Hepatitis E Virus in Croatia in the “One-Health” Context
Abstract
1. Introduction
2. Methods
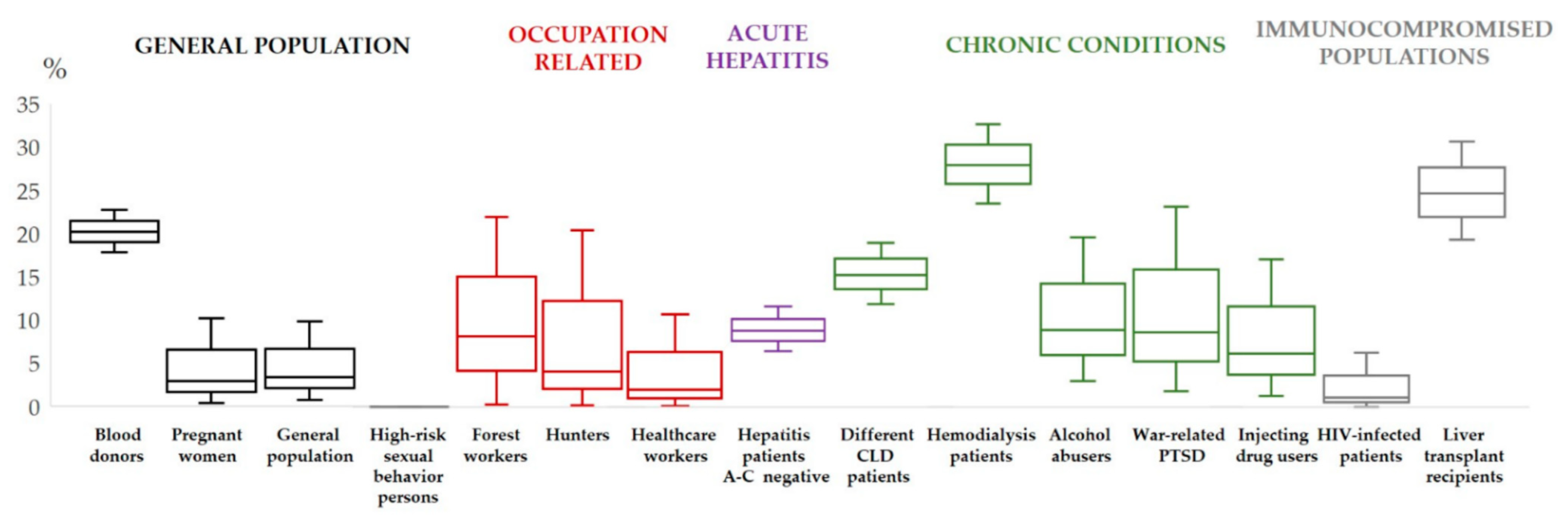
3. Hepatitis E Virus in Croatia–Human Studies
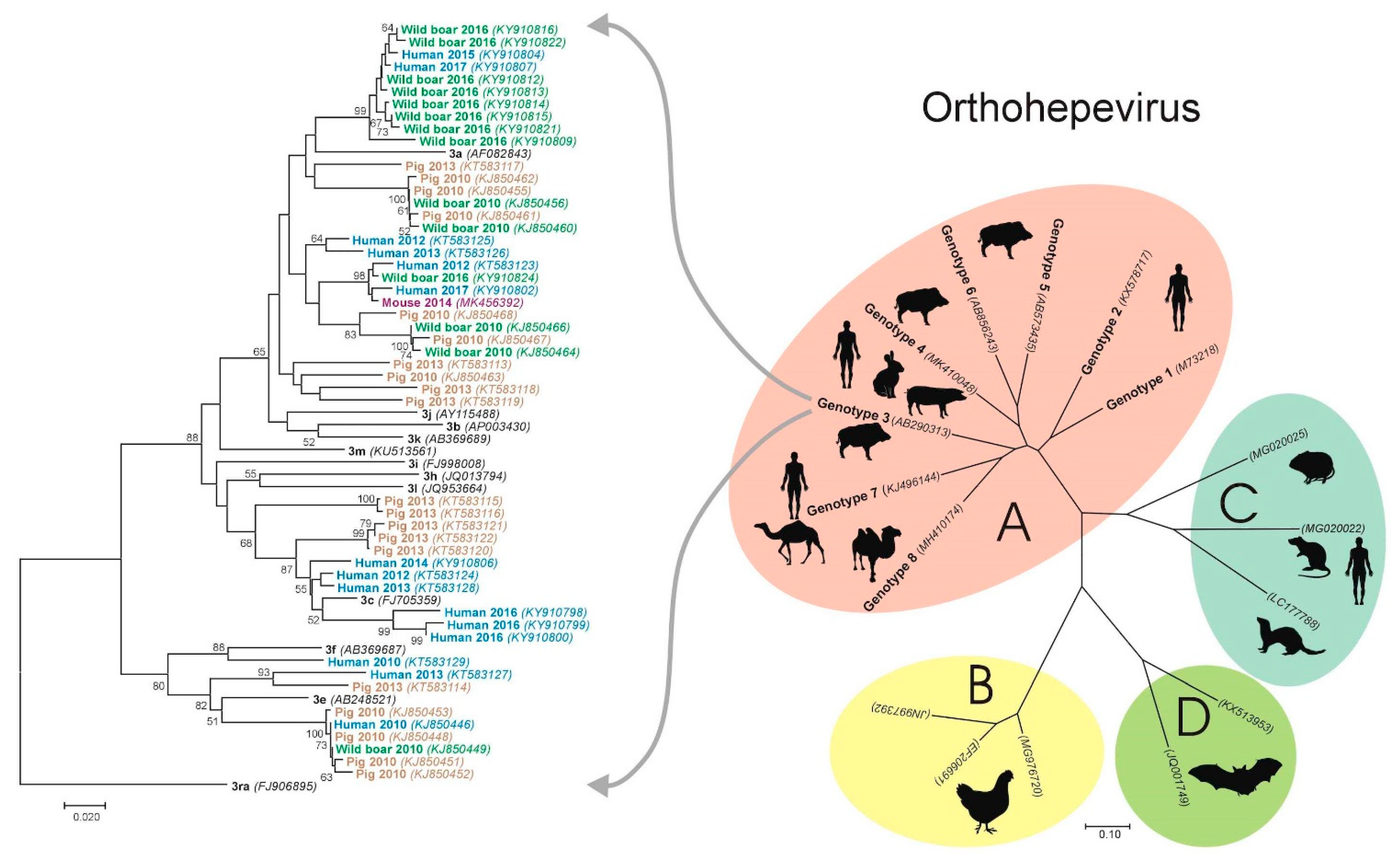
4. Hepatitis E Virus in Croatia—Animal Studies
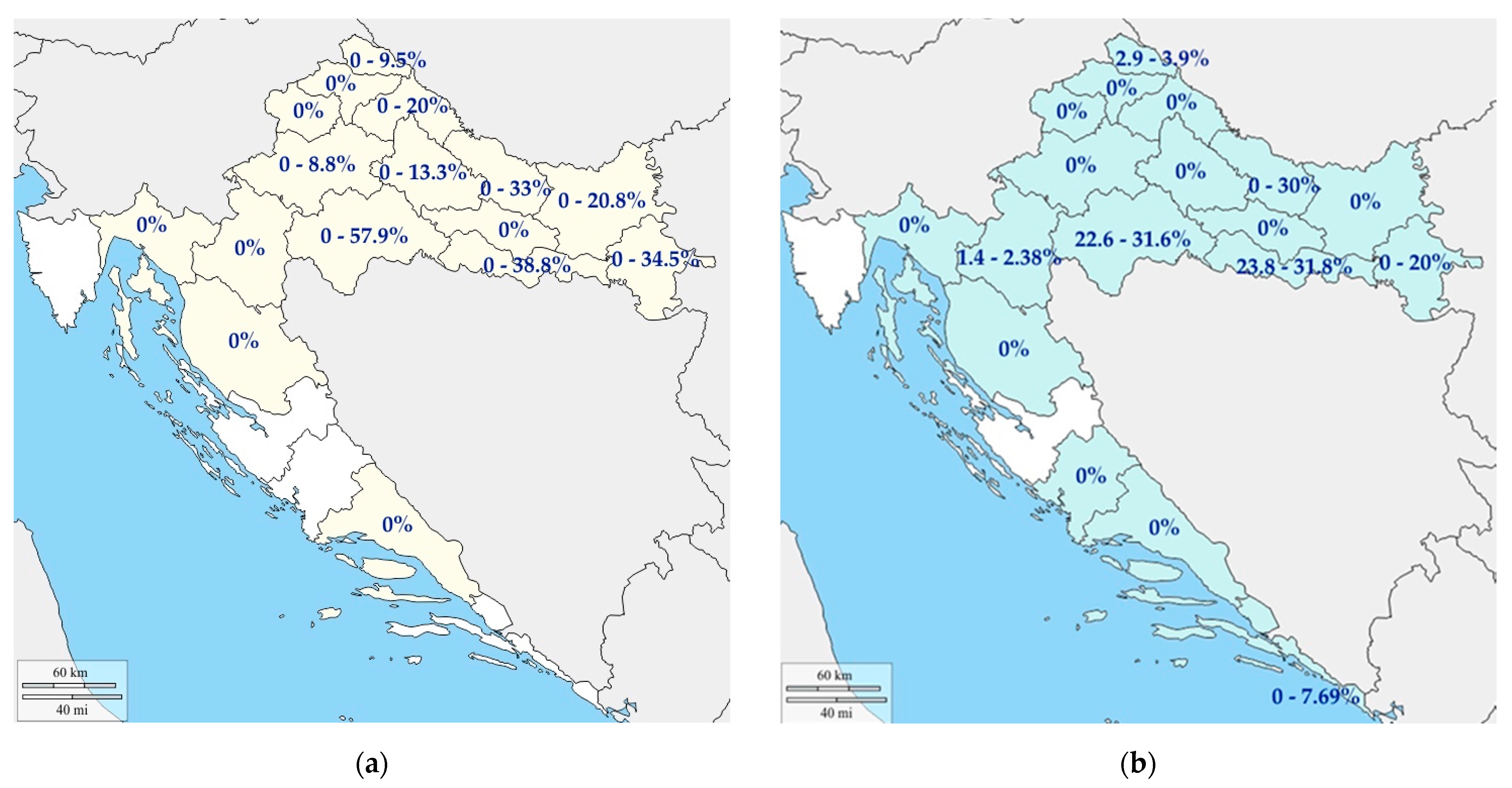
4.1. Pigs
4.2. Wild Boars
4.3. Birds
4.4. Shellfish
4.5. Other Investigated Species
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020, 40, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Teng, J.L.L.; Chiu, T.H.; Lau, S.K.P.; Woo, P.C.Y. Hepatitis E Virus Genotypes and Evolution: Emergence of Camel Hepatitis E Variants. Int. J. Mol. Sci. 2017, 8, 869. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis, E. Lancet 2012, 30, 2477–2488. [Google Scholar] [CrossRef]
- Primadharsini, P.P.; Nagashima, S.; Okamoto, H. Genetic Variability and Evolution of Hepatitis E Virus. Viruses 2019, 18, 456. [Google Scholar] [CrossRef]
- Dziedzinska, R.; Krzyzankova, M.; Bena, M.; Vasickova, P. Evidence of Hepatitis E Virus in Goat and Sheep Milk. Viruses 2020, 12, 1429. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kang, J.H.; Ohnishi, S.; Hino, K.; Miyakawa, H.; Miyakawa, Y.; Maekubo, H.; Mishiro, S. Full-length sequences of six hepatitis E virus isolates of genotypes III and IV from patients with sporadic acute or fulminant hepatitis in Japan. Intervirology 2003, 46, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Sato, H.; Naka, K.; Furuya, S.; Tsukiji, H.; Kitagawa, K.; Sonoda, Y.; Usui, T.; Sakamoto, H.; Yoshino, S.; et al. A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: Identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch. Virol. 2011, 156, 1345–1358. [Google Scholar] [CrossRef]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S.; Kamar, N.; Abravanel, F.; Guérin, J.L. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg. Infect. Dis. 2012, 18, 1274–1281. [Google Scholar] [CrossRef]
- Takahashi, K.; Kitajima, N.; Abe, N.; Mishiro, S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 2004, 20, 501–505. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Elkhawaga, A.A.; Sayed, I.M. Assessment of hepatitis E virus (HEV) in the edible goat products pointed out a risk for human infection in Upper Egypt. Int. J. Food Microbiol. 2020, 330, 108784. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology 2016, 64, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Nishizawa, T.; Nagashima, S.; Jirintai, S.; Kawakami, M.; Sonoda, Y.; Suzuki, T.; Yamamoto, S.; Shigemoto, K.; Ashida, K.; et al. Molecular characterization of a novel hepatitis E virus (HEV) strain obtained from a wild boar in Japan that is highly divergent from the previously recognized HEV strains. Virus Res. 2014, 13, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tsang, A.K.L.; Joseph, M.; Wong, E.Y.M.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Cao, K.Y.; Wernery, U.; Schountz, T.; Chiu, T.H.; Tsang, A.K.; Wong, P.C.; Wong, E.Y.; et al. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China, 2013. Emerg. Infect. Dis. 2016, 22, 2219–2221. [Google Scholar] [CrossRef] [PubMed]
- Sayed, I.M.; Elkhawaga, A.A.; El-Mokhtar, M.A. In vivo models for studying Hepatitis E virus infection; Updates and applications. Virus Res. 2019, 274, 197765. [Google Scholar] [CrossRef]
- Gouilly, J.; Chen, Q.; Siewiera, J.; Cartron, G.; Levy, C.; Dubois, M.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N.; El Costa, H. Genotype specific pathogenicity of hepatitis E virus at the human maternal-fetal interface. Nat. Commun. 2018, 12, 4748. [Google Scholar] [CrossRef]
- Marion, O.; Lhomme, S.; Nayrac, M.; Dubois, M.; Pucelle, M.; Requena, M.; Migueres, M.; Abravanel, F.; Peron, J.M.; Carrere, N.; et al. Hepatitis E virus replication in human intestinal cells. Gut 2020, 69, 901–910. [Google Scholar] [CrossRef] [PubMed]
- El-Mokhtar, M.A.; Othman, E.R.; Khashbah, M.Y.; Ismael, A.; Ghaliony, M.A.; Seddik, M.I.; Sayed, I.M. Evidence of the Extrahepatic Replication of Hepatitis E Virus in Human Endometrial Stromal Cells. Pathogens 2020, 17, 295. [Google Scholar] [CrossRef] [PubMed]
- Sayed, I.M.; Seddik, M.I.; Gaber, M.A.; Saber, S.H.; Mandour, S.A.; El-Mokhtar, M.A. Replication of Hepatitis E Virus (HEV) in Primary Human-Derived Monocytes and Macrophages In Vitro. Vaccines 2020, 21, 239. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Seddik, M.I.; Osman, A.; Adel, S.; Abdel Aziz, E.M.; Mandour, S.A.; Mohammed, N.; Zarzour, M.A.; Abdel-Wahid, L.; Radwan, E.; et al. Hepatitis E Virus Mediates Renal Injury via the Interaction between the Immune Cells and Renal Epithelium. Vaccines 2020, 14, 454. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Govindarajan, S.; Herbert, R.; St Claire, M.; Elkins, W.R.; Cook, A.; Shaver, C.; Beauregard, M.; Swerczek, J.; et al. Pathobiology of hepatitis E: Lessons learned from primate models. Emerg. Microbes. Infect. 2013, 2, e9. [Google Scholar] [CrossRef]
- Rogée, S.; Le Gall, M.; Chafey, P.; Bouquet, J.; Cordonnier, N.; Frederici, C.; Pavio, N. Quantitative proteomics identifies host factors modulated during acute hepatitis E virus infection in the swine model. J. Virol. 2015, 89, 129–143. [Google Scholar] [CrossRef]
- Cao, D.; Cao, Q.M.; Subramaniam, S.; Yugo, D.M.; Heffron, C.L.; Rogers, A.J.; Kenney, S.P.; Tian, D.; Matzinger, S.R.; Overend, C.; et al. Pig model mimicking chronic hepatitis E virus infection in immunocompromised patients to assess immune correlates during chronicity. Proc. Natl. Acad. Sci. USA 2017, 114, 6914–6923. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Romanet, P.; Moal, V.; Borentain, P.; Purgus, R.; Benezech, A.; Motte, A.; Gérolami, R. Autochthonous infections with hepatitis E virus genotype 4, France. Emerg. Infect. Dis. 2012, 18, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Midgley, S.; Vestergaard, H.T.; Dalgaard, C.; Enggaard, L.; Fischer, T.K. Hepatitis E virus genotype 4, Denmark, 2012. Emerg. Infect. Dis. 2014, 20, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Boxall, E.; Herborn, A.; Kochethu, G.; Pratt, G.; Adams, D.; Ijaz, S.; Teo, C.G. Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus. Med. 2006, 16, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Garrouste, C.; Haagsma, E.B.; Garrigue, V.; Pischke, S.; Chauvet, C.; Dumortier, J.; Cannesson, A.; Cassuto-Viguier, E.; Thervet, E.; et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011, 140, 1481–1489. [Google Scholar] [CrossRef]
- De Schryver, A.; De Schrijver, K.; François, G.; Hambach, R.; van Sprundel, M.; Tabibi, R.; Colosio, C. Hepatitis E virus infection: An emerging occupational risk? Occup. Med. 2015, 65, 667–672. [Google Scholar] [CrossRef]
- Prpić, J.; Černi, S.; Škorić, D.; Keros, T.; Brnić, D.; Cvetnić, Ž.; Jemeršić, L. Distribution and molecular characterization of Hepatitis E virus in domestic animals and wildlife in Croatia. Food Environ. Virol. 2015, 7, 195–205. [Google Scholar] [CrossRef]
- Jemeršić, L.; Prpić, J.; Brnić, D.; Keros, T.; Pandak, N.; Đaković Rode, O. Genetic diversity of hepatitis E virus (HEV) strains derived from humans, swine and wild boars in Croatia from 2010 to 2017. BMC Infect. Dis. 2019, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Perrin, H.; Madden, R.G.; Stanley, A.; Crossan, C.; Hunter, J.G.; Vine, L.; Lane, K.; Devooght-Johnson, N.; McLaughlin, C.; Petrik, J.; et al. Hepatitis E virus in patients with decompensated chronic liver disease: A prospective UK/French study. Aliment. Pharmacol. Ther. 2015, 42, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Sayed, I.M.; El-Mokhtar, M.A.; Mahmoud, M.A.R.; Elkhawaga, A.A.; Gaber, S.; Seddek, N.H.; Abdel-Wahid, L.; Ashmawy, A.M.; Alkareemy, E.A.R. Clinical Outcomes and Prevalence of Hepatitis E Virus (HEV) Among Non-A-C Hepatitis Patients in Egypt. Infect. Drug Resist. 2021, 12, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Mansuy, J.M.; Cointault, O.; Selves, J.; Abravanel, F.; Danjoux, M.; Otal, P.; Esposito, L.; Durand, D.; Izopet, J.; et al. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am. J. Transplant. 2008, 8, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Ingiliz, P.; Mayr, C.; Obermeier, M.; Herbst, H.; Polywka, S.; Pischke, S. Persisting hepatitis E virus infection leading to liver cirrhosis despite recovery of the immune system in an HIV-infected patient. Clin. Res. Hepatol. Gastroenterol. 2016, 40, e23–e25. [Google Scholar] [CrossRef]
- Dalton, H.R.; van Eijk, J.J.J.; Cintas, P.; Madden, R.G.; Jones, C.; Webb, G.W.; Norton, B.; Pique, J.; Lutgens, S.; Devooght-Johnson, N.; et al. Hepatitis E virus infection and acute non-traumatic neurological injury: A prospective multicentre study. J. Hepatol. 2017, 67, 925–932. [Google Scholar] [CrossRef]
- Pischke, S.; Hartl, J.; Pas, S.D.; Lohse, A.W.; Jacobs, B.C.; Van der Eijk, A.A. Hepatitis E virus: Infection beyond the liver? J. Hepatol. 2017, 66, 1082–1095. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Kamili, S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J. Viral. Hepat. 2003, 10, 61–69. [Google Scholar] [CrossRef]
- Patra, S.; Kumar, A.; Trivedi, S.S.; Puri, M.; Sarin, S.K. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann. Intern. Med. 2007, 147, 28–33. [Google Scholar] [CrossRef]
- Lachish, T.; Erez, O.; Daudi, N.; Shouval, D.; Schwartz, E. Acute hepatitis E virus in pregnant women in Israel and in other industrialized countries. J. Clin. Virol. 2015, 73, 20–24. [Google Scholar] [CrossRef]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Jemersic, L.; Prpic, J.; Barbic, L.; Savic, V.; Stevanovic, V.; Vilibic-Cavlek, T. Epidemiology of hepatitis E in South-East Europe in the “One Health” concept. World J. Gastroenterol. 2019, 7, 3168–3182. [Google Scholar] [CrossRef]
- Tsachev, I.; Baymakova, M.; Ciccozzi, M.; Pepovich, R.; Kundurzhiev, T.; Marutsov, P.; Dimitrov, K.K.; Gospodinova, K.; Pishmisheva, M.; Pekova, L. Seroprevalence of hepatitis E virus infection in pigs from Southern Bulgaria. Vector. Borne Zoonotic Dis. 2019, 19, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Tsachev, I.; Baymakova, M.; Pepovich, R.; Palova, N.; Marutsov, P.; Gospodinova, K.; Kundurzhiev, T.; Ciccozzi, M. High seroprevalence of hepatitis E virus infection among East Balkan swine (Sus scrofa) in Bulgaria: Preliminary results. Pathogens 2020, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Milojevic, L.; Velebit, B.; Teodorovic, V.; Kirbis, A.; Petrovic, T.; Karabasil, N.; Dimitrijevic, M. Screening and molecular characterization of hepatitis E virus in slaughter pigs in Serbia. Food Environ. Virol. 2019, 11, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Zele, D.; Barry, A.F.; Hakze-van der Honing, R.W.; Vengust, G.; van der Poel, W.H. Prevalence of anti-hepatitis E virus antibodies and first detection of hepatitis E virus in wild boar in Slovenia. Vector. Borne Zoonotic Dis. 2016, 16, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Porea, D.; Anita, A.; Demange, A.; Raileanu, C.; Oslobanu Ludu, L.; Anita, D.; Savuta, G.; Pavio, N. Molecular detection of hepatitis E virus in wild boar population in Eastern Romania. Transbound. Emerg. Dis. 2018, 65, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, T.; Lupulovic, D.; Jimenez de Oya, N.; Vojvodic, S.; Blazquez, A.B.; Escribano-Romero, E.; Martin-Acebes, M.A.; Potkonjak, A.; Milosevic, V.; Lazic, S.; et al. Prevalence of hepatitis E virus (HEV) antibodies in Serbian blood donors. J. Infect. Dev. Ctries. 2014, 8, 1322–1327. [Google Scholar] [CrossRef]
- Baymakova, M.; Terzieva, K.; Popov, R.; Grancharova, E.; Kundurzhiev, T.; Pepovich, R.; Tsachev, I. Seroprevalence of hepatitis E virus infection among blood donors in Bulgaria. Viruses 2021, 13, 492. [Google Scholar] [CrossRef]
- Porea, D.; Anita, A.; Vata, A.; Teodor, D.; Crivei, L.; Raileanu, C.; Gotu, V.; Ratoi, I.; Cozma, A.; Anita, D.; et al. Common European origin of hepatitis E virus in human population from Eastern Romania. Front. Public Health 2020, 8, 578163. [Google Scholar] [CrossRef]
- Pittaras, T.; Valsami, S.; Mavrouli, M.; Kapsimali, V.; Tsakris, A.; Politou, M. Seroprevalence of hepatitis E virus in blood donors in Greece. Vox Sang. 2014, 106, 387. [Google Scholar] [CrossRef]
- Čivljak, R.; Rode Đaković, O.; Jemeršić, L.; Topić, A.; Turalija, I.; Kuzman, I.; Čačić, I. Autochthonous hepatitis E in patient from Zagreb: A case report. Croat. J. Infect. 2013, 33, 35–39. [Google Scholar]
- Đaković Rode, O.; Jemeršić, L.; Brnić, D.; Pandak, N.; Mikulić, R.; Begovac, J.; Vince, A. Hepatitis E in patients with hepatic disorders and HIV-infected patients in Croatia: Is one diagnostic method enough for hepatitis E diagnosis? Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Vilibic, M.; Kolaric, B.; Jemersic, L.; Kucinar, J.; Barbic, L.; Bagaric, A.; Stevanovic, V.; Tabain, I.; Sviben, M.; et al. Seroepidemiology of hepatitis E in selected population groups in Croatia: A prospective pilot study. Zoonoses Public Health 2016, 63, 494–502. [Google Scholar] [CrossRef]
- Miletić, M.; Vuk, T.; Hećimović, A.; Stojić Vidović, M.; Jemeršić, L.; Jukić, I. Estimation of the hepatitis E assay-dependent seroprevalence among Croatian blood donors. Transfus. Clin. Biol. 2019, 26, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Jeličić, P.; Vilibić-Čavlek, T.; Vilibić, M.; Jemeršić, L.; Kolarić, B.; Kučinar, J.; Barbić, L.; Stevanović, V.; Janev-Holcer, N.; Tabain, I.; et al. Seroprevalence of hepatitis E in different population groups in Croatia. In Proceedings of the 7th Congress of Slovenian Microbiological Society, Bled, Slovenia, 20–22 September 2017. [Google Scholar]
- Jeličić, P.; Jemeršić, L.; Brumen, V.; Janev-Holcer, N.; Prohić, A.; Barbić, L.; Stevanović, V.; Vilibić-Čavlek, T. Seroprevalence of hepatitis E in professionally exposed groups in Croatia: Preliminary results. In Proceedings of the 7th International Congress “Veterinary Science and Profession”, Zagreb, Croatia, 5–7 October 2017. [Google Scholar]
- Haffar, S.; Bazerbachi, F.; Leise, M.D.; Dillon, J.J.; Albright, R.C.; Murad, M.H.; Kamath, P.S.; Watt, K.D. Systematic review with meta-analysis: The association between hepatitis E seroprevalence and haemodialysis. Aliment. Pharmacol. Ther. 2017, 46, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Knotek, M.; Kudumija, B.; Ilic, M.; Gulin, M.; Zibar, L.; Hrstic, I.; Jurekovic, Z.; Kolaric, B.; et al. Seroepidemiology of hepatitis E in patients on haemodialysis in Croatia. Int. Urol. Nephrol. 2020, 52, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Jemersic, L.; Vilibic-Cavlek, T. The Burden of Hepatitis E Infection in Chronic Liver Diseases in Croatia. Vector. Borne Zoonotic Dis. 2020, 21, 67–68. [Google Scholar] [CrossRef]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Vilibic-Cavlek, T.; Jemersic, L.; Prpic, J.; Dakovic-Rode, O.; Kolaric, B.; Vince, A. Hepatitis E seroprevalence and associated risk factors in Croatian liver transplant recipients. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190302. [Google Scholar] [CrossRef]
- Jemeršić, L.; Roić, B.; Balatinec, J.; Keros, T. Hepatitis E—Are we threatened? Hepatitis E—Jesmo li ugroženi? Vet. Stanica 2010, 41, 383–397. [Google Scholar]
- Halbur, P.G.; Kasorndorkbua, C.; Gilbert, C.; Guenette, D.; Potters, M.B.; Purcell, R.H.; Emerson, S.U.; Toth, T.E.; Meng, X.J. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 2001, 39, 918–923. [Google Scholar] [CrossRef]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.J.; Norder, H.; Okamoto, H.; van der Poel, W.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef]
- Jemeršić, L.; Keros, T.; Maltar, L.; Barbić, L.; Vilibić-Čavlek, T.; Jeličić, P.; Đaković-Rode, O.; Prpić, J. Differences in hepatitis E virus (HEV) presence in naturally infected seropositive domestic pigs and wild boars—An indication of wild boars having an important role in HEV epidemiology. Vet. Arh. 2017, 87, 651–663. [Google Scholar] [CrossRef]
- Jemeršić, L.; Prpić, J.; Roić, B.; Želježić, D.; Keros, T. The wild boar (Sus Scrofa)—A victim and ally of the most important viral infections in Europe. Vet. Stanica 2019, 50, 137–148. [Google Scholar]
- Kozyra, I.; Jabłonski, A.; Bigoraj, E.; Rzezutka, A. Wild Boar as a Sylvatic Reservoir of Hepatitis E Virus in Poland: A Cross-Sectional Population Study. Viruses 2020, 12, 1113. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, R.; She, R.; Zhang, C.; Shi, R.; Li, W.; Du, F.; Wu, Q.; Hu, F.; Zhang, Y.; et al. Case Report Associated with Aspergillosis and Hepatitis E Virus Coinfection in Himalayan Griffons. Biomed. Res. Int. 2015, 2015, 287315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bilic, I.; Troxler, S.; Hess, M. Evidence of genotypes 1 and 3 of avian hepatitis E virus in wild birds. Virus Res. 2017, 228, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Amšel Zelenika, T.; Prpić, J.; Tišljar, M.; Savić, V.; Balenović, M.; Jurinović, L.; Jemeršić, L. Ptičji Hepatitis E—Potencijalna opasnost za peradarsku proizvodnju?! (Avian Hepatitis E—A Potential Risk for Poultry Production?!). In Proceedings of the 10th Scientific-Professional Symposium “Poultry Days 2013” with International Participation, Šibenik, Croatia, 15–18 May 2013; pp. 86–90. [Google Scholar]
- Prpić, J.; Keros, T.; Vucelja, M.; Bjedov, L.; Đaković-Rode, O.; Margaletić, J.; Habrun, B.; Jemeršić, L. First evidence of hepatitis E virus infection in a small mammal (yellow-necked mouse) from Croatia. PLoS ONE 2019, 14, e0225583. [Google Scholar] [CrossRef]
| Species | Years of Investigation | N Tested | HEV RNA Prevalence % Positive (95% CI) | HEV IgG Prevalence % Positive (95% CI) | Reference |
|---|---|---|---|---|---|
| Domestic pigs | 2010–2017 | 1419 | 15.2% (13.5–17.2) | ND 1 | [30] |
| 2016 and 2017 | 1424 | NT 2 | 32.9% (30.5–35.4%) | [63] | |
| 2016 and 2017 | 670 | 0 | NT 2 | [63] | |
| 2009 and 2010 | 1092 | 24.5% (21.7–27.6%) | ND 1 | [29] | |
| Wild boars | 2010–2017 | 720 | 11.5% (9.4–14.1%) | ND 1 | [30] |
| 2016 and 2017 | 1000 | - | 31.1% (28.3–34.0%) | [63] | |
| 2016 and 2017 | 150 | 31.10% (28.31–34.04%) | NT 2 | [63] | |
| 2009 and 2010 | 536 | 12.3% (9.7–15.4%) | ND 1 | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrzljak, A.; Jemersic, L.; Savic, V.; Balen, I.; Ilic, M.; Jurekovic, Z.; Pavicic-Saric, J.; Mikulic, D.; Vilibic-Cavlek, T. Hepatitis E Virus in Croatia in the “One-Health” Context. Pathogens 2021, 10, 699. https://doi.org/10.3390/pathogens10060699
Mrzljak A, Jemersic L, Savic V, Balen I, Ilic M, Jurekovic Z, Pavicic-Saric J, Mikulic D, Vilibic-Cavlek T. Hepatitis E Virus in Croatia in the “One-Health” Context. Pathogens. 2021; 10(6):699. https://doi.org/10.3390/pathogens10060699
Chicago/Turabian StyleMrzljak, Anna, Lorena Jemersic, Vladimir Savic, Ivan Balen, Maja Ilic, Zeljka Jurekovic, Jadranka Pavicic-Saric, Danko Mikulic, and Tatjana Vilibic-Cavlek. 2021. "Hepatitis E Virus in Croatia in the “One-Health” Context" Pathogens 10, no. 6: 699. https://doi.org/10.3390/pathogens10060699
APA StyleMrzljak, A., Jemersic, L., Savic, V., Balen, I., Ilic, M., Jurekovic, Z., Pavicic-Saric, J., Mikulic, D., & Vilibic-Cavlek, T. (2021). Hepatitis E Virus in Croatia in the “One-Health” Context. Pathogens, 10(6), 699. https://doi.org/10.3390/pathogens10060699










