) | 75 a,d,e | |
| BNZ 100 mg/kg/day—40 days | 8/8 (100%) | 1/8 (22%) | 0/7 (0%) | 0/8 (0%) | 0/8 (0%) | 1/8 (13%) | 87 a,b,d,e | |
| BNZ 40 mg/kg/day—20 days | 8/8 (100%) | 7/8 (88%) | 1/1 (100%) | 1/7 (14%) | 7/7 (100%) | 8/8 (100%) | 0 | |
| BNZ 40 mg/kg/day—40 days | 7/8 (88%) | 7/7 (100%) | - | 0/7 (0%) | 7/7 (100%) | 7/7 (100%) | 0 b | |
| VL-10 | Untreated | 8/8 (100%) | 8/8 (100%) | - | 8/8 (100%) | 8/8 (100%) | 8/8 (100%) | 0 |
| BNZ 100 mg/kg/day—20 days | 7/8 (88%) | 6/7 (88%) | 0/1 (0%) | 5/7 (71%) | 7/7 (100%) | 7/7 (100%) | 0 | |
| BNZ 100 mg/kg/day—40 days | 8/8 (100%) | 5/8 (63%) | 0/3 (0%) | 4/8 (50%) | 7/8 (88%) | 7/8 (88%) | 12 a,b,d,e | |
| BNZ 40 mg/kg/day—20 days | 8/8 (100%) | 8/8 (100%) | - | 8/8 (100%) | 8/8 (100%) | 8/8 (100%) | 0 | |
| BNZ 40 mg/kg/day—40 days | 7/8 (88%) | 7/7 (100%) | - | 6/6 ** (100%) | 6/6 (100%) | 7/7 (100%) | 0 | |
| Strain £ | Group | Survival § | Positive | Positive qPCR ɸ | Total | Cure # | ||
|---|---|---|---|---|---|---|---|---|
| FBE ¶ | Blood | Heart | Colon | Positive mice | (%) | |||
| VL-10 | Untreated | 8/8 (100%) | 5/8 (63%) | 2/3 (67%) | 4/5 * (80%) | 2/5 (40%) | 8/8 (100%) | 0 |
| BNZ 100 mg/kg/day—20 days | 8/8 (100%) | 5/8 (63%) | 2/3 (67%) | 2/7 * (29%) | 4/7 (57%) | 8/8 (100%) | 0 | |
| BNZ 100 mg/kg/day—40 days | 8/8 (100%) | 2/8 (25%) | 0/6 (0%) | 0/8 (0%) | 1/8 (13%) | 3/8 (38%) | 62 a,b | |
| BNZ 40 mg/kg/day—20 days | 8/8 (100%) | 4/8 (50%) | 3/4 (75%) | 1/7 * (14%) | 1/7 (14%) | 8/8 (100%) | 0 c | |
| BNZ 40 mg/kg/day—40 days | 8/8 (100%) | 6/8 (75%) | 0/2 (0%) | 0/5 * (0%) | 0/5 (0%) | 6/8 (75%) | 25 a,b,c,d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, K.d.S.; Perin, L.; de Paiva, N.C.N.; da Silva, B.C.; Duarte, T.H.C.; Marques, F.d.S.; Costa, G.d.P.; Molina, I.; Correa-Oliveira, R.; Vieira, P.M.d.A.; et al. Benznidazole Treatment: Time- and Dose-Dependence Varies with the Trypanosoma cruzi Strain. Pathogens 2021, 10, 729. https://doi.org/10.3390/pathogens10060729
Fonseca KdS, Perin L, de Paiva NCN, da Silva BC, Duarte THC, Marques FdS, Costa GdP, Molina I, Correa-Oliveira R, Vieira PMdA, et al. Benznidazole Treatment: Time- and Dose-Dependence Varies with the Trypanosoma cruzi Strain. Pathogens. 2021; 10(6):729. https://doi.org/10.3390/pathogens10060729
Chicago/Turabian StyleFonseca, Kátia da Silva, Luísa Perin, Nívia Carolina Nogueira de Paiva, Beatriz Cristiane da Silva, Thays Helena Chaves Duarte, Flávia de Souza Marques, Guilherme de Paula Costa, Israel Molina, Rodrigo Correa-Oliveira, Paula Melo de Abreu Vieira, and et al. 2021. "Benznidazole Treatment: Time- and Dose-Dependence Varies with the Trypanosoma cruzi Strain" Pathogens 10, no. 6: 729. https://doi.org/10.3390/pathogens10060729
APA StyleFonseca, K. d. S., Perin, L., de Paiva, N. C. N., da Silva, B. C., Duarte, T. H. C., Marques, F. d. S., Costa, G. d. P., Molina, I., Correa-Oliveira, R., Vieira, P. M. d. A., & Carneiro, C. M. (2021). Benznidazole Treatment: Time- and Dose-Dependence Varies with the Trypanosoma cruzi Strain. Pathogens, 10(6), 729. https://doi.org/10.3390/pathogens10060729