Application of Microbiome Management in Therapy for Clostridioides difficile Infections: From Fecal Microbiota Transplantation to Probiotics to Microbiota-Preserving Antimicrobial Agents
Abstract
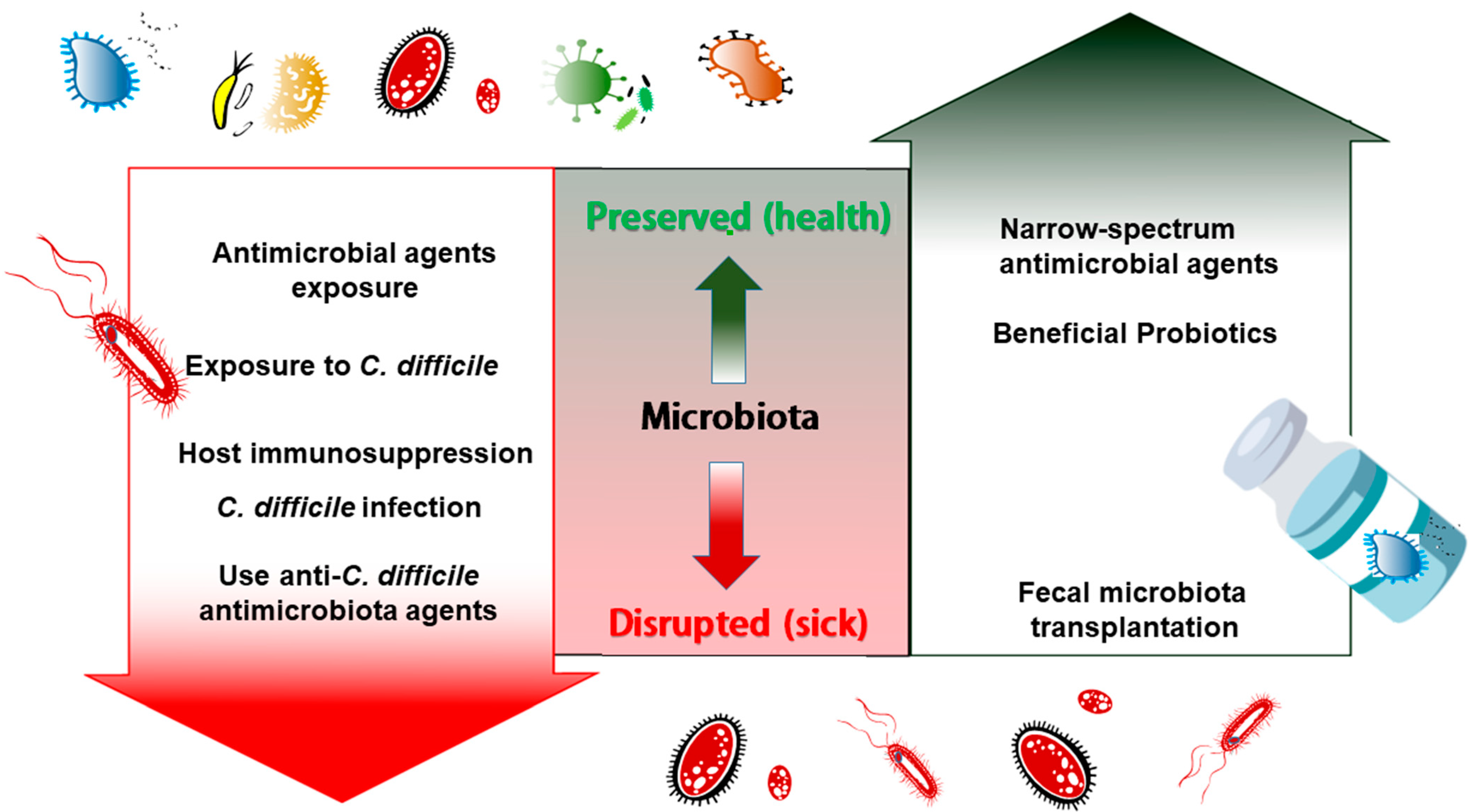
1. Disruption of Gut Microbiota after Antibiotic Exposure Results in Recurrent C. difficile Infection
2. Probiotics Supply to Restore the Disturbed Microbiota in CDI
3. Therapeutic Agents Preserving Microbiota during C. difficile Infection Treatment
4. Experimental C. difficile Animal Models for Microbiota Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuijper, E.J.; van Dissel, J.T.; Wilcox, M.H. Clostridium difficile: Changing epidemiology and new treatment options. Curr. Opin. Infect. Dis. 2007, 20, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Man, Y.X.; Bai, Y.Y.; Shao, C.H.; Liu, C.M.; Wang, C.H.; Lei, Y.X.; Wang, Y.; Jin, Y. Molecular characterization of Clostridioides difficile ribotype 027 in a major Chinese hospital. J. Microbiol. Immunol. Infect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hung, Y.P.; Tsai, B.Y.; Tsai, P.J.; Ko, W.C. Severe Clostridium difficile infections in intensive care units: Diverse clinical presentations. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.H.; Hsu, W.Y.; Yang, T.I.; Lu, C.Y.; Hsueh, P.R.; Chen, J.M.; Lee, P.I.; Huang, L.M.; Chang, L.Y. Increased age and proton pump inhibitors are associated with severe Clostridium difficile infections in children. J. Microbiol. Immunol. Infect. 2020, 53, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Lee, J.C.; Tsai, B.Y.; Wu, J.L.; Liu, H.C.; Liu, H.C.; Lin, H.J.; Tsai, P.J.; Ko, W.C. Risk factors of Clostridium difficile-associated diarrhea in hospitalized adults: Vary by hospitalized duration. J. Microbiol. Immunol. Infect. 2019. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.B.; Buffie, C.G.; Carter, R.A.; Leiner, I.; Toussaint, N.C.; Miller, L.C.; Gobourne, A.; Ling, L.; Pamer, E.G. Loss of Microbiota-Mediated Colonization Resistance to Clostridium difficile Infection With Oral Vancomycin Compared With Metronidazole. J. Infect. Dis. 2015, 212, 1656–1665. [Google Scholar] [CrossRef]
- Kelly, C.P.; LaMont, J.T. Clostridium difficile--more difficult than ever. N. Engl. J. Med. 2008, 359, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Umemura, T.; Ota, A.; Mutoh, Y.; Norizuki, C.; Mizuno, T.; Kozaki, K.; Ikeda, Y.; Ichihara, T. Efficacy of prolonged tapered and pulsed vancomycin regimen on recurrent Clostridioides difficile infection in the Japanese setting: A case control study. J. Pharm. Health Care Sci. 2019, 5, 19. [Google Scholar] [CrossRef]
- Giuffre, M.; Campigotto, M.; Campisciano, G.; Comar, M.; Croce, L.S. A story of liver and gut microbes: How does the intestinal flora affect liver disease? A review of the literature. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G889–G906. [Google Scholar] [CrossRef]
- Giuffre, M.; Moretti, R.; Campisciano, G.; da Silveira, A.B.M.; Monda, V.M.; Comar, M.; Di Bella, S.; Antonello, R.M.; Luzzati, R.; Croce, L.S. You Talking to Me? Says the Enteric Nervous System (ENS) to the Microbe. How Intestinal Microbes Interact with the ENS. J. Clin. Med. 2020, 9, 3705. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Sakamoto, K.; Seo, S.U.; Pickard, J.M.; Gillilland, M.G., 3rd; Pudlo, N.A.; Hoostal, M.; Li, X.; Wang, T.D.; Feehley, T.; et al. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science 2017, 356, 315–319. [Google Scholar] [CrossRef]
- Davenport, E.R.; Mizrahi-Man, O.; Michelini, K.; Barreiro, L.B.; Ober, C.; Gilad, Y. Seasonal variation in human gut microbiome composition. PLoS ONE 2014, 9, e90731. [Google Scholar] [CrossRef] [PubMed]
- Wuethrich, I.; Pelzer, B.W.; Khodamoradi, Y.; Vehreschild, M.J. The role of the human gut microbiota in colonization and infection with multidrug-resistant bacteria. Gut Microbes 2021, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; van Beurden, Y.H.; Terveer, E.M.; Goorhuis, A.; Bauer, M.P.; Keller, J.J.; Mulder, C.J.J.; Kuijper, E.J. Update of treatment algorithms for Clostridium difficile infection. Clin. Microbiol. Infect. 2018, 24, 452–462. [Google Scholar] [CrossRef]
- Mintz, M.; Khair, S.; Grewal, S.; LaComb, J.F.; Park, J.; Channer, B.; Rajapakse, R.; Bucobo, J.C.; Buscaglia, J.M.; Monzur, F.; et al. Longitudinal microbiome analysis of single donor fecal microbiota transplantation in patients with recurrent Clostridium difficile infection and/or ulcerative colitis. PLoS ONE 2018, 13, e0190997. [Google Scholar] [CrossRef]
- Chilton, C.H.; Pickering, D.S.; Freeman, J. Microbiologic factors affecting Clostridium difficile recurrence. Clin. Microbiol. Infect. 2018, 24, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.D.; Ajami, N.J.; Petrosino, J.F.; Jun, G.; Hanis, C.L.; Shah, M.; Hochman, L.; Ankoma-Sey, V.; DuPont, A.W.; Wong, M.C.; et al. Randomised clinical trial: Faecal microbiota transplantation for recurrent Clostridum difficile infection—fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol. Ther. 2017, 45, 899–908. [Google Scholar] [CrossRef]
- Staley, C.; Kaiser, T.; Vaughn, B.P.; Graiziger, C.; Hamilton, M.J.; Kabage, A.J.; Khoruts, A.; Sadowsky, M.J. Durable Long-Term Bacterial Engraftment following Encapsulated Fecal Microbiota Transplantation To Treat Clostridium difficile Infection. mBio 2019, 10. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Jenq, R.R.; Ajami, N.J.; Petrosino, J.F.; Alexander, A.A.; Ke, S.; Iqbal, T.; DuPont, A.W.; Muldrew, K.; Shi, Y.; et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: A randomized clinical trial. PLoS ONE 2018, 13, e0205064. [Google Scholar] [CrossRef]
- Fareed, S.; Sarode, N.; Stewart, F.J.; Malik, A.; Laghaie, E.; Khizer, S.; Yan, F.; Pratte, Z.; Lewis, J.; Immergluck, L.C. Applying fecal microbiota transplantation (FMT) to treat recurrent Clostridium difficile infections (rCDI) in children. PeerJ 2018, 6, e4663. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Ortiz, A.; Gutierrez-Delgado, E.M.; Garcia-Mazcorro, J.F.; Mendoza-Olazaran, S.; Martinez-Melendez, A.; Palau-Davila, L.; Baines, S.D.; Maldonado-Garza, H.; Garza-Gonzalez, E. Randomized clinical trial to evaluate the effect of fecal microbiota transplant for initial Clostridium difficile infection in intestinal microbiome. PLoS ONE 2017, 12, e0189768. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Vaughn, B.P.; Graiziger, C.T.; Singroy, S.; Hamilton, M.J.; Yao, D.; Chen, C.; Khoruts, A.; Sadowsky, M.J. Community dynamics drive punctuated engraftment of the fecal microbiome following transplantation using freeze-dried, encapsulated fecal microbiota. Gut Microbes 2017, 8, 276–288. [Google Scholar] [CrossRef]
- Jalanka, J.; Mattila, E.; Jouhten, H.; Hartman, J.; de Vos, W.M.; Arkkila, P.; Satokari, R. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med. 2016, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Millan, B.; Park, H.; Hotte, N.; Mathieu, O.; Burguiere, P.; Tompkins, T.A.; Kao, D.; Madsen, K.L. Fecal Microbial Transplants Reduce Antibiotic-resistant Genes in Patients With Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2016, 62, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Hamilton, M.J.; Khoruts, A.; Kilburn, A.; Unno, T.; Paliy, O.; Sadowsky, M.J. Species and genus level resolution analysis of gut microbiota in Clostridium difficile patients following fecal microbiota transplantation. Microbiome 2014, 2, 13. [Google Scholar] [CrossRef]
- Jouhten, H.; Ronkainen, A.; Aakko, J.; Salminen, S.; Mattila, E.; Arkkila, P.; Satokari, R. Cultivation and Genomics Prove Long-Term Colonization of Donor’s Bifidobacteria in Recurrent Clostridioides difficile Patients Treated With Fecal Microbiota Transplantation. Front. Microbiol. 2020, 11, 1663. [Google Scholar] [CrossRef]
- Girotra, M.; Garg, S.; Anand, R.; Song, Y.; Dutta, S.K. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection in the Elderly: Long-Term Outcomes and Microbiota Changes. Dig. Dis. Sci. 2016, 61, 3007–3015. [Google Scholar] [CrossRef]
- Dutta, S.K.; Girotra, M.; Garg, S.; Dutta, A.; von Rosenvinge, E.C.; Maddox, C.; Song, Y.; Bartlett, J.G.; Vinayek, R.; Fricke, W.F. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin. Gastroenterol. Hepatol. 2014, 12, 1572–1576. [Google Scholar] [CrossRef]
- Song, Y.; Garg, S.; Girotra, M.; Maddox, C.; von Rosenvinge, E.C.; Dutta, A.; Dutta, S.; Fricke, W.F. Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection. PLoS ONE 2013, 8, e81330. [Google Scholar] [CrossRef]
- Brown, J.R.; Flemer, B.; Joyce, S.A.; Zulquernain, A.; Sheehan, D.; Shanahan, F.; O’Toole, P.W. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol. 2018, 18, 131. [Google Scholar] [CrossRef]
- Anand, R.; Song, Y.; Garg, S.; Girotra, M.; Sinha, A.; Sivaraman, A.; Phillips, L.; Dutta, S.K. Effect of Aging on the Composition of Fecal Microbiota in Donors for FMT and Its Impact on Clinical Outcomes. Dig. Dis. Sci. 2017, 62, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Koziel, J.; Dieterich, W.; Haziri, D.; Wirtz, S.; Glowczyk, I.; Konturek, K.; Neurath, M.F.; Zopf, Y. Successful therapy of Clostridium difficile infection with fecal microbiota transplantation. J. Physiol. Pharmacol. 2016, 67, 859–866. [Google Scholar] [CrossRef]
- Langdon, A.; Schwartz, D.J.; Bulow, C.; Sun, X.; Hink, T.; Reske, K.A.; Jones, C.; Burnham, C.D.; Dubberke, E.R.; Dantas, G.; et al. Microbiota restoration reduces antibiotic-resistant bacteria gut colonization in patients with recurrent Clostridioides difficile infection from the open-label PUNCH CD study. Genome Med. 2021, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; McDonald, J.A.K.; Pechlivanis, A.; Allegretti, J.R.; Kao, D.; Barker, G.F.; Kapila, D.; Petrof, E.O.; Joyce, S.A.; Gahan, C.G.M.; et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut 2019, 68, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, T.; Mullish, B.H.; Patterson, J.; Wong, G.K.; Marchesi, J.R.; Xu, H.; Jilani, T.; Kao, D. Effective fecal microbiota transplantation for recurrent Clostridioides difficile infection in humans is associated with increased signalling in the bile acid-farnesoid X receptor-fibroblast growth factor pathway. Gut Microbes 2019, 10, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Solbach, P.; Chhatwal, P.; Woltemate, S.; Tacconelli, E.; Buhl, M.; Gerhard, M.; Thoeringer, C.K.; Vehreschild, M.; Jazmati, N.; Rupp, J.; et al. BaiCD gene cluster abundance is negatively correlated with Clostridium difficile infection. PLoS ONE 2018, 13, e0196977. [Google Scholar] [CrossRef] [PubMed]
- Seekatz, A.M.; Theriot, C.M.; Rao, K.; Chang, Y.M.; Freeman, A.E.; Kao, J.Y.; Young, V.B. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe 2018, 53, 64–73. [Google Scholar] [CrossRef]
- Ianiro, G.; Valerio, L.; Masucci, L.; Pecere, S.; Bibbo, S.; Quaranta, G.; Posteraro, B.; Curro, D.; Sanguinetti, M.; Gasbarrini, A.; et al. Predictors of failure after single faecal microbiota transplantation in patients with recurrent Clostridium difficile infection: Results from a 3-year, single-centre cohort study. Clin. Microbiol. Infect. 2017, 23, 337.e1–e3. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Phelps, E.; Ganapini, V.; Khan, N.; Ouyang, F.; Xu, H.; Khanna, S.; Tariq, R.; Friedman-Moraco, R.J.; Woodworth, M.H.; et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: A multicenter experience. Am. J. Transplant. 2018, 19, 501–511. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Pochapin, M. The effect of probiotics on Clostridium difficile diarrhea. Am. J. Gastroenterol. 2000, 95, S11–S13. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.A.; Karmirantzou, M.; Snel, B.; Vilanova, D.; Berger, B.; Pessi, G.; Zwahlen, M.C.; Desiere, F.; Bork, P.; Delley, M.; et al. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 2002, 99, 14422–14427. [Google Scholar] [CrossRef]
- Trejo, F.M.; Minnaard, J.; Perez, P.F.; De Antoni, G.L. Inhibition of Clostridium difficile growth and adhesion to enterocytes by Bifidobacterium supernatants. Anaerobe 2006, 12, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Gargari, G.; Taverniti, V.; Balzaretti, S.; Ferrario, C.; Gardana, C.; Simonetti, P.; Guglielmetti, S. Consumption of a Bifidobacterium bifidum Strain for 4 Weeks Modulates Dominant Intestinal Bacterial Taxa and Fecal Butyrate in Healthy Adults. Appl. Environ. Microbiol. 2016, 82, 5850–5859. [Google Scholar] [CrossRef]
- Barker, A.K.; Duster, M.; Valentine, S.; Hess, T.; Archbald-Pannone, L.; Guerrant, R.; Safdar, N. A randomized controlled trial of probiotics for Clostridium difficile infection in adults (PICO). J. Antimicrob. Chemother. 2017, 72, 3177–3180. [Google Scholar] [CrossRef]
- Maziade, P.J.; Pereira, P.; Goldstein, E.J. A Decade of Experience in Primary Prevention of Clostridium difficile Infection at a Community Hospital Using the Probiotic Combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin. Infect. Dis. 2015, 60 (Suppl. S2), S144–S147. [Google Scholar] [CrossRef]
- Kujawa-Szewieczek, A.; Adamczak, M.; Kwiecien, K.; Dudzicz, S.; Gazda, M.; Wiecek, A. The Effect of Lactobacillus plantarum 299v on the Incidence of Clostridium difficile Infection in High Risk Patients Treated with Antibiotics. Nutrients 2015, 7, 10179–10188. [Google Scholar] [CrossRef] [PubMed]
- Hickson, M.; D’Souza, A.L.; Muthu, N.; Rogers, T.R.; Want, S.; Rajkumar, C.; Bulpitt, C.J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: Randomised double blind placebo controlled trial. BMJ 2007, 335, 80. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Verstraelen, H.; Osowska, S.; Doerffel, Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology 2008, 135, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Koon, H.W.; Su, B.; Xu, C.; Mussatto, C.C.; Tran, D.H.; Lee, E.C.; Ortiz, C.; Wang, J.; Lee, J.E.; Ho, S.; et al. Probiotic Saccharomyces boulardii CNCM I-745 prevents outbreak-associated Clostridium difficile-associated cecal inflammation in hamsters. Am. J. Physiol. Gastrointest Liver Physiol. 2016, 311, G610–G623. [Google Scholar] [CrossRef]
- Surawicz, C.M.; McFarland, L.V.; Greenberg, R.N.; Rubin, M.; Fekety, R.; Mulligan, M.E.; Garcia, R.J.; Brandmarker, S.; Bowen, K.; Borjal, D.; et al. The search for a better treatment for recurrent Clostridium difficile disease: Use of high-dose vancomycin combined with Saccharomyces boulardii. Clin. Infect. Dis. 2000, 31, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Fekety, R.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L.; Noorani, Z.; et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994, 271, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, Y.; Huang, Y.; Xiao, Y.; Song, L.; Lu, S.; Ren, Z. A strain of Bacteroides thetaiotaomicron attenuates colonization of Clostridioides difficile and affects intestinal microbiota and bile acids profile in a mouse model. Biomed. Pharmacother. 2021, 137, 111290. [Google Scholar] [CrossRef]
- Deng, H.; Yang, S.; Zhang, Y.; Qian, K.; Zhang, Z.; Liu, Y.; Wang, Y.; Bai, Y.; Fan, H.; Zhao, X.; et al. Bacteroides fragilis Prevents Clostridium difficile Infection in a Mouse Model by Restoring Gut Barrier and Microbiome Regulation. Front. Microbiol. 2018, 9, 2976. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.C.; McCartney, A.L.; Gibson, G.R. Intestinal microflora of human infants and current trends for its nutritional modulation. Br. J. Nutr. 2002, 87, 405–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oka, K.; Osaki, T.; Hanawa, T.; Kurata, S.; Sugiyama, E.; Takahashi, M.; Tanaka, M.; Taguchi, H.; Kamiya, S. Establishment of an Endogenous Clostridium difficile Rat Infection Model and Evaluation of the Effects of Clostridium butyricum MIYAIRI 588 Probiotic Strain. Front. Microbiol. 2018, 9, 1264. [Google Scholar] [CrossRef]
- Seki, H.; Shiohara, M.; Matsumura, T.; Miyagawa, N.; Tanaka, M.; Komiyama, A.; Kurata, S. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr. Int. 2003, 45, 86–90. [Google Scholar] [CrossRef]
- Nagamine, T.; Matsumoto, Y.; Nakamura, M. Combination probiotics may prevent Clostridium difficile infection among elderly patients undergoing an orthopedic surgery. Biosci. Microbiota Food Health 2019, 38, 31–33. [Google Scholar] [CrossRef]
- Khoruts, A. Targeting the microbiome: From probiotics to fecal microbiota transplantation. Genome Med. 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Hoffmann, D.E.; Khoruts, A. Probiotics and the Microbiome-How Can We Help Patients Make Sense of Probiotics? Gastroenterology 2021, 160, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.; Mian, S.; Paolino, K.; Siddique, Z. Lactobacillus masticator abscess after probiotics consumption. Proc. (Bayl. Univ. Med. Cent.) 2020, 34, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Chen, C.L.; Feng, Y.; Chen, C.C.; Lien, R.; Chiu, C.H. Lactobacillus rhamnosus sepsis associated with probiotic therapy in an extremely preterm infant: Pathogenesis and a review for clinicians. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sendil, S.; Shrimanker, I.; Mansoora, Q.; Goldman, J.; Nookala, V.K. Lactobacillus rhamnosus Bacteremia in an Immunocompromised Renal Transplant Patient. Cureus 2020, 12, e6887. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Konishi, H.; Aoki, K.; Ishii, Y.; Chono, K.; Tateda, K. The gut microbiome diversity of Clostridioides difficile-inoculated mice treated with vancomycin and fidaxomicin. J. Infect. Chemother 2020, 26, 483–491. [Google Scholar] [CrossRef]
- Ajami, N.J.; Cope, J.L.; Wong, M.C.; Petrosino, J.F.; Chesnel, L. Impact of Oral Fidaxomicin Administration on the Intestinal Microbiota and Susceptibility to Clostridium difficile Colonization in Mice. Antimicrob. Agents Chemother 2018, 62. [Google Scholar] [CrossRef]
- Guery, B.; Menichetti, F.; Anttila, V.J.; Adomakoh, N.; Aguado, J.M.; Bisnauthsing, K.; Georgopali, A.; Goldenberg, S.D.; Karas, A.; Kazeem, G.; et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): A randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect. Dis. 2018, 18, 296–307. [Google Scholar] [CrossRef]
- Louie, T.J.; Cannon, K.; Byrne, B.; Emery, J.; Ward, L.; Eyben, M.; Krulicki, W. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin. Infect. Dis. 2012, 55 (Suppl. S2), S132–S142. [Google Scholar] [CrossRef]
- Qian, X.; Yanagi, K.; Kane, A.V.; Alden, N.; Lei, M.; Snydman, D.R.; Vickers, R.J.; Lee, K.; Thorpe, C.M. Ridinilazole, a narrow spectrum antibiotic for treatment of Clostridioides difficile infection, enhances preservation of microbiota-dependent bile acids. Am. J. Physiol. Gastrointest Liver Physiol 2020, 319, G227–G237. [Google Scholar] [CrossRef]
- Vickers, R.J.; Tillotson, G.S.; Nathan, R.; Hazan, S.; Pullman, J.; Lucasti, C.; Deck, K.; Yacyshyn, B.; Maliakkal, B.; Pesant, Y.; et al. Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: A phase 2, randomised, double-blind, active-controlled, non-inferiority study. Lancet Infect Dis. 2017, 17, 735–744. [Google Scholar] [CrossRef]
- Gerding, D.N.; Cornely, O.A.; Grill, S.; Kracker, H.; Marrast, A.C.; Nord, C.E.; Talbot, G.H.; Buitrago, M.; Gheorghe Diaconescu, I.; Murta de Oliveira, C.; et al. Cadazolid for the treatment of Clostridium difficile infection: Results of two double-blind, placebo-controlled, non-inferiority, randomised phase 3 trials. Lancet Infect Dis. 2019, 19, 265–274. [Google Scholar] [CrossRef]
- Chilton, C.H.; Crowther, G.S.; Baines, S.D.; Todhunter, S.L.; Freeman, J.; Locher, H.H.; Athanasiou, A.; Wilcox, M.H. In vitro activity of cadazolid against clinically relevant Clostridium difficile isolates and in an in vitro gut model of C. difficile infection. J. Antimicrob Chemother 2014, 69, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.B. Anti-Sense Antibiotic Agents as Treatment for Bacterial Infections. Surg. Infect. 2018, 19, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, J.P.; Krzeminski, J.; Sharma, A.K.; Guzman-Villanueva, D.; Weissig, V.; Stewart, D.B., Sr. Bolaamphiphile-based nanocomplex delivery of phosphorothioate gapmer antisense oligonucleotides as a treatment for Clostridium difficile. Int. J. Nanomedicine 2016, 11, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.K.; Harris, H.C.; Smith, M.; Giles, J.; Polak, O.; Buckley, A.M.; Clark, E.; Ewin, D.; Moura, I.B.; Spitall, W.; et al. A Novel, Orally Delivered Antibody Therapy and Its Potential to Prevent Clostridioides difficile Infection in Pre-clinical Models. Front. Microbiol. 2020, 11, 578903. [Google Scholar] [CrossRef]
- Dzunkova, M.; D’Auria, G.; Xu, H.; Huang, J.; Duan, Y.; Moya, A.; Kelly, C.P.; Chen, X. The Monoclonal Antitoxin Antibodies (Actoxumab-Bezlotoxumab) Treatment Facilitates Normalization of the Gut Microbiota of Mice with Clostridium difficile Infection. Front. Cell Infect. Microbiol. 2016, 6, 119. [Google Scholar] [CrossRef]
- Jones, J.A.; Prior, A.M.; Marreddy, R.K.R.; Wahrmund, R.D.; Hurdle, J.G.; Sun, D.; Hevener, K.E. Small-Molecule Inhibition of the C. difficile FAS-II Enzyme, FabK, Results in Selective Activity. ACS Chem. Biol. 2019, 14, 1528–1535. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Lundberg, R.; Toft, M.F.; August, B.; Hansen, A.K.; Hansen, C.H. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes 2016, 7, 68–74. [Google Scholar] [CrossRef]
- Hazenberg, M.P.; Bakker, M.; Verschoor-Burggraaf, A. Effects of the human intestinal flora on germ-free mice. J. Appl. Bacteriol. 1981, 50, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Im, S.H. Of men in mice: The development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 2020, 52, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Raibaud, P.; Ducluzeau, R.; Dubos, F.; Hudault, S.; Bewa, H.; Muller, M.C. Implantation of bacteria from the digestive tract of man and various animals into gnotobiotic mice. Am. J. Clin. Nutr. 1980, 33, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Mallett, A.K.; Bearne, C.A.; Rowland, I.R.; Farthing, M.J.; Cole, C.B.; Fuller, R. The use of rats associated with a human faecal flora as a model for studying the effects of diet on the human gut microflora. J. Appl. Bacteriol. 1987, 63, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Palm, N.W. Causal effects of the microbiota on immune-mediated diseases. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Feng, H.; Parry, N.; Tzipori, S. Piglet models of acute or chronic Clostridium difficile illness. J. Infect. Dis. 2010, 201, 428–434. [Google Scholar] [CrossRef]
- Krych, L.; Hansen, C.H.; Hansen, A.K.; van den Berg, F.W.; Nielsen, D.S. Quantitatively different, yet qualitatively alike: A meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS ONE 2013, 8, e62578. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Burz, S.D.; Abraham, A.L.; Fonseca, F.; David, O.; Chapron, A.; Beguet-Crespel, F.; Cenard, S.; Le Roux, K.; Patrascu, O.; Levenez, F.; et al. A Guide for Ex Vivo Handling and Storage of Stool Samples Intended for Fecal Microbiota Transplantation. Sci. Rep. 2019, 9, 8897. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Country | Recipient Numbers | Procedures of FMT | Effects on CDIs | Influence on Receipt Microbiota | Reference |
|---|---|---|---|---|---|---|---|
| Amy Langdon et al. | 2013 | USA | 29 | RBX2660, a liquid suspension of donor microbiota via enema | 41.3% patients had no recurrence after a single dose; others had a recurrence and required a repeat dose | Reduced the abundance of antibiotic-resistant Enterobacteriaceae in two months | [34] |
| Yang Song et al. | 2013 | USA | 14 | Combined enteroscopy and colonoscopy | rCDI treated successfully by FMT | Members of Streptococci or Enterobacteriaceae were significantly decreased and putative butyrate producers, such as Lachnospiraceae and Ruminococcaceae, were significantly increased. | [30] |
| Sudhir K Dutta et al. | 2014 | USA | 27 | Combined enteroscopy and colonoscopy | All recipients had clinical resolution | Increased microbial diversity, increasing proportions of Lachnospiraceae (phylum Firmicutes) and reducing proportions of Enterobacteriaceae. | [29] |
| Anna M. Seekatz et al. | 2014 | USA | 6 | Colonoscopy | All recipients had clinical resolution of CDI following FMT and were recurrence-free up to six months. | Metabolites that increased following FMT were associated with bacteria classified within the Lachnospiraceae, Ruminococcaceae, and unclassified Clostridiales families. | [38] |
| Vijay Shankar et al. | 2014 | Finland | 3 | Colonoscopy | All patients had reduced diarrheal symptoms | Rich in members of Blautia, Coprococcus, and Faecalibacterium. | [26] |
| Michael Mintz et al. | 2013–2016 | USA | 11 | Colonoscopy | No rCDI after one year of follow-up | Reduced beta diversity differences between the donors and recipients, and increased in relative abundance of F. prausnitzii. | [16] |
| P C Konturek et al. | 2014–2016 | German | 17 | Colonoscopy | The healing rate of CDI was 94%. In all successfully treated patients no recurrent CDI was observed during follow-up (16 months) | Elevated abundance of beneficial bacterial species such as Lactobacillaceae, Ruminococcaceae, Desulfovibrionaceae, Sutterellaceae, and Porphyromonodacea after FMT. | [33] |
| Jonna Jalanka et al. | 2016 | Finland | 14 | The fecal suspension was infused into the cecum | The FMT treatment cleared rCDI from all patients | Dominated by Clostridium clusters IV and XIVa, with increased Bacteroidetes. | [24] |
| Braden Millan et al. | 2016 | Canada | 20 | Colonoscopy | FMT resulted in a resolution of symptoms | Decreased number and diversity of antibiotic resistance genes and increased Bacteroidetes and Firmicutes with reduced Proteobacteria. | [25] |
| Mohit Girotra et al. | 2016 | USA | 29 | Combined colonoscopy and nasojejunal tube | Marked improvement in all clinical parameters | Microbiota diversity increased with Proteobacteria decreased. | [28] |
| Christopher Staley et al. | 2016 | USA | 39 | Prepared as capsule and oral intake | Rapid resolution of rCDI symptoms | Taxa within the Firmicutes showed rapid increases in relative abundance and did not vary significantly over time. Bacteroidetes taxa only showed significant increases in abundance after one-month post-FMT. | [23] |
| Adrián Camacho-Ortiz et al. | 2017 | United Kingdom | 7 | Either colonoscopy or nasojejunal tube | Symptoms resolved in 57.1% patients after the first FMT and in 71.4% after the second dose. | The bacterial composition was dominated by Firmicutes, Bacteroidetes, and Proteobacteria at all-time points, and the microbiota were remarkably stable over time. | [22] |
| Z D Jiang et al. | 2017 | USA | 72 | Randomized to receive fresh, frozen or lyophilized FMT product via colonoscopy | Overall resolution of CDI was 87% during 2 months of follow-up. | Microbial diversity was reconstituted by day 7 with fresh or frozen product; by 30 days with lyophilized material. | [18] |
| Shaaz Fareed et al. | 2017 | USA | 15 | Either colonoscopy or nasojejunal tube | Prevented recurrent CDI for minimum of 3 months post-FMT in all patients. | Increased levels of Bacteroidetes and decreased levels of Proteobacteria | [21] |
| Jillian R-M Brown et al. | 2018 | Ireland | 10 | Both esophago-gastro-duodenoscopy and full colonoscopy | Nine out of ten patients improved clinically and remained C. difficile toxin negative for between 6 months and 2 years after a single FMT | FMT moves the microbiota of recipients towards that of the donor and improves bacterial diversity. | [31] |
| Zhi-Dong Jiang et al. | 2018 | USA | 65 | Encapsulated lyophilized fecal microbiota (n = 31) or frozen FMT (n = 34) by enema. | CDI recurrence rate after FMT: 84% in capsule group; 88% in FMT enema group, p = 0.76 | Both products normalized fecal microbiota diversity while the lyophilized orally administered product was less effective in repleting Bacteroidia and Verrucomicrobia classes compared to frozen product via enema. | [20] |
| Christopher Staley et al. | 2019 | USA | 18 | encapsulated lyophilized fecal microbiota orally | All recovered clinically and were free of CDI | Members of the genera Bacteroides, Parabacteroides, and Faecalibacterium were positively correlated with donor similarity. | [19] |
| Hanne Jouhten et al. | 2020 | Finland | 13 | ND | Only recipients with rCDI successfully treated with FMT were included, | Specific donor-derived bifidobacterium can colonize rCDI patients for at least one year. | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-W.; Tsai, P.-J.; Lee, C.-C.; Ko, W.-C.; Hung, Y.-P. Application of Microbiome Management in Therapy for Clostridioides difficile Infections: From Fecal Microbiota Transplantation to Probiotics to Microbiota-Preserving Antimicrobial Agents. Pathogens 2021, 10, 649. https://doi.org/10.3390/pathogens10060649
Chiu C-W, Tsai P-J, Lee C-C, Ko W-C, Hung Y-P. Application of Microbiome Management in Therapy for Clostridioides difficile Infections: From Fecal Microbiota Transplantation to Probiotics to Microbiota-Preserving Antimicrobial Agents. Pathogens. 2021; 10(6):649. https://doi.org/10.3390/pathogens10060649
Chicago/Turabian StyleChiu, Chun-Wei, Pei-Jane Tsai, Ching-Chi Lee, Wen-Chien Ko, and Yuan-Pin Hung. 2021. "Application of Microbiome Management in Therapy for Clostridioides difficile Infections: From Fecal Microbiota Transplantation to Probiotics to Microbiota-Preserving Antimicrobial Agents" Pathogens 10, no. 6: 649. https://doi.org/10.3390/pathogens10060649
APA StyleChiu, C.-W., Tsai, P.-J., Lee, C.-C., Ko, W.-C., & Hung, Y.-P. (2021). Application of Microbiome Management in Therapy for Clostridioides difficile Infections: From Fecal Microbiota Transplantation to Probiotics to Microbiota-Preserving Antimicrobial Agents. Pathogens, 10(6), 649. https://doi.org/10.3390/pathogens10060649







