Unusual Presentation of Feline Leprosy Caused by Mycobacterium lepraemurium in the Alpine Region
Abstract
1. Introduction
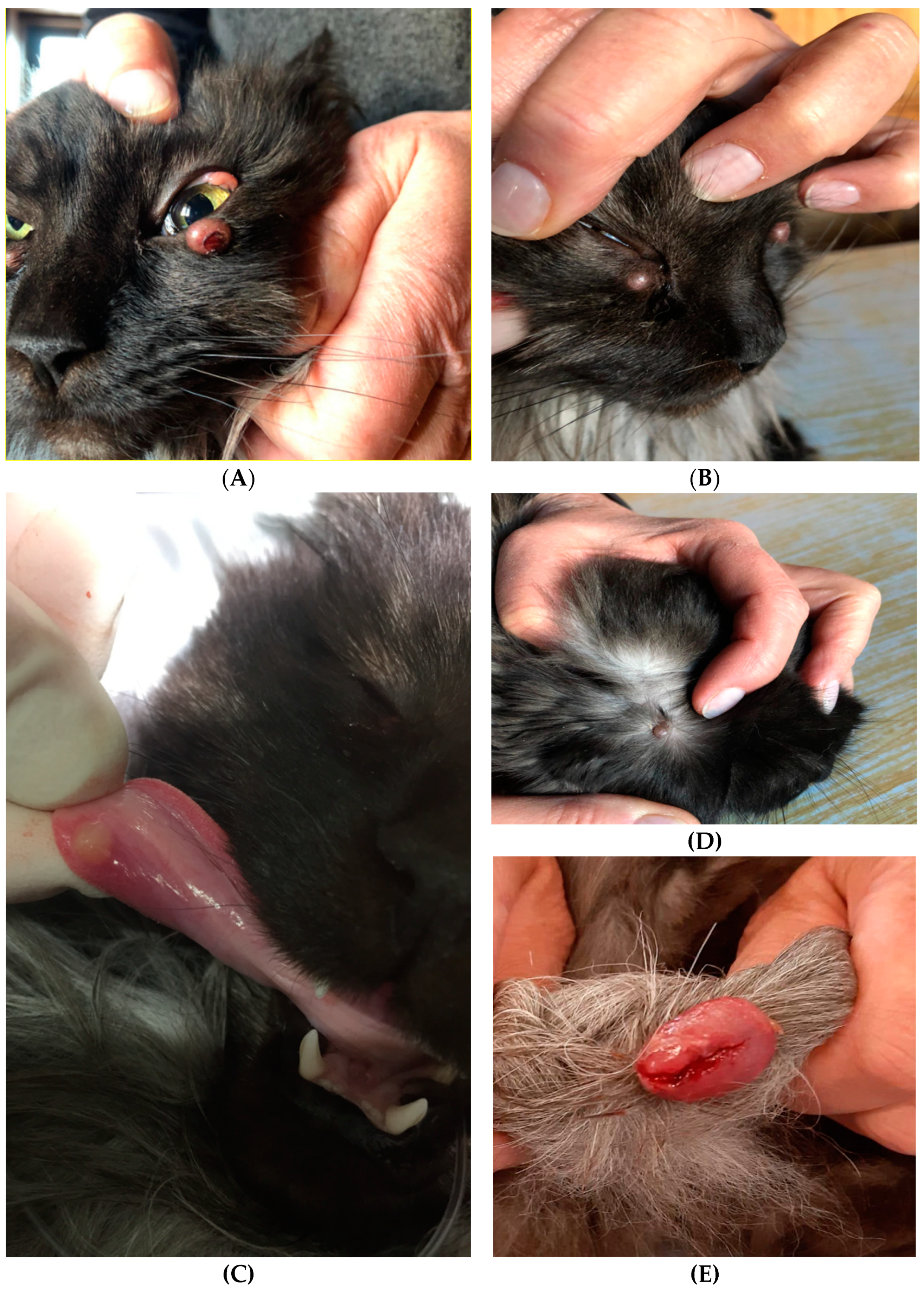
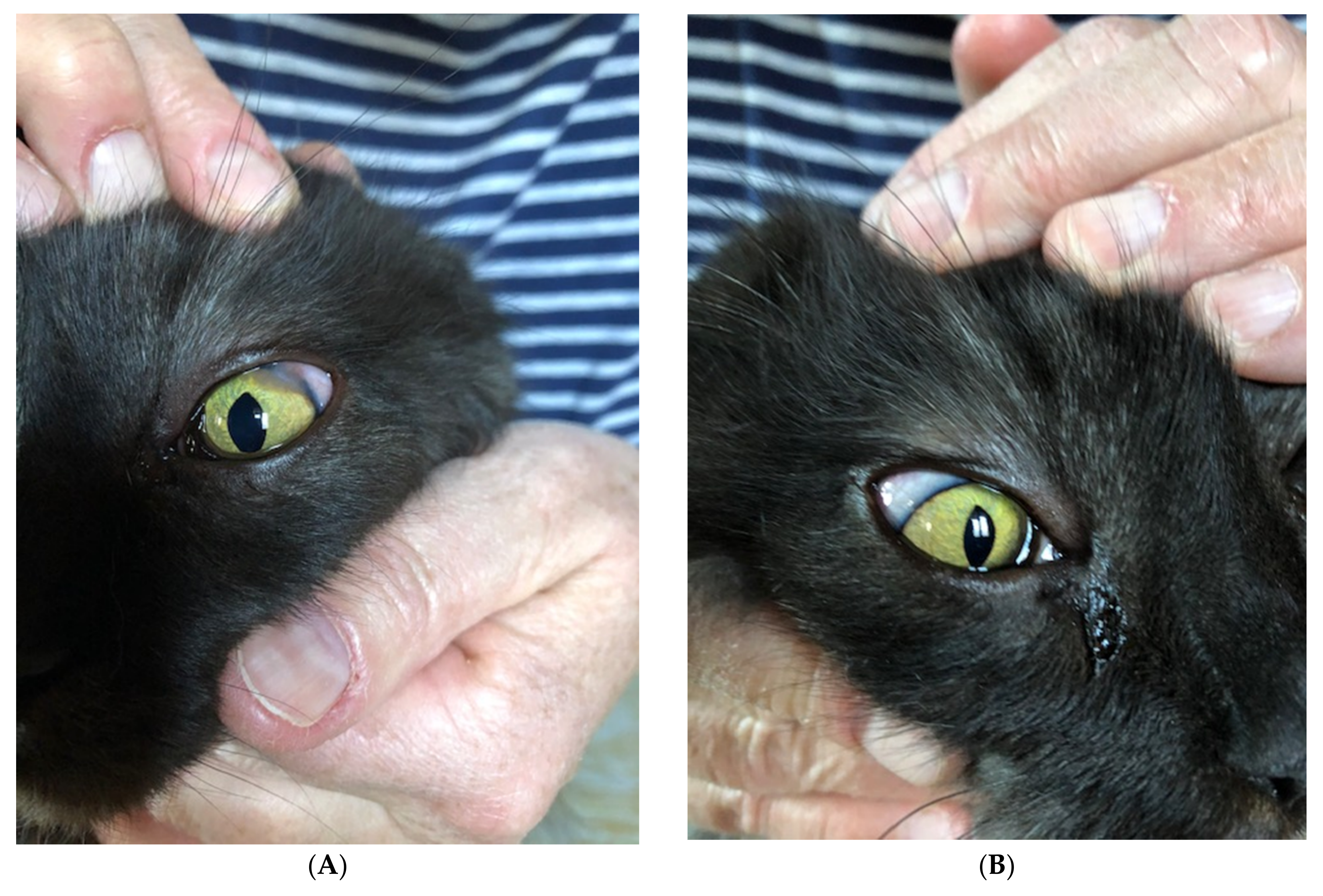
2. Case Description
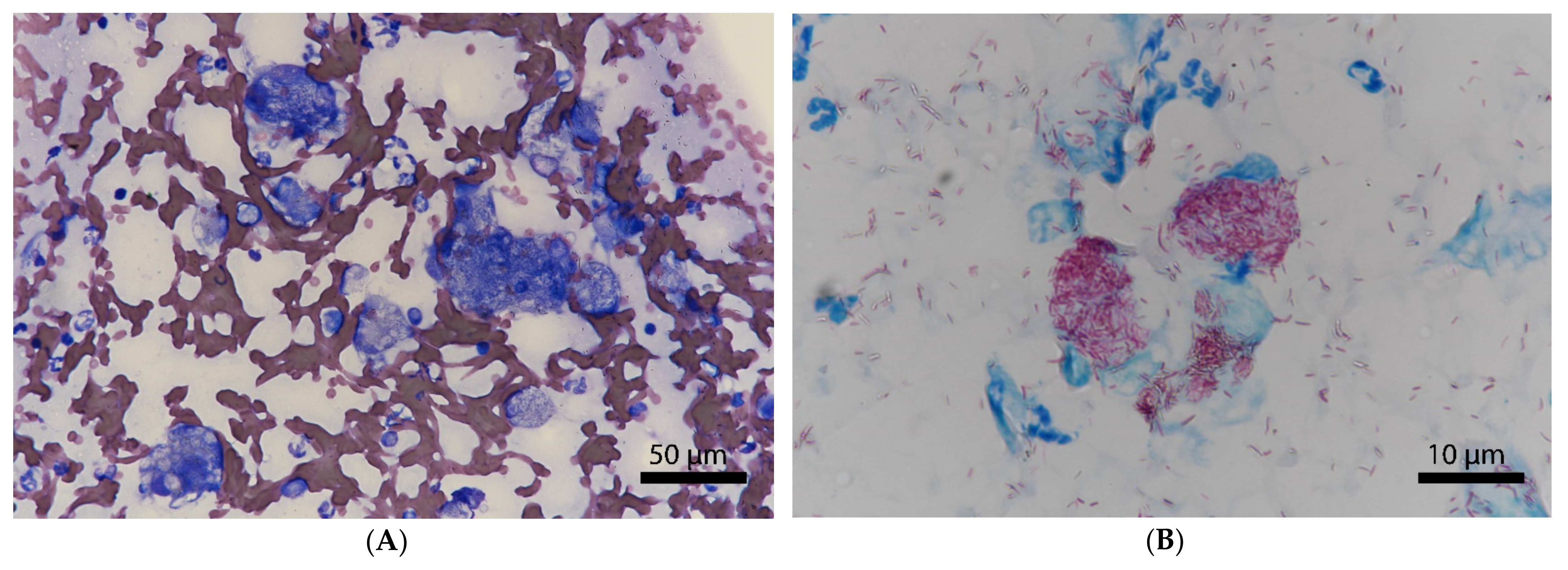
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunn-Moore, D.A.; Gaunt, C.; Shaw, D.J.; Gunn-Moore, D. Incidence of mycobacterial infections in cats in Great Britain: Estimate from feline tissue samples Ssubmitted to diagnostic laboratories. Transbound. Emerg. Dis. 2013, 60, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Hoefsloot, W.; Van Ingen, J.; Andrejak, C.; Ängeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Herrmann, J.-L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Genet. 2020, 18, 392–407. [Google Scholar] [CrossRef]
- Gunn-Moore, D.A. Feline mycobacterial infections. Vet. J. 2014, 201, 230–238. [Google Scholar] [CrossRef]
- Frye, F.L.; Carney, J.D.; Loughman, W.D. Feline lepromatous leprosy. Vet. Med. Small Anim. Clin. 1974, 69, 1272–1273. [Google Scholar]
- McIntosh, D. Feline Leprosy: A Review of Forty-four Cases from Western Canada. Can. Vet. J. Rev. Vet. Can. 1982, 23, 291–295. [Google Scholar]
- Schiefer, B.; Gee, B.R.; Ward, G.E. A disease resembling feline leprosy in western Canada. J. Am. Vet. Med. Assoc. 1974, 165, 1085–1087. [Google Scholar] [PubMed]
- Malik, R.; Hughes, M.; James, G.; Martin, P.; Wigney, D.; Canfield, P.; Chen, S.; Mitchell, D.; Love, D. Feline leprosy: Two different clinical syndromes. J. Feline Med. Surg. 2002, 4, 43–59. [Google Scholar] [CrossRef]
- O’Brien, C.; Malik, R.; Globan, M.; Reppas, G.; McCowan, C.; Fyfe, J.A. Feline leprosy due to Candidatus ‘Mycobacterium lepraefelis’: Further clinical and molecular characterisation of eight previously reported cases and an additional 30 cases. J. Feline Med. Surg. 2017, 19, 919–932. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; Malik, R.; Globan, M.; Reppas, G.; McCowan, C.; Fyfe, J.A. Feline leprosy due to Mycobacterium lepraemurium: Further clinical and molecular characterisation of 23 previously reported cases and an additional 42 cases. J. Feline Med. Surg. 2017, 19, 737–746. [Google Scholar] [CrossRef]
- O’Brien, C.; Malik, R.; Globan, M.; Reppas, G.; McCowan, C.; Fyfe, J.A. Feline leprosy due to Candidatus ‘Mycobacterium tarwinense’: Further clinical and molecular characterisation of 15 previously reported cases and an additional 27 cases. J. Feline Med. Surg. 2017, 19, 498–512. [Google Scholar] [CrossRef]
- Robinson, M. Skin granuloma of cats associated with acid-fast bacilli. J. Small Anim. Pract. 1975, 16, 563–567. [Google Scholar] [CrossRef]
- Poelma, F.G.; Leiker, D.L. Cat leprosy in the Netherlands. Int. J. Lepr. Mycobact. Dis. 1974, 42, 307–311. [Google Scholar]
- Laprie, C.; Duboy, J.; Malik, R.; Fyfe, J. Feline cutaneous mycobacteriosis: A review of clinical, pathological and molecular charac-terization of one case of Mycobacterium microti skin infection and nine cases of feline leprosy syndrome from France and New Caledonia. Vet. Dermatol. 2013, 24, e133–e134. [Google Scholar] [CrossRef]
- Courtin, F.; Huerre, M.; Fyfe, J.; Dumas, P.; Boschiroli, M.L. A case of feline leprosy caused by Mycobacterium lepraemurium originating from the island of Kythira (Greece): Diagnosis and treatment. J. Feline Med. Surg. 2007, 9, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Lamagna, B.; Paciello, O.; Ragozzino, M.; Papparella, S.; Montagnaro, S.; Lamagna, F. Isolated lepromatous conjunctivo-corneal granuloma in a cat from Italy. Vet. Ophthalmol. 2009, 12, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.A.; McCowan, C.; O’Brien, C.R.; Globan, M.; Birch, C.; Revill, P.; Barrs, V.R.D.; Wayne, J.; Hughes, M.S.; Holloway, S. Molecular characterization of a novel fastidious Mycobacterium causing lepromatous lesions of the skin, subcutis, cornea, and conjunctiva of cats living in Victoria, Australia. J. Clin. Microbiol. 2008, 46, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Benjak, A.; Honap, T.P.; Avanzi, C.; Becerril-Villanueva, E.; Estrada-García, I.; Rojas-Espinosa, O.; Stone, A.C.; Cole, S.T. Insights from the genome sequence of Mycobacterium lepraemurium: Massive gene decay and reductive evolution. mBio 2017, 8. [Google Scholar] [CrossRef]
- Dean, G. A disease of the rat caused by an acid-fast bacillus. Cent. F Bakteriol. 1903, 34, 222–224. [Google Scholar]
- O’Connor, C.M.; Abid, M.; Walsh, A.L.; Behbod, B.; Roberts, T.; Booth, L.V.; Thomas, H.L.; Smith, N.H.; Palkopoulou, E.; Dale, J.; et al. Cat-to-human transmission of Mycobacterium bovis, United Kingdom. Emerg. Infect. Dis. 2019, 25, 2284–2286. [Google Scholar] [CrossRef]
- Černá, P.; Mitchell, J.L.; Lodzinska, J.; Cazzini, P.; Varjonen, K.; Gunn-Moore, D.A. Systemic Mycobacterium kansasii Infection in Two Related Cats. Pathogens 2020, 9, 959. [Google Scholar] [CrossRef]
- Munro, M.J.L.; Byrne, B.A.; Sykes, J.E. Feline mycobacterial disease in northern California: Epidemiology, clinical features, and antimicrobial susceptibility. J. Vet. Intern. Med. 2021, 35, 273–283. [Google Scholar] [CrossRef]
- Niederhäuser, S.; Klauser, L.; Bolliger, J.; Friedel, U.; Schmitt, S.; Ruetten, M.; Greene, C.E.; Ghielmetti, G. First report of nodular skin lesions caused by Mycobacterium nebraskense in a 9-year-old cat. J. Feline Med. Surg. Open Rep. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, K.M.; Rohrbach, B.W. A Retrospective study of eyelid tumors from 43 Cats. Vet. Pathol. 2009, 46, 916–927. [Google Scholar] [CrossRef]
- Leal, R.O.; Pereira, H.; Cartaxeiro, C.; Delgado, E.; Peleteiro, M.D.C.; Da Fonseca, I.P. Granulomatous rhinitis secondary to feline leishmaniosis: Report of an unusual presentation and therapeutic complications. J. Feline Med. Surg. Open Rep. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Chaitman, J.; Van Der Woerdt, A.; Bartick, T.E. Multiple eyelid cysts resembling apocrine hidrocystomas in three Persian cats and one Himalayan cat. Vet. Pathol. 1999, 36, 474–476. [Google Scholar] [CrossRef]
- Zemljič, T.; Matheis, F.L.; Venzin, C.; Makara, M.; Grest, P.; Spiess, B.M.; Pot, S. Orbito-nasal cyst in a young European short-haired cat. Vet. Ophthalmol. 2011, 14, 122–129. [Google Scholar] [CrossRef]
- Ghielmetti, G.; Friedel, U.; Scherrer, S.; Sarno, E.; Landolt, P.; Dietz, O.; Hilbe, M.; Zweifel, C.; Stephan, R. Non-tuberculous Mycobacteria isolated from lymph nodes and faecal samples of healthy slaughtered cattle and the abattoir environment. Transbound. Emerg. Dis. 2017, 65, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Ghielmetti, G.; Scherrer, S.; Friedel, U.; Frei, D.; Suter, D.; Perler, L.; Wittenbrink, M.M. Epidemiological tracing of bovine tuberculosis in Switzerland, multilocus variable number of tandem repeat analysis of Mycobacterium bovis and Mycobacterium caprae. PLoS ONE 2017, 12, e0172474. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Ed.; John Wiley & Sons.: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Biet, F.; Boschiroli, M.L. Non-tuberculous mycobacterial infections of veterinary relevance. Res. Vet. Sci. 2014, 97, S69–S77. [Google Scholar] [CrossRef]
- Tortoli, E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin. Microbiol. Rev. 2014, 27, 727–752. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; Shalloo, L. Invited review: The economic impact and control of paratuberculosis in cattle. J. Dairy Sci. 2015, 98, 5019–5039. [Google Scholar] [CrossRef] [PubMed]
- Glawischnig, W.; Steineck, T.; Spergser, J. Infections caused by Mycobacterium avium subspecies avium, hominissuis, and paratuberculosis in free-ranging red deer (Cervus elaphus hippelaphus) in Austria, 2001–2004. J. Wildlife Dis. 2006, 42, 724–731. [Google Scholar] [CrossRef]
- Miller, M.A.; Davey, S.C.; Van Helden, L.S.; Kettner, F.; Weltan, S.M.; Last, R.; Grewar, J.; Botha, L.; Van Helden, P.D. Paratuberculosis in a domestic dog in South Africa. J. S. Afr. Vet. Assoc. 2017, 88, 1–5. [Google Scholar] [CrossRef]
- Stefansky, W.K. Eine lepraähnliche Erkrankung der Haut und der Lymphdrüsen bei Wanderratten. Zent. Bakteriol. Parasitenkd. Infekt. Hyg. 1903, 33, 481–487. [Google Scholar]
- Ogawa, T. Some observations on cultures of Mycobacterium lepraemurium. Int. J. Lepr. Mycobact. Dis. 1976, 44, 537. [Google Scholar]
- Ogawa, T.; Motomura, K. Studies on murine leprosy bacillus. I. Attempt to cultivate in vitro the Hawaiian strain of Mycobacterium lepraemurium. Kitasato Arch. Exp. Med. 1970, 43, 65–80. [Google Scholar]
- Saito, H.; Matsuo, Y. Characterization of Mycobacterium lepraemurium cultured in NC-5 medium. Hiroshima J. Med. Sci. 1974, 23, 77–81. [Google Scholar]
- Juarez-Ortega, M.; Hernandez, V.G.; Arce-Paredes, P.; Villanueva, E.B.; Aguilar-Santelises, M.; Rojas-Espinosa, O. Induction and treatment of anergy in murine leprosy. Int. J. Exp. Pathol. 2014, 96, 31–41. [Google Scholar] [CrossRef]
- Helgert, N.D.; Miller, D.L.; Whittemore, J.C.; Sula, M.-J.M. Intrahistiocytic storage of clofazimine crystals in a cat. Vet. Pathol. 2021, 58, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Baral, R.M.; Metcalfe, S.S.; Krockenberger, M.B.; Caft, M.J.; Barrs, V.R.; McWhirter, C. Disseminated Mycobacterium avium infection in young cats: Overrepresentation of Abyssinian cats. J. Feline Med. Surg. 2006, 8, 23–44. [Google Scholar] [CrossRef]
- Ghielmetti, G.; Giger, U. Mycobacterium avium: An emerging pathogen for dog breeds with hereditary immunodeficiencies. Curr. Clin. Microbiol. Rep. 2020, 7, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Smits, B.; Reppas, G.; Laprie, C.; O’Brien, C.; Fyfe, J. Ulcerated and nonulcerated nontuberculous cutaneous mycobacterial granulomas in cats and dogs. Vet. Dermatol. 2013, 24, 146–153.e32–33. [Google Scholar] [CrossRef] [PubMed]
- Roccabianca, P.; Caniatti, M.; Scanziani, E.; Penati, V. Feline leprosy: Spontaneous remission in a cat. J. Am. Anim. Hosp. Assoc. 1996, 32, 189–193. [Google Scholar] [CrossRef]
- Rojas-Espinosa, O.; Rodríguez-Paez, L.; González-Cruz, O.; Estrada-Parra, S. Phagocytosis in leprosy. 5. The effect of the infection with Mycobacterium lepraemurium on the level of diverse hydrolytic lysosomal enzymes of murine peritoneal macrophages. Int. J. Lepr. Mycobact. Dis. 1982, 50, 306–315. [Google Scholar]
- Rojas-Espinosa, O.; Lovik, M. Mycobacterium leprae and Mycobacterium lepraemurium infections in domestic and wild animals. Rev. Sci. Tech. OIE 2001, 20, 219–251. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghielmetti, G.; Schmitt, S.; Friedel, U.; Guscetti, F.; Walser-Reinhardt, L. Unusual Presentation of Feline Leprosy Caused by Mycobacterium lepraemurium in the Alpine Region. Pathogens 2021, 10, 687. https://doi.org/10.3390/pathogens10060687
Ghielmetti G, Schmitt S, Friedel U, Guscetti F, Walser-Reinhardt L. Unusual Presentation of Feline Leprosy Caused by Mycobacterium lepraemurium in the Alpine Region. Pathogens. 2021; 10(6):687. https://doi.org/10.3390/pathogens10060687
Chicago/Turabian StyleGhielmetti, Giovanni, Sarah Schmitt, Ute Friedel, Franco Guscetti, and Ladina Walser-Reinhardt. 2021. "Unusual Presentation of Feline Leprosy Caused by Mycobacterium lepraemurium in the Alpine Region" Pathogens 10, no. 6: 687. https://doi.org/10.3390/pathogens10060687
APA StyleGhielmetti, G., Schmitt, S., Friedel, U., Guscetti, F., & Walser-Reinhardt, L. (2021). Unusual Presentation of Feline Leprosy Caused by Mycobacterium lepraemurium in the Alpine Region. Pathogens, 10(6), 687. https://doi.org/10.3390/pathogens10060687





