Synergistic Activation of Bovine CD4+ T Cells by Neutrophils and IL-12
Abstract
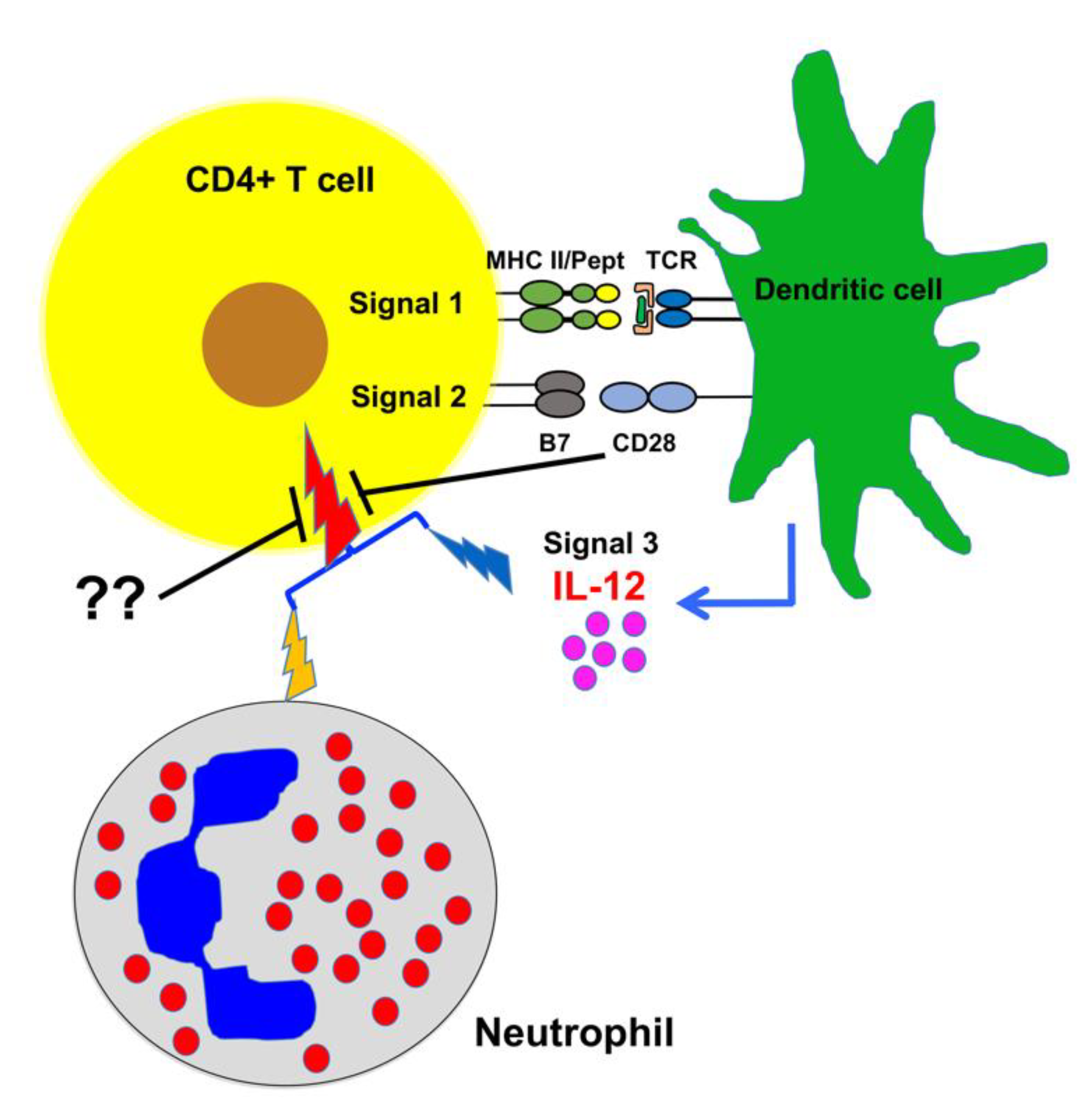
1. Introduction
2. Results
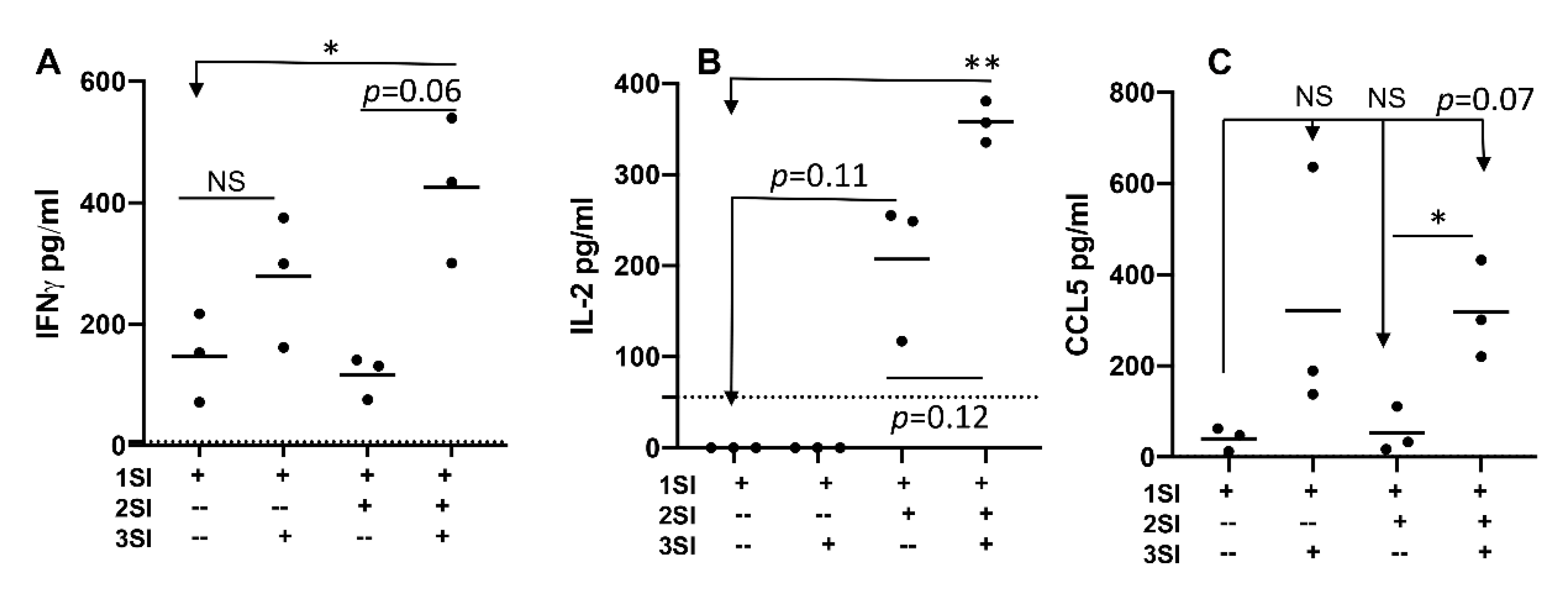
2.1. Bovine CD4+ T Cell Activation Requires Third Signal Cytokine
2.2. Bovine Naïve CD4+ T Cell Activation Leads to Cytokine Secretion
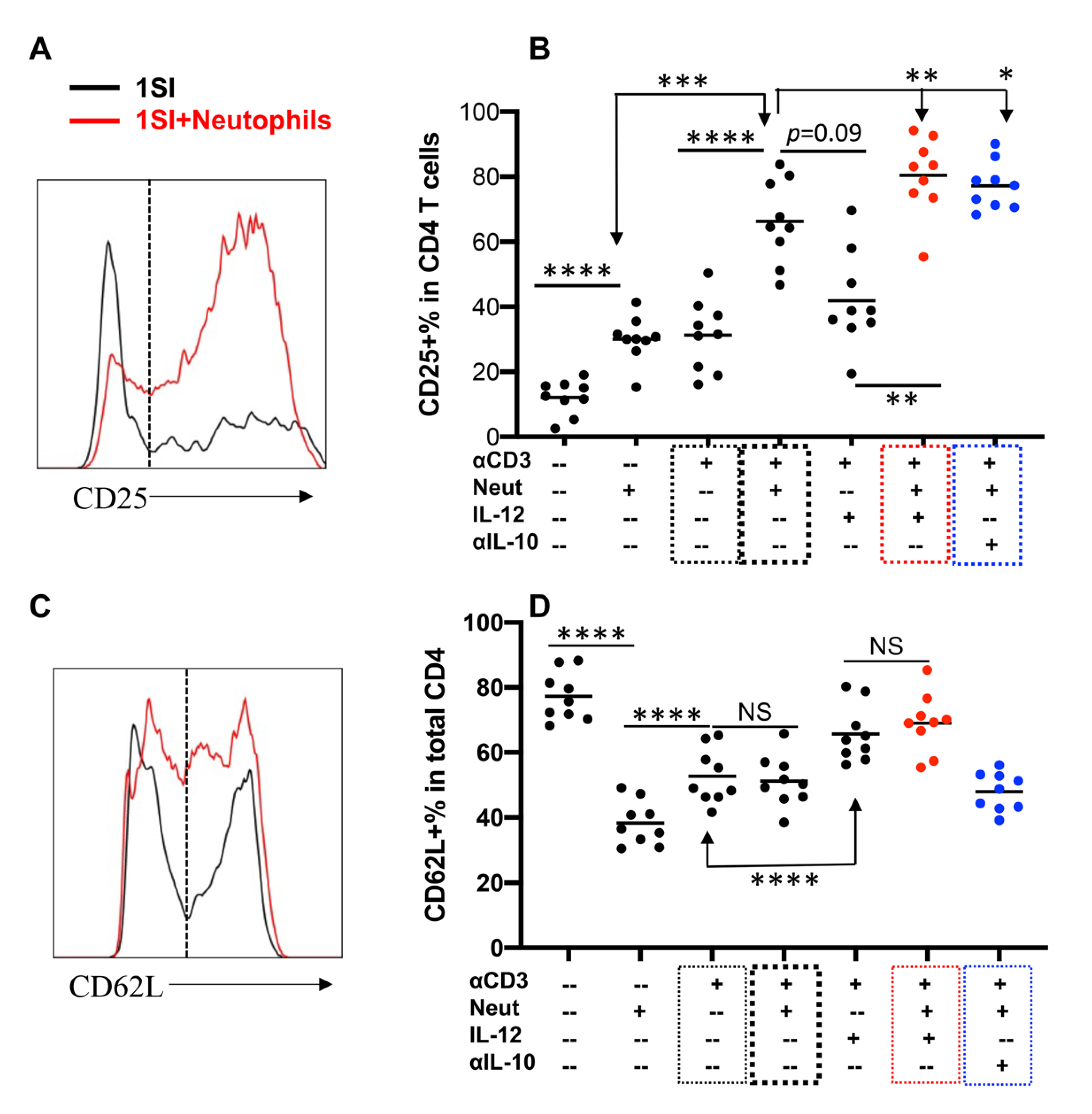
2.3. Neutrophils and IL-12 Synergistically Activate Bovine CD4+ T Cells
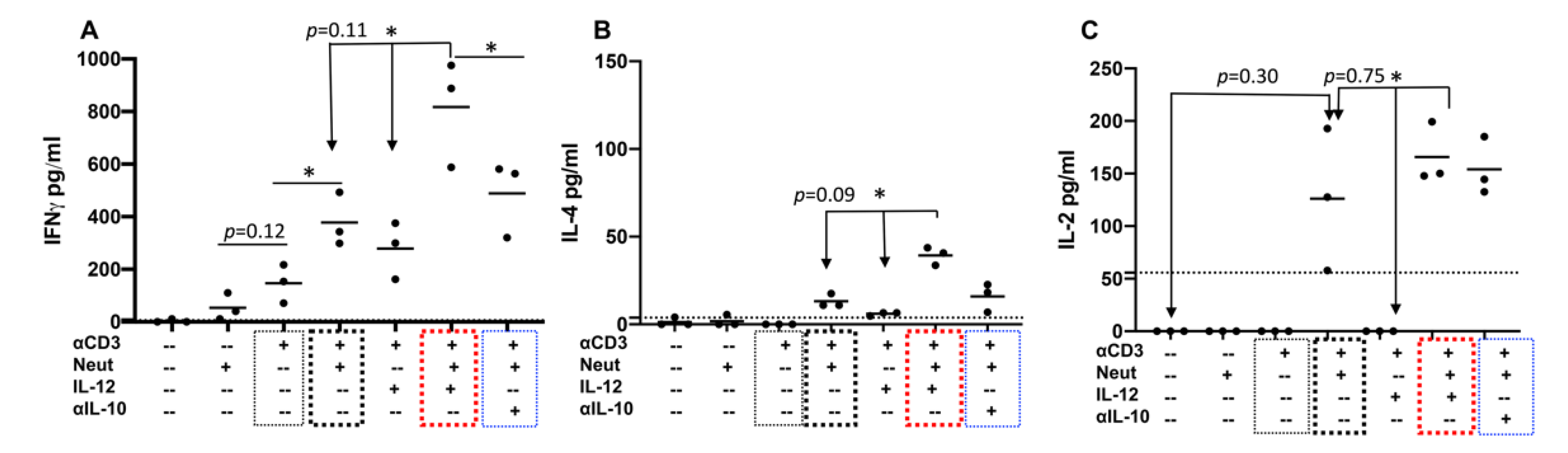
2.4. Neutrophils and IL-12 Synergistically Stimulate the Secretion of Cytokines by Activated Bovine CD4+ T Cells
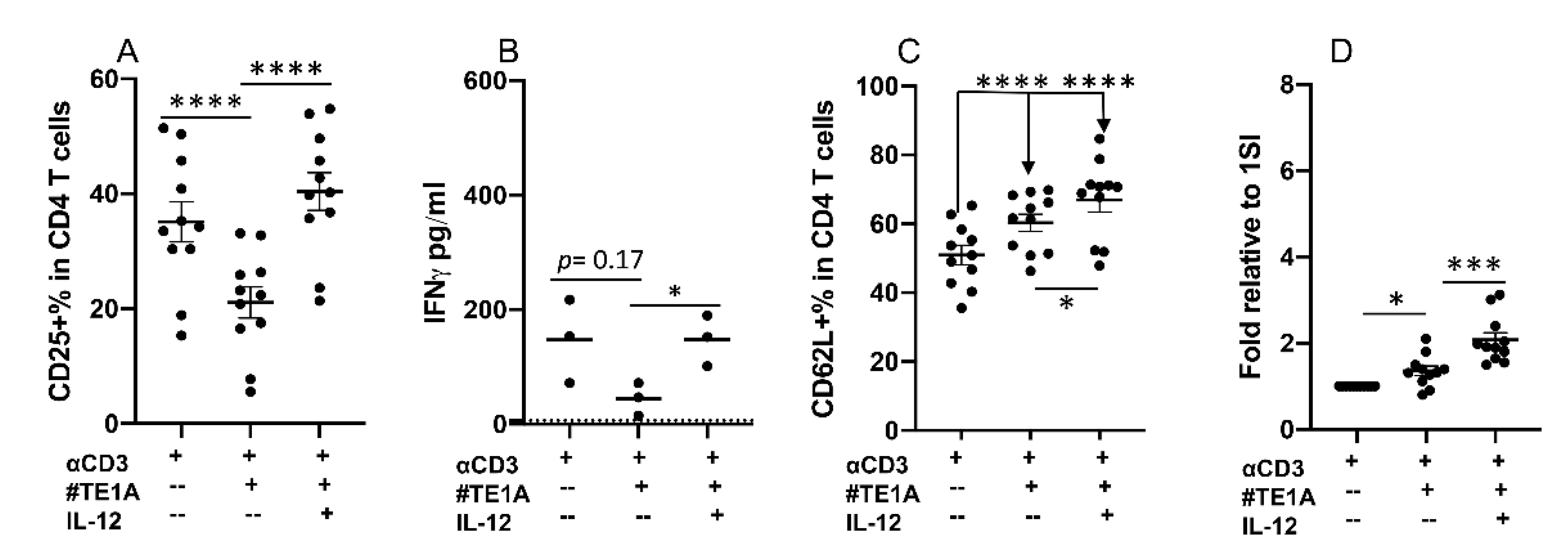
2.5. CD28 Can Deliver Inhibitory Signaling to Bovine CD4+ T Cells
2.6. Synergy between Neutrophils and IL-12 Can Be Regulated by Inhibitory Signaling from CD28
3. Discussion
4. Materials and Methods
4.1. Cattle
4.2. Bovine Neutrophil Isolation
4.3. CD4+ T Cell Isolation
4.4. Stimulation of CD4+ T Cells
4.5. Bovine Cytokine Array
4.6. Flow Cytometry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Pulendran, B. Variegation of the Immune Response with Dendritic Cells and Pathogen Recognition Receptors. J. Immunol. 2005, 174, 2457–2465. [Google Scholar] [CrossRef]
- Harty, J.T.; Badovinac, V.P. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 2008, 8, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.J. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 2004, 4, 595–602. [Google Scholar] [CrossRef]
- Mescher, M.F.; Curtsinger, J.M.; Agarwal, P.; Casey, K.A.; Gerner, M.; Hammerbeck, C.D.; Popescu, F.; Xiao, Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006, 211, 81–92. [Google Scholar] [CrossRef]
- Itano, A.A.; Jenkins, M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 2003, 4, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Kambayashi, T.; Laufer, T.M. Atypical MHC class II-expressing antigen-presenting cells: Can anything replace a dendritic cell? Nat. Rev. Immunol. 2014, 14, 719–730. [Google Scholar] [CrossRef]
- Steinman, R.M. Dendritic cells: Understanding immunogenicity. Eur. J. Immunol. 2007, 37, S53–S60. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 2010, 238, 247–262. [Google Scholar] [CrossRef]
- Mescher, M.F.; Agarwal, P.; Casey, K.A.; Hammerbeck, C.D.; Xiao, Z.; Curtsinger, J.M. Molecular basis for checkpoints in the CD8 T cell response: Tolerance versus activation. Semin. Immunol. 2007, 19, 153–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharpe, A.H. Mechanisms of costimulation. Immunol. Rev. 2009, 229, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Baitsch, L.; Legat, A.; Barba, L.; Marraco, S.A.F.; Rivals, J.-P.; Baumgaertner, P.; Christiansen-Jucht, C.; Bouzourene, H.; Rimoldi, D.; Pircher, H.; et al. Extended Co-Expression of Inhibitory Receptors by Human CD8 T-Cells Depending on Differentiation, Antigen-Specificity and Anatomical Localization. PLoS ONE 2012, 7, e30852. [Google Scholar]
- Kane, L.P. T Cell Ig and Mucin Domain Proteins and Immunity. J. Immunol. 2010, 184, 2743–2749. [Google Scholar] [CrossRef]
- Simpson, T.R.; A Quezada, S.; Allison, J.P. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS). Curr. Opin. Immunol. 2010, 22, 326–332. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Kruger, P.; Saffarzadeh, M.; Weber, A.; Rieber, N.; Radsak, M.; Von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PLoS Pathog. 2015, 11, e1004651. [Google Scholar] [CrossRef]
- Leliefeld, P.H.; Koenderman, L.; Pillay, J. How Neutrophils Shape Adaptive Immune Responses. Front. Immunol. 2015, 6, 471. [Google Scholar] [CrossRef] [PubMed]
- Dancey, J.T.; Deubelbeiss, K.A.; Harker, L.A.; Finch, C.A. Neutrophil kinetics in man. J. Clin. Investig. 1976, 58, 705–715. [Google Scholar] [CrossRef]
- McMillan, R.; Scott, J.L. Leukocyte labeling with 51-Chromium. I. Technic and results in normal subjects. Blood 1968, 32, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Re, F.; Polentarutti, N.; Sozzani, S.; Mantovani, A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 1922, 80, 2012–2020. [Google Scholar] [CrossRef]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Chtanova, T.; Schaeffer, M.; Han, S.-J.; van Dooren, G.G.; Nollmann, M.; Herzmark, P.; Chan, S.W.; Satija, H.; Camfield, K.; Aaron, H.; et al. Dynamics of Neutrophil Migration in Lymph Nodes during Infection. Immunity 2008, 29, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Pesce, J.T.; Liu, Z.; Hamed, H.; Alem, F.; Whitmire, J.; Lin, H.; Liu, Q.; Urban, J.F.; Gause, W.C. Neutrophils Clear Bacteria Associated with Parasitic Nematodes Augmenting the Development of an Effective Th2-Type Response. J. Immunol. 2008, 180, 464–474. [Google Scholar] [CrossRef]
- Maletto, B.A.; Ropolo, A.S.; Alignani, D.O.; Liscovsky, M.V.; Ranocchia, R.P.; Moron, V.G.; Pistoresi-Palencia, M.C. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood 2006, 108, 3094–3102. [Google Scholar] [CrossRef]
- Beauvillain, C.; Delneste, Y.; Scotet, M.; Peres, A.; Gascan, H.; Guermonprez, P.; Barnaba, V.; Jeannin, P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood 2007, 110, 2965–2973. [Google Scholar] [CrossRef]
- Lok, L.S.C.; Dennison, T.W.; Mahbubani, K.M.; Saeb-Parsy, K.; Chilvers, E.R.; Clatworthy, M.R. Phenotypically distinct neutrophils patrol uninfected human and mouse lymph nodes. Proc. Natl. Acad. Sci. USA 2019, 116, 19083–19089. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Si, H.; Wu, S.-W.; Mendez, J.O.; Zarlenga, D.; Tuo, W.; Xiao, Z. Characterization of IL-10-producing neutrophils in cattle infected with Ostertagia ostertagi. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Elhmouzi-Younes, J.; Storset, A.K.; Boysen, P.; Laurent, F.; Drouet, F. Bovine neonate natural killer cells are fully functional and highly responsive to interleukin-15 and to NKp46 receptor stimulation. Vet. Res. 2009, 40, 54. [Google Scholar] [CrossRef]
- Goff, W.L.; Storset, A.K.; Johnson, W.C.; Brown, W.C. Bovine splenic NK cells synthesize IFN-gamma in response to IL-12-containing supernatants from Babesia bovis-exposed monocyte cultures. Parasite Immunol. 2006, 28, 221–228. [Google Scholar] [CrossRef]
- Obar, J.J.; Lefrançois, L. Early Signals during CD8+ T Cell Priming Regulate the Generation of Central Memory Cells. J. Immunol. 2010, 185, 263–272. [Google Scholar] [CrossRef]
- Van Wely, C.A.; Beverley, P.; Brett, S.J.; Britten, C.J.; Tite, J.P. Expression of L-selectin on Th1 cells is regulated by IL-12. J. Immunol. 1999, 163, 1214–1221. [Google Scholar]
- Díaz-Montero, C.M.; El Naggar, S.; Al Khami, A.; El Naggar, R.; Montero, A.J.; Cole, D.J.; Salem, M.L. Priming of naive CD8+ T cells in the presence of IL-12 selectively enhances the survival of CD8+CD62Lhi cells and results in superior anti-tumor activity in a tolerogenic murine model. Cancer Immunol. Immunother. 2007, 57, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, R.; Ullmann, S.; Proulx, S.T.; Pytowski, B.; Alitalo, K.; Detmar, M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J. Exp. Med. 2010, 207, 2255–2269. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014, 5, 4172. [Google Scholar] [CrossRef]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef]
- Bousso, P. T-cell activation by dendritic cells in the lymph node: Lessons from the movies. Nat. Rev. Immunol. 2008, 8, 675–684. [Google Scholar] [CrossRef]
- Jenkins, M.K.; Ashwell, J.D.; Schwartz, R.H. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J. Immunol. 1988, 140, 3324–3330. [Google Scholar]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Clark, E.A.; Ledbetter, J.A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc. Natl. Acad. Sci. USA 1990, 87, 5031–5035. [Google Scholar] [CrossRef]
- Engelhardt, J.J.; Sullivan, T.J.; Allison, J.P. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J. Immunol. 2006, 177, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, P.A.; Davis, S.J. Molecular interactions mediating T cell antigen recognition. Annu. Rev. Immunol. 2003, 21, 659–684. [Google Scholar] [CrossRef]
- Andrews, L.P.; Yano, H.; Vignali, D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat. Immunol. 2019, 20, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Mitsuiki, N.; Schwab, C.; Grimbacher, B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol. Rev. 2019, 287, 33–49. [Google Scholar] [CrossRef]
- Eder, J.M.; Gorden, P.J.; Lippolis, J.D.; Reinhardt, T.A.; Sacco, R.E. Lactation stage impacts the glycolytic function of bovine CD4+ T cells during ex vivo activation. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Thakur, A.; Riber, U.; Davis, W.C.; Jungersen, G. Increasing the ex vivo antigen-specific IFN-γ production in subpopulations of T cells and NKp46+ cells by anti-CD28, anti-CD49d and recombinant IL-12 costimulation in cattle vaccinated with recombinant proteins from Mycobacterium avium subspecies paratuberculosis. Vet. Immunol. Immunopathol. 2013, 155, 276–283. [Google Scholar]
- Lv, Y.; Mattson, E.; BhaduriHauck, A.; Garcia, K.; Li, L.; Xiao, Z. Effector functions of memory CTLs can be affected by signals received during reactivation. Immunol. Res. 2017, 65, 841–852. [Google Scholar] [CrossRef]
- Hogg, A.E.; Parsons, K.; Taylor, G.; Worth, A.; Beverley, P.; Howard, C.J.; Villarreal-Ramos, B. Characterization of age-related changes in bovine CD8+ T-cells. Vet. Immunol. Immunopathol. 2011, 140, 47–54. [Google Scholar] [CrossRef]
- Brown, W.C.; Rice-Ficht, A.C.; Estes, D. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 1998, 63, 45–55. [Google Scholar] [CrossRef]
- DeKuiper, J.L.; Coussens, P.M. Inflammatory Th17 responses to infection with Mycobacterium avium subspecies paratuberculosis (MAP) in cattle and their potential role in development of Johne’s disease. Vet. Immunol. Immunopathol. 2019, 218, 109954. [Google Scholar] [CrossRef]
- Brackenbury, L.S.; Carr, B.V.; Stamataki, Z.; Prentice, H.; Lefevre, E.A.; Howard, C.J.; Charleston, B. Identification of a Cell Population That Produces Alpha/Beta Interferon In Vitro and In Vivo in Response to Noncytopathic Bovine Viral Diarrhea Virus. J. Virol. 2005, 79, 7738–7744. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, N.P.; Bielefeldt-Ohmann, H.; Van Campen, H.; Austin, K.J.; Han, H.; Montgomery, D.L.; Shoemaker, M.L.; van Olphen, A.L.; Hansen, T.R. Acute non-cytopathic bovine viral diarrhea virus infection induces pronounced type I interferon response in pregnant cows and fetuses. Virus Res. 2008, 132, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R.; Pharr, G.; Boyd, B.; Pinchuk, L. Bovine viral diarrhea viruses modulate toll-like receptors, cytokines and co-stimulatory molecules genes expression in bovine peripheral blood monocytes. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 403–418. [Google Scholar] [CrossRef]
- Hansen, T.R.; Smirnova, N.P.; Webb, B.T.; Bielefeldt-Ohmann, H.; Sacco, R.E.; Van Campen, H. Innate and adaptive immune responses to in utero infection with bovine viral diarrhea virus. Anim. Health Res. Rev. 2015, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Dar, P.A.; Hajam, I.A.; Suryanarayana, V.S.; Kishore, S.; Kondabattula, G. Kinetics of cytokine expression in bovine PBMCs and whole blood after in vitro stimulation with foot-and-mouth disease virus (FMDV) antigen. Cytokine 2015, 72, 58–62. [Google Scholar] [CrossRef]
- Skinner Margot, A.M. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 2003, 71, 4901–4907. [Google Scholar] [CrossRef]
- Thacker, T.C.; Palmer, M.V.; Waters, W.R. Associations between cytokine gene expression and pathology in Mycobacterium bovis infected cattle. Vet. Immunol. Immunopathol. 2007, 119, 204–213. [Google Scholar] [CrossRef]
- Muskens, J.; van Zijderveld, F.; Eger, A.; Bakker, D. Evaluation of the long-term immune response in cattle after vaccination against paratuberculosis in two Dutch dairy herds. Vet. Microbiol. 2002, 86, 269–278. [Google Scholar] [CrossRef]
- Koo Hye Cheong, H.C. Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves. Infect. Immun. 2004, 72, 6870–6883. [Google Scholar]
- Coussens, P.M.; Verman, N.; Coussens, M.A.; Elftman, M.D.; McNulty, A.M. Cytokine Gene Expression in Peripheral Blood Mononuclear Cells and Tissues of Cattle Infected with Mycobacterium avium subsp. paratuberculosis: Evidence for an Inherent Proinflammatory Gene Expression Pattern. Infect. Immun. 2004, 72, 1409–1422. [Google Scholar] [CrossRef]
- Stabel, J.; Bannantine, J. Divergent antigen-specific cellular immune responses during asymptomatic subclinical and clinical states of disease in cows naturally infected with Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 2019, 88, e00650-19. [Google Scholar] [CrossRef] [PubMed]
- Magombedze, G.; Shiri, T.; Eda, S.; Stabel, J.R. Inferring biomarkers for Mycobacterium avium subsp. paratuberculosis infection and disease progression in cattle using experimental data. Sci. Rep. 2017, 7, 44765. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; McElwain, T.F.; Hötzel, I.; Suarez, C.E.; Palmer, G.H. Helper T-Cell Epitopes Encoded by the Babesia bigemina rap-1 Gene Family in the Constant and Variant Domains Are Conserved among Parasite Strains. Infect. Immun. 1998, 66, 1561–1569. [Google Scholar] [CrossRef]
- Brown, W.C.; McElwain, T.F.; Palmer, G.H.; Chantler, S.E.; Estes, D.M. Bovine CD4+ T-Lymphocyte Clones Specific for Rhoptry-Associated Protein 1 of Babesia bigemina Stimulate Enhanced Immunoglobulin G1 (IgG1) and IgG2 Synthesis. Infect. Immun. 1999, 67, 155–164. [Google Scholar] [CrossRef]
- Rodríguez, S.D.; Palmer, G.H.; McElwain, T.F.; McGuire, T.C.; Ruef, B.J.; Chitko-McKown, M.G.; Brown, W.C. CD4+ T-helper lymphocyte responses against Babesia bigemina rhoptry-associated protein I. Infect. Immun. 1996, 64, 2079–2087. [Google Scholar] [CrossRef]
- Begg, D.J.; De Silva, K.; Carter, N.; Plain, K.M.; Purdie, A.; Whittington, R.J. Does a Th1 over Th2 dominancy really exist in the early stages of Mycobacterium avium subspecies paratuberculosis infections? Immunobiology 2011, 216, 840–846. [Google Scholar] [CrossRef]
- Graham-Brown, J.; Hartley, C.; Clough, H.; Kadioglu, A.; Baylis, M.; Williams, D.J. Dairy heifers naturally exposed to Fasciola hepatica develop a type 2 immune response and concomitant suppression of leukocyte proliferation. Infect. Immun. 2018, 86, e00607–e00617. [Google Scholar] [CrossRef]
- Peckham, R.K.; Brill, R.; Foster, D.S.; Bowen, A.L.; Leigh, J.A.; Coffey, T.J.; Flynn, R.J. Two distinct populations of Bovine IL-17+ T-cells can be induced and WC1+IL-17+γδ T-cells are effective killers of protozoan parasites. Sci. Rep. 2015, 4, 5431. [Google Scholar] [CrossRef]
- Corrêa, F.; Hidalgo, C.; Stoore, C.; Jiménez, M.; Hernández, M.; Paredes, R. Cattle co-infection of Echinococcus granulosus and Fasciola hepatica results in a different systemic cytokine profile than single parasite infection. PLoS ONE 2020, 15, e0238909. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pelayo, L.; García-Sánchez, M.; Collantes-Fernández, E.; Regidor-Cerrillo, J.; Horcajo, P.; Gutiérrez-Expósito, D.; Espinosa, J.; Benavides, J.; Osoro, K.; Pfarrer, C.; et al. Crosstalk between Neospora caninum and the bovine host at the maternal-foetal interface determines the outcome of infection. Vet. Res. 2020, 51, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.; Sun, D.; Tuo, W.; Xiao, Z. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite Ostertagia ostertagi. Sci. Rep. 2018, 8, 17598. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.S.A.; Egan, C.E.; Butcher, B.A.; Denkers, E.Y. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int. Immunol. 2011, 23, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, F.; Yu, Y.; Luo, J.; Hu, Q.; Bequette, B.J.; Vi, R.L.B.; Liu, G.; Zan, L.; Updike, M.S.; et al. Muscle transcriptomic analyses in Angus cattle with divergent tenderness. Mol. Biol. Rep. 2011, 39, 4185–4193. [Google Scholar] [CrossRef]
- Carrillo, J.A.; He, Y.; Li, Y.; Liu, J.; Erdman, R.A.; Sonstegard, T.S.; Song, J. Integrated metabolomic and transcriptome analyses reveal finishing forage affects metabolic pathways related to beef quality and animal welfare. Sci. Rep. 2016, 6, 25948. [Google Scholar] [CrossRef]
- Tuo, W.; Li, L.; Lv, Y.; Carrillo, J.; Brown, D.; Davis, W.C.; Song, J.; Zarlenga, D.; Xiao, Z. Abomasal mucosal immune responses of cattle with limited or continuous exposure to pasture-borne gastrointestinal nematode parasite infection. Vet. Parasitol. 2016, 229, 118–125. [Google Scholar] [CrossRef]
- García, M.; Elsasser, T.; Biswas, D.; Moyes, K. The effect of citrus-derived oil on bovine blood neutrophil function and gene expression in vitro. J. Dairy Sci. 2015, 98, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, U.K.; Moore, T.T.; Joo, H.-G.; Tanaka, Y.; Herrmann, V.; Doherty, G.; Drebin, J.A.; Strasberg, S.M.; Eberlein, T.J.; Goedegebuure, P.S.; et al. Prevalence of Regulatory T Cells Is Increased in Peripheral Blood and Tumor Microenvironment of Patients with Pancreas or Breast Adenocarcinoma. J. Immunol. 2002, 169, 2756–2761. [Google Scholar] [CrossRef]
- Hanby-Flarida, M.D.; Trask, O.J.; Yang, T.J.; Baldwin, C.L. Modulation of WC1, a lineage-specific cell surface molecule of gamma/delta T cells augments cellular proliferation. Immunology 1996, 88, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kwong, L.; Hope, J.; Thom, M.; Sopp, P.; Duggan, S.; Bembridge, G.; Howard, C. Development of an ELISA for bovine IL-10. Vet. Immunol. Immunopathol. 2002, 85, 213–223. [Google Scholar] [CrossRef]
- Guzman, E.; Hope, J.; Taylor, G.; Smith, A.L.; Cubillos-Zapata, C.; Charleston, B. Bovine γδ T cells are a major regulatory T cell subset. J. Immunol. 2014, 193, 208–222. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Kandel, A.; Li, L. Synergistic Activation of Bovine CD4+ T Cells by Neutrophils and IL-12. Pathogens 2021, 10, 694. https://doi.org/10.3390/pathogens10060694
Xiao Z, Kandel A, Li L. Synergistic Activation of Bovine CD4+ T Cells by Neutrophils and IL-12. Pathogens. 2021; 10(6):694. https://doi.org/10.3390/pathogens10060694
Chicago/Turabian StyleXiao, Zhengguo, Anmol Kandel, and Lei Li. 2021. "Synergistic Activation of Bovine CD4+ T Cells by Neutrophils and IL-12" Pathogens 10, no. 6: 694. https://doi.org/10.3390/pathogens10060694
APA StyleXiao, Z., Kandel, A., & Li, L. (2021). Synergistic Activation of Bovine CD4+ T Cells by Neutrophils and IL-12. Pathogens, 10(6), 694. https://doi.org/10.3390/pathogens10060694






